Introduction
Hepatocellular carcinoma (HCC) is the most common
form of primary liver cancer and the third most deadly malignancy
worldwide, particularly in China where the mortality rate
associated with HCC accounts for more than 50% of the worldwide
rate (1). Metastasis is one of the
main obtacles to improving the survival rate and long-term
prognosis of patients with HCC. It is well known that the final
site of metastasis of tumor cells is not random but organ-specific,
in which chemokine receptors play important roles in the chemotaxis
of tumor cells to target organs (2,3).
Identification of these metastasis-related chemokine receptors may
provide potential targets for use in tumor therapy.
CXCR7, a recently identified orphan receptor, has
been demonstrated to have the definitive ligand stromal
cell-derived factor-1 (SDF-1) and truncated I-TAC (CXCL11)
(4,5). CXCR7 has been found crucial for
proper migration of primordial germ cells toward their targets in
zebra fish (6). Recently, high
expression of CXCR7 has been shown to be correlated with highly
aggressive prostate tumors. (7) In
addition, higher expression of CXCR7 was found to be linked to
early and metastatic recurrence in pathological stage I non-small
cell lung cancer (8). Most
recently, research has reveaked the high expression of CXCR7 in the
HCC cell line SMMC-7721 and overexpression in tumor tissues
(9). However, whether CXCR7
participates in metastasis of HCC is yet unclear.
HCC is prone to metastasize to the lung, bone and
brain; in particular lung metastasis occurs in 30% of patients with
HCC. In the present study, expression of CXCR7 in HCC cell lines
with different lung metastatic potential was observed. Furthermore,
the effects of targeting CXCR7 on the proliferation and metastasis
of a highly metastatic HCC cell line were observed in vitro
and in vivo. Additionally, expression of CXCR7 in specimens
from patients with metastatic HCC was evaluated.
Materials and methods
Cell lines
Three human HCC cell lines were used. HCCLM3 (100%
lung metastatic potential) and MHCC97-L (low metastatic potential)
(10) were established in the
Liver Cancer Institute of Fudan University. SMMC-7721 (without lung
metastatic potential) was obtained from the Chinese Academy of
Science (Shanghai, China). All cell lines were cultured in high
glucose DMEM (Gibco-BRL, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (Hyclone, UT, USA).
Animals
Male 6- to 8-week-old BALB/c nu/nu mice were
obtained from the Chinese Academy of Sciences and maintained in
accordance with the recommendations of the NIH Guidelines for the
Care and Use of Laboratory Animals.
Patients
One hundred and sixteen specimens were used for the
tissue microarray (TMA) preparation and consisted of paraffin
tissue blocks from HCC patients. These patients underwent hepatic
resection at the Liver Cancer Institute, Zhongshan Hospital of
Fudan University from January 2000 to July 2004, and were followed
up by pulmonary CT regularly until July 2009. All patients
presented with pathological type HCC.
Gene microarray
Oligo GEArray® human chemokines and a
chemokine receptor chip (SuperArray Bioscience, Frederick, MD, USA)
was repeated twice to compare the profiles of the HCCLM3, MHCC97-L
and SMMC-7721 cells. Total RNA was extracted, isolated and purified
according to the protocol of the TRIzol reagent (Invitrogen Corp.,
Carlsbad, CA). Subsequent procedures were carried out according to
protocols included in the SuperArray reagent packaging.
Real-time reverse transcription-PCR
analyses
Real-time reverse transcription-PCR (RT-PCR) was
carried out using TaqMan PCR reagents and the ABI PRISM 7700
sequence detection system (Applied Biosystems, Foster, CA) in
accordance with a previously described protocol (11). Reactions were performed in
20-μl volumes, each containing 2 μl cDNA derived from
50 ng RNA using the following primer pairs: CXCR7 sense,
5′-GGGATGCAGCGGATAGTCAA-3′ and antisense,
5′-CGGTCGTTGTCCACATCCA-3′; Taqman probe,
5′-TCGGTCTCTCCCTGCCCGTCCT-3′.
Western blotting
Western blotting was performed according to the
protocol of the Bio-Rad wet transfer using the Bio-Rad Transfer
Cell system (Bio-Rad, Mississauga, Ontario, Canada). Rabbit
anti-human CXCR7 IgG antibody (1:800, Novus Biologicals, Littleton,
CO, USA) followed by 1:3,000 horseradish peroxidase-conjugated goat
anti-rabbit IgG F(ab’)2 antibody (Jackson ImmunoResearch, West
Grove, PA) were used.
Chemotaxis assay and antibody inhibition
assay
Lung extractions were prepared as described
previously from nude mice (12).
The chemotaxis assay was performed using a 24-well Transwell
(Corning Costar Corporation, Corning, NY) in line with the protocol
described by Hujanen and Terranova (13). In brief, the upper parts with
polycarbonate filters (8 μm pore size) were coated with
fibronectin (5.0 μg/l; 37°C, 2 h). HCCLM3 cells
(1.0×103 in 100 μl DMEM) were collected and added
to the pre-coated wells. DMEM (600 μl) containing SDF-1α was
added to the lower compartment. SDF-1α (1 to 100 μg/l) or
lung extractions at 1 g/l (12)
were used. After 24 h at 37°C, the cells migrating to the membrane
were enumerated with Giemsa staining. Rabbit anti-human CXCR4 IgG
antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 20
μg/ml (14) was added when
performing the inhibition assay. Each assay was performed three
times independently.
RNA interference (RNAi)
CXCR7 expression in HCCLM3 cells was down-regulated
by small interference RNAs (siRNAs) which were designed and
synthesized by GenePharma Corp. (Shanghai, China) as follows:
siCXCR7-241, 5′-CGG UGA UGU GUC CCA ACA UdTdT-3′ (position at 241
of CXCR7 mRNA); siCXCR7-286, 5′-CGC UCU CCU UCA UUU ACA UdTdT-3′
(position at 286); siCXCR7-362, 5′-GGC CAA GAC CAC AGG CUA UdTdT-3′
(position at 362); siCXCR7-1133, 5′-GGC CUU CAU CUU CAA GUA
CdTdT-3′ (position at 1133); negative control siRNA, 5′-UUC UCC GAA
CGU GUC ACG UTT-3′; glyceraldehyde phosphate dehydrogenase (GAPDH)
positive control siRNA, 5′-GUA UGA CAA CAG CCU CAA GTT-3′. SiRNA
transfection of HCCLM3 cells was performed according to
Lipofectamine 2000 protocol (15).
Cell proliferation assay
Cells (3×103/well) were plated in 96-well
plates 18 h prior to CXCR7 siRNA transfection. Transfection was
carried out according to Lipofectamine 2000 96-well protocol. After
incubation for 0–4 days, the cells were assayed for proliferation
by the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide assay (Chemicon, Temecula, CA, USA) following the
manufacturer’s protocol. Each assay was performed three times
independently.
Invasion assay
In 24-well Transwell chambers, the upper parts with
polycarbonate filters (8-μm pore size; Costar, Acton, MA,
USA) were coated with 50 μl of Matrigel (0.8 mg/ml, 37°C, 2
h) (BD Biosciences, San Diego, CA, USA). After siRNA transfection
for 24 h, HCCLM3 cells (1.0×103 in 100 μl DMEM)
were collected and added to the pre-coated wells. The cells were
then allowed to migrate toward the lower compartment containing
conditioned medium as a chemoattractant (37°C, 40 h). The cells
migrating to the membrane were enumerated with Giemsa staining. The
assay was performed three times independently.
Gelatin zymogram assay
HCCLM3 cells were transfected with siRNA of CXCR7
for 24 and 48 h prior to the gelatin zymogram assay. Analyses of
matrix metalloproteinases (MMP)-2 and MMP-9 were performed on Novex
zymogram gels (Invitrogen) as described by Jacob et al
(16) except that 20 μg
supernatant/lane was run on a 10% Tris-glycine polyacrylamide gel
containing 0.1% gelatin. Each assay was carried out thrice
independently.
In vivo study
HCCLM3 tumors were established by subcutaneous
injection of the tumor cells with or without CXCR7 siRNA
transfection into the dorsal flanks of nude mice
(1×107/mouse, n=6). The RNAi strategy of CXCR7 in nude
mice is described in Table I. On
the 49th day after implantation, mice were sacrificed to examine
tumor growth and metastases. Tumor size (tumor volume =
ab2/2 in mm3, where a and b are the longest
and the shortest perpendicular diameters of the tumor,
respectively) and tumor weight were measured. Lung metastasis was
evaluated using sequential sections of paraffin-embedded
tissue.
 | Table I.RNA interference strategy of CXCR7
in vivo. |
Table I.
RNA interference strategy of CXCR7
in vivo.
| Group | Pre-treatment | Drug | Dose | Application |
|---|
| 1 | None | None | None | None |
| 2 | siRNA-286 | None | None | None |
| 3 | siRNA-286 | Chemically modified
siRNA-286 | 0.5 μg/g in
100 μl | i.v., twice per
week, continuous 7 weeks from inoculation, or days 1, 4, 8, 11, 15,
18, 22, 25, 29, 32, 36, 39, 43, 46 |
TMA construction and immunohistochemical
detection
Specimens from the HCC patients were divided into
two groups: with or without lung metastasis. TMA was constructed in
accordance with standard procedures as previously described
(17,18). Immunohistochemical detection of the
TMA was carried out using the avidin-biotin complex method (ABC,
Vector Laboratories, Burlingame, CA, USA). Rabbit anti-human CXCR7
IgG at 1:200 and rabbit anti-human CXCR4 IgG (Santa Cruz
Biotechnology) at 1:100 were used as the primary antibodies for
detection. Detection without a primary antibody was considered the
negative control. The immunoassay was defined by the staining
intensity (0–3) and the percentage of positive tumor cells as
described previously (18). Two
pathologists observed the results independently.
Statistical analysis
SPSS 15.0 Statistical Package (SPSS Inc., Chicago,
IL, USA) was used to analyze the data. Results are expressed as the
mean ± SD. One-way ANOVA was used for intergroup comparisons. When
two groups of cells or animals were compared, analysis was
performed with the Student’s t-test. Pearson Chi-square test or
Fisher’s exact test was used to compare qualitative variables.
Differences between experimental results were considered to be
significant at a P-value <0.05.
Results
Expression of CXCR7 is significantly
higher in a highly metastatic HCC cell line
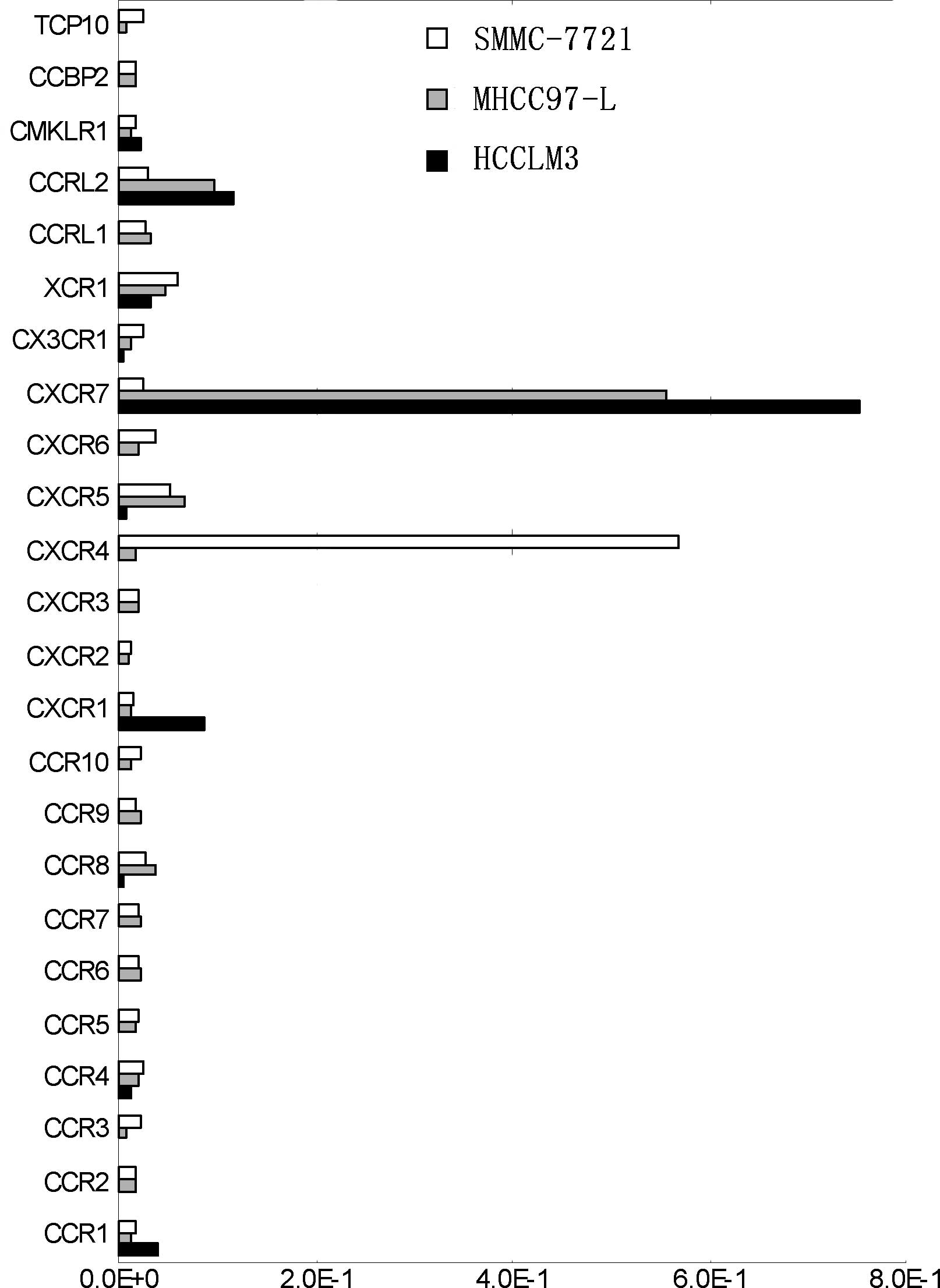
The chemokine receptor gene chip showed that the
expression of CXCR7, CXCR1 and CCR1 was up-regulated markedly in
the highly metastatic HCCLM3 cells, particularly CXCR7 which
demonstrated a significantly high expression (Fig. 1). CXCR4, on the other hand, was
nearly undetectable and CXCR7 exhibited a reverse trend of
expression (Fig. 1). Furthermore,
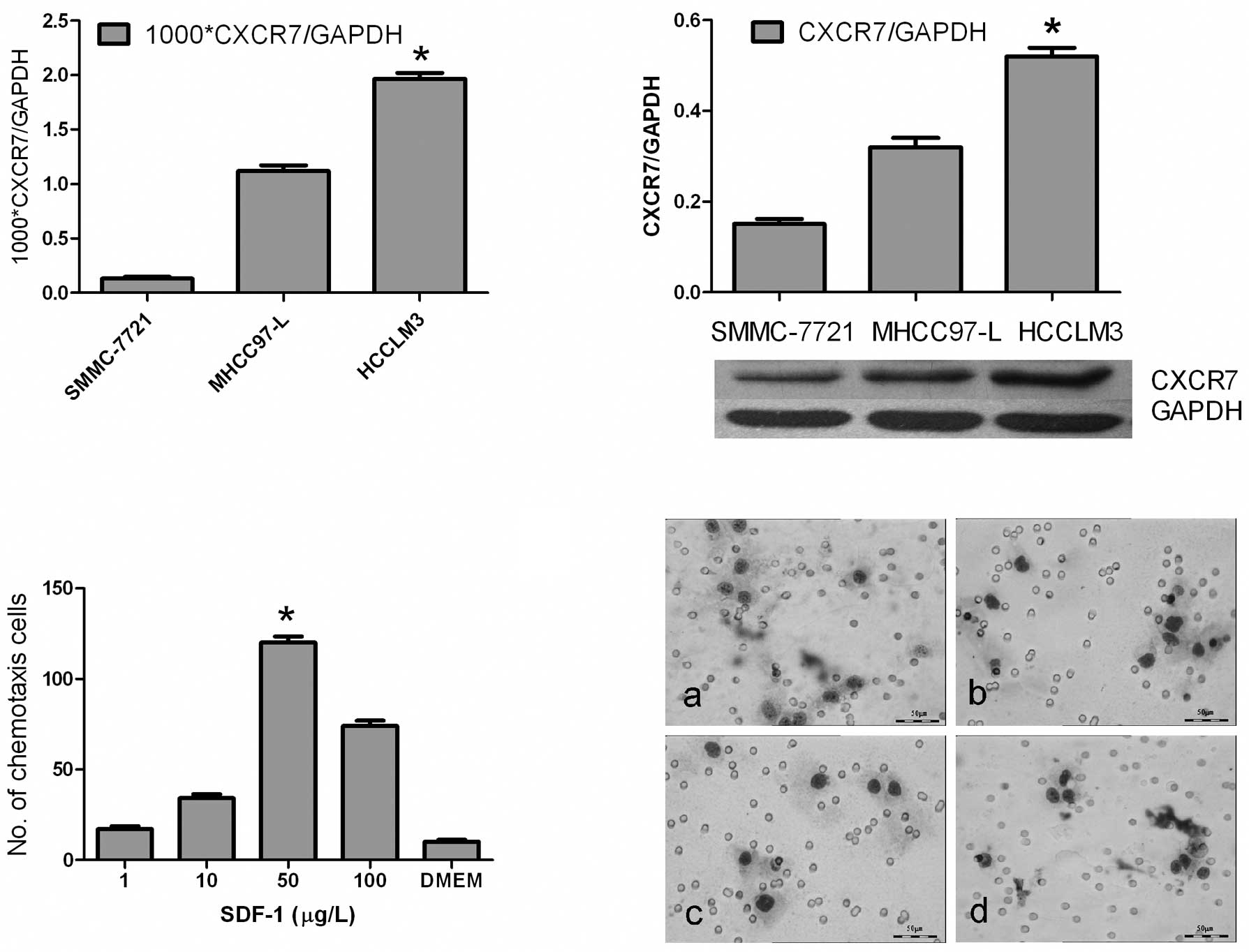
real-time PCR showed that the Ct value of CXCR7 relative to GAPDH
was more markedly up-regulated in the HCCLM3 cell line than values
in MHCC97-L and SMMC-7721 (1.96±0.15×10−3 vs.
1.12±0.22×10−3 and 0.13±0.10×10−3,
respectively; P<0.01) (Fig.
2A). Western blotting showed that the protein level of CXCR7
was in accordance with the mRNA level (P<0.05)(Fig. 2B).
HCCLM3 cells functionally respond to
SDF-1α and lung extractions
Chemotaxis assay showed that the HCCLM3 cells
responded to SDF-1α from 1 to 100 ng/ml, particularly at the
concentration of 50 ng/ml (P<0.01) (Fig. 2C). However, addition of the CXCR4
antibody (20 μg/ml) did not inhibit the response of HCCLM3
cells to SDF-1α at the optimal concentration of 50 ng/ml
(112±9/field vs. 118±7/field, P>0.05). Also, HCCLM3 cells were
observed to respond better to lung extractions under x400
microscopy than MHCC97-L and DMEM (12±2/field vs. 7±1/field and
4±1/field, respectively; P<0.05). Addition of the CXCR4 antibody
(20 μg/ml), however, did not inhibit the response of HCCLM3
cells to the lung extractions (12±2/field vs. 11±2/field;
P>0.05) (Fig. 2D).
RNAi down-regulates CXCR7 expression in
HCCLM3 cells
To further study the function of CXCR7 in the growth
and metastasis of HCC, gene silencing of CXCR7 in highly metastatic
HCCLM3 cells was carried out by RNAi. Four pairs of siRNAs of CXCR7
were designed. Real-time PT-PCR and Western blotting demonstrated
that siCXCR7-286 had the strongest inhibitory effect (65% at 48 h,
mRNA level; and 72% at 72 h, protein level, respectively).
Therefore, siCXCR7-286 was used in the subsequent experiment.
Inhibition of the proliferation and
metastatic potential of HCCLM3 cells by targeting CXCR7 in
vitro
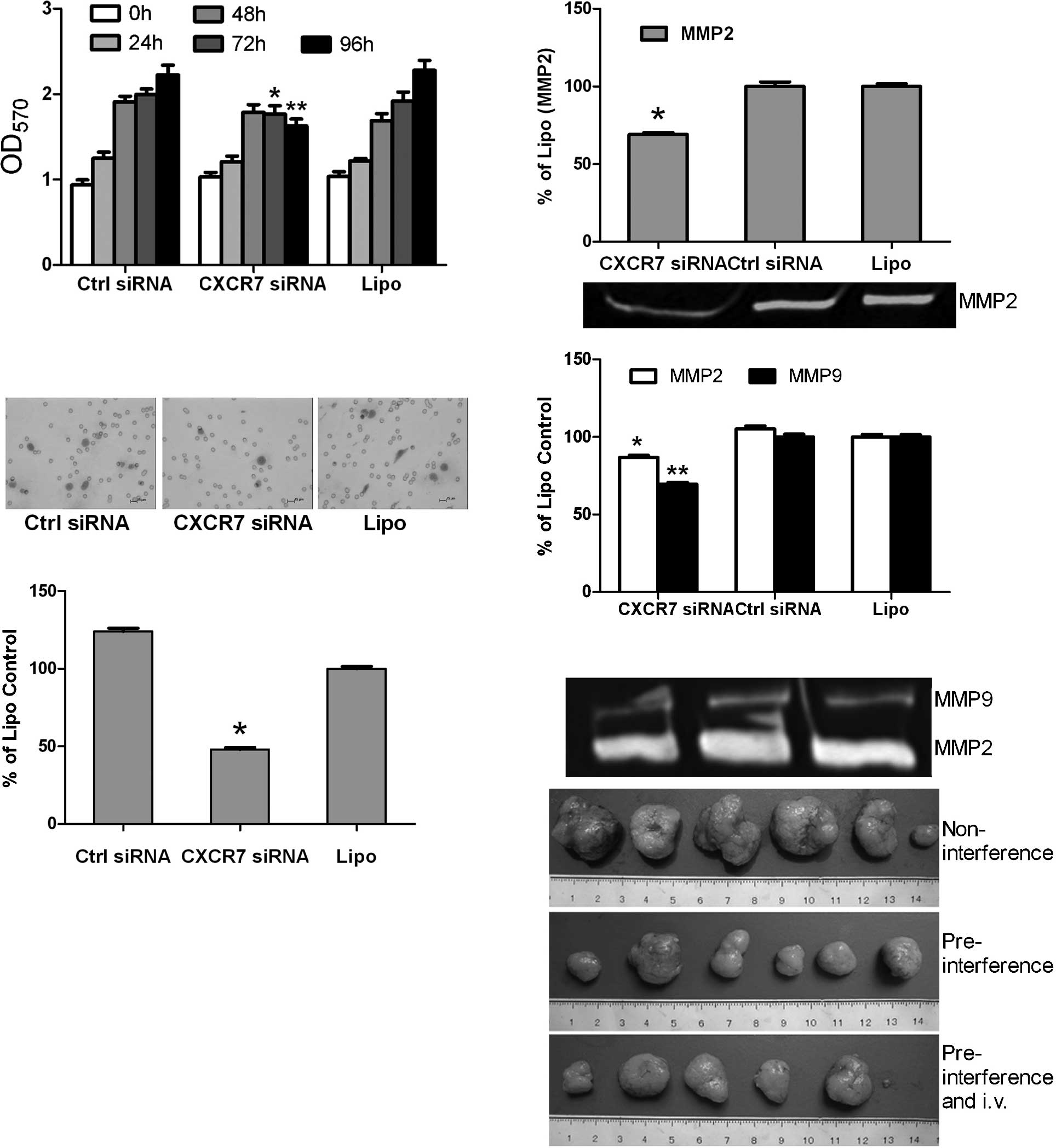
The cell proliferation assay revealed that the
growth of the HCCLM3 cells was not obviously affected at 0, 24 and
48 h after gene silencing of CXCR7 (P>0.05). However, cell
growth decreased in the CXCR7 siRNA group compared to the siRNA
control and liposome groups at 72 and 96 h, respectively
(P<0.01) (Fig. 3A). The
invasion assay revealed that the HCCLM3 cells in the CXCR7 siRNA
group exhibited a more significantly decreased invasive ability
than those in the siRNA control and liposome groups (4.0±1.0/field
vs. 8.3±1.5/field and 10.3±1.5/field, respectively; P<0.01)
(Fig. 3B). After RNA interference
at 24 and 48 h, the gelatin zymogram assay revealed a decreased
expression of MMP2 and MMP9 in the CXCR7 siRNA group in comparison
with that of the siRNA control and liposome groups (P<0.05), and
down-regulation of MMP2 and MMP9 was stronger at 24 h than at 48 h
after RNAi (Fig. 3C).
Gene silencing of CXCR7 inhibits the
growth and lung metastasis of HCCLM3 tumors in vivo
Dissection of the subcutaneous tumors and evaluation
of the lung metastases in mice were carried out on the 49th day.
Results showed the complete formation of tumors in all mice
(Fig. 3D). The volume and weight
of tumors in the pre-interference group with and without regular
intravenous injections were significantly smaller than those in the
non-interference group (Table II).
The group with pre-interference and regular intravenous injections
showed slower tumor growth (volume and weight of tumors) than the
pre-interference group without intravenous injections, which
however had no significant difference (P>0.05) (Table II).
 | Table II.CXCR7 siRNA treatment reduces HCCLM3
tumor size, weight and metastasis. |
Table II.
CXCR7 siRNA treatment reduces HCCLM3
tumor size, weight and metastasis.
| Treatment
groups | Tumor size
| Lung metastasis
|
|---|
| Volume
(mm3) | Weight (g) | Cases (%) | Metastasis
(no.) |
|---|
|
Non-interference |
1,265.42±900.01 | 3.02±1.68 | 6 of 6 (100) | 8.0±2.8 |
|
Pre-interference |
372.83±265.02a | 1.20±0.75a | 4 of 6 (66.7) | 2.5±2.0a |
| Pre-interferece and
i.v. |
355.42±383.10b | 1.18±0.77b | 3 of 6 (50) | 2.3±2.2b |
Lung metastasis was evaluated based on both the
positive metastases in the lungs and the average number of
metastases in the lungs per positive case. Sequential sections of
the lung tissues showed that the metastatic nodules in the
pre-interference groups were significantly fewer than those in the
non-interference group (P<0.01) (Table II). The number of cases presenting
lung metastases in the group receiving pre-interference and regular
intravenous injections was less than that in the pre-interference
group (3 of 6 vs. 4 of 6) (Table
II).
CXCR7 is expressed highly in the tumor
tissues of HCC patients with lung metastasis
TMA showed that the group with lung metastasis
presented higher CXCR7 expression than the group without (P=0.017),
and CXCR7 in tumor tissues was expressed much higher than that in
the para-cancerous tissues (P=0.006) (Table III). In addition, CXCR4 expressin
was shown to be lower in the tumor tissues than in the
para-cancerous tissues (P=0.034), and there was no significant
difference between patients with and without lung metastasis
(P>0.05) (Table III).
 | Table III.Expression of CXCR7 and CXCR4 in the
tissue microarray. |
Table III.
Expression of CXCR7 and CXCR4 in the
tissue microarray.
| No. of cases | CXCR7 expression
| P-value | CXCR4 expression
| P-value |
|---|
| Weak + | Moderate ++ | Strong +++ | Weak + | Moderate ++ | Strong +++ |
|---|
| Tumor | 116 | 31 | 31 | 54 | 0.006a | 17 | 7 | 3 | |
| Lung metastasis
(yes) | 58 | 16 | 9 | 33 | 0.017b | 55 | 27 | 5 | NSb |
| Lung metastasis
(no) | 58 | 15 | 22 | 21 | | 44 | 24 | 15 | |
| Adjacent-tumor | 47 | 6 | 30 | 11 | | 10 | 5 | 2 | 0.034a |
| Lung metastasis
(yes) | 23 | 3 | 12 | 8 | NSc | 28 | 11 | 6 | NSc |
| Lung metastasis
(no) | 24 | 3 | 18 | 3 | | 20 | 31 | 16 | |
Discussion
In the present study, CXCR7 was demonstrated to play
a critical role in metastatic HCC. First, we found increased
expression of CXCR7 in a highly metastatic HCC cell line. Also,
knockdown of CXCR7 in HCCLM3 inhibited the proliferation and
invasion of tumor cells, as well as the decreased expression of
MMP-2 and MMP-9. Furthermore, gene silencing of CXCR7 decreased the
growth and lung metastasis of HCCLM3 tumors in nude mice. Finally,
the TMA showed that CXCR7 was expressed higher in the tumor tissues
of HCC patients with lung metastasis. Together, these findings
suggest the potential roles of CXCR7 in both the growth and
metastasis of HCC. Our finding that CXCR7 was associated with the
growth and lung metastasis of HCC is partly in accordance with
recently reported observations (9). In the present study, however,
SMMC-7721 was used as a cell line with low metastatic potential due
to its nearly non-metastatic ability to the lung. Expression of
CXCR7 in SMMC-7721 cells was notably lower than that in HCCLM3 with
highly metastatic potential in this study.
The chemokine receptor gene chip, in this study,
showed marked up-regulated expression of CXCR7, CXCR1 and CCR1 and
down-regulated expression of CXCR4. Our previous study showed that
CCR1 is tightly related with local dissemination in the portal vein
system (19). Down-regulation of
the expression of CCR1 by RNAi decreases the invasive and
metastatic ability in vitro. Therefore, the chemokine
receptor expression pattern observed in this study suggests that
HCC, with high metastasis potential, exhibits a unique expression
of chemokine receptors itself. Strongly up-regulated expression of
CXCR7 and CCR1 may be a unique feature of metastatic HCC.
In the present study, CXCR7 expression was much
stronger in the highly metastatic HCC cell line, whereas CXCR4 was
not, which is partly in accordance with previous observation that
CXCR4 expression is low in HCC cell lines with metastatic potential
(20,21). Schimanski et al (22) demonstrated a relationship between
CXCR4 and lymphatic metastasis as well as dissemination. Recent
research revealed that CXCR4 expression in HCC patients increases
the risk of bone metastases and poor survival (23). SDF-1α was also found to directly
control the migration of both the leading and trailing edges of a
tissue through the activation of two independent receptors, CXCR4b
and CXCR7, in a zebrafish model, respectively (24). Since CXCR7 and CXCR4 share the same
ligand or SDF-1, we believe that this unique expression style of
CXCR7 and CXCR4 observed in our study suggests various potential
unknown control mechanisms.
In this study, an in vitro cell culture
demonstrated the growth inhibition of HCCLM3 cells by RNAi of
CXCR7. Furthermore, the size of primary xenografted HCCLM3 tumors
was markedly smaller in the pre-interference and pre-interference +
i.v. groups in comparison with the non-interference group. This
suggests that the pre-treatment of CXCR7 siRNA markedly reduces
tumor growth in vitro and in vivo. Thus, tumor growth
inhibition may be a major factor in the reduction of the number of
metastatic lung tumors, which indicates that the anti-proliferative
effect of the down-regulation of CXCR7 may be an important factor
in the anti-metastasis mechanism. These findings are similar to
observations in breast and lung models (25). In an osteoarthritis model (26), overexpressed CXCR7 in chondrocyte
cells induced increased expression of osteopontin, interleukin-8,
MMP2 and vascular endothelial growth factor, suggesting the
potential roles of CXCR7 in the metastasis of HCC due to the close
correlations between these genes and metastatic HCC. Recently,
research has shown that ligand binding to CXCR7 does not result in
the activation of signaling pathways typical of G proteins but does
activate MAP kinases through β-arrestins, which suggests the role
of CXCR7 in the non-classic pathway as a seven-transmembrane
receptor (27). The mechanisms of
CXCR7 participating in the growth and metastasis of HCC warrant
further investigation.
In conclusion, the present study demonstrated the
critical roles of CXCR7 in tumor growth and lung metastasis of HCC.
RNAi of CXCR7 inhibited the growth and invasion of HCC cells,
suggesting the potential therapeutic benefit of CXCR7 as a novel
molecular target for HCC.
Acknowledgements
This study was supported by a State
Key Basic Research Program Grant from the Ministry of Science and
Technology, China (no. 2004CB518708)
References
|
1.
|
DM ParkinF BrayJ FerlayP PisaniEstimating
the world cancer burden: Globocan 2000Int J
Cancer94153156200110.1002/ijc.144011668491
|
|
2.
|
A Ben-BaruchOrgan selectivity in
metastasis: regulation by chemokines and their receptorsClin Exp
Metastasis25345356200810.1007/s10585-007-9097-317891505
|
|
3.
|
T KakinumaST HwangChemokines, chemokine
receptors, and cancer metastasisJ Leukoc
Biol79639651200610.1189/jlb.110563316478915
|
|
4.
|
K BalabanianB LaganeS InfantinoThe
chemokine SDF-1/CXCL12 binds to and signals through the orphan
receptor RDC1 in T lymphocytesJ Biol
Chem2803576035766200510.1074/jbc.M50823420016107333
|
|
5.
|
JM BurnsBC SummersY WangA novel chemokine
receptor for SDF-1 and I-TAC involved in cell survival, cell
adhesion, and tumor developmentJ Exp
Med20322012213200610.1084/jem.2005214416940167
|
|
6.
|
B BoldajipourH MahabaleshwarE
KardashControl of chemokine-guided cell migration by ligand
sequestrationCell132463473200810.1016/j.cell.2007.12.03418267076
|
|
7.
|
J WangY ShiozawaY WangThe role of
CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate
cancerJ Biol Chem28342834294200810.1074/jbc.M70746520018057003
|
|
8.
|
S IwakiriN MinoT TakahashiHigher
expression of chemokine receptor CXCR7 is linked to early and
metastatic recurrence in pathological stage I non-small cell lung
cancerCancer11525802593200910.1002/cncr.2428119309748
|
|
9.
|
K ZhengHY LiXL SuChemokine receptor CXCR7
regulates the invasion, angiogenesis and tumor growth of human
hepatocellular carcinoma cellsJ Exp Clin Cancer
Res2931201010.1186/1756-9966-29-3120380740
|
|
10.
|
J TianZY TangSL YeNew human hepatocellular
carcinoma (HCC) cell line with highly metastatic potential (MHCC97)
and its expressions of the factors associated with metastasisBr J
Cancer81814821199910.1038/sj.bjc.669076910555751
|
|
11.
|
P StordeurLF PoulinL CraciunCytokine mRNA
quantification by real-time PCRJ Immunol
Methods2595564200210.1016/S0022-1759(01)00489-611730841
|
|
12.
|
XN JiSL YeY LiContributions of lung tissue
extracts to invasion and migration of human hepatocellular
carcinoma cells with various metastatic potentialsJ Cancer Res Clin
Oncol129556564200310.1007/s00432-003-0475-112942314
|
|
13.
|
ES HujanenVP TerranovaMigration of tumor
cells to organ-derived chemoattractantsCancer
Res453517352119854016733
|
|
14.
|
A MullerB HomeyH SotoInvolvement of
chemokine receptors in breast cancer
metastasisNature4105056200110.1038/3506501611242036
|
|
15.
|
B DalbyS CatesA HarrisAdvanced
transfection with Lipofectamine 2000 reagent: primary neurons,
siRNA, and high-throughput
applicationsMethods3395103200410.1016/j.ymeth.2003.11.02315121163
|
|
16.
|
K JacobM WebberD BenayahuHK
KleinmanOsteonectin promotes prostate cancer cell migration and
invasion: a possible mechanism for metastasis to boneCancer
Res5944534457199910485497
|
|
17.
|
R SimonM MirlacherG SauterTissue
microarraysMethods Mol Med973773892004
|
|
18.
|
A LugliH SpichtinR MaurerEphB2 expression
across 138 human tumor types in a tissue microarray: high levels of
expression in gastrointestinal cancersClin Cancer
Res1164506458200510.1158/1078-0432.CCR-04-245816166419
|
|
19.
|
X WuJ FanX WangDownregulation of CCR1
inhibits human hepatocellular carcinoma cell invasionBiochem
Biophys Res
Commun355866871200710.1016/j.bbrc.2007.01.19917336272
|
|
20.
|
NA BegumK ShibutaM MoriGF BarnardReduced
expression of the CXCR4 receptor mRNA in hepatocellular carcinoma
and lack of inducibility of its ligand α-chemokine hIRH/SDF1α/PBSF
in vitroInt J Oncol14927934199910200343
|
|
21.
|
P MitraA DeMF EthierLoss of chemokine
SDF-1alpha-mediated CXCR4 signalling and receptor internalization
in human hepatoma cell line HepG2Cell
Signal13311319200110.1016/S0898-6568(01)00156-511369512
|
|
22.
|
CC SchimanskiR BahreI GockelDissemination
of hepatocellular carcinoma is mediated via chemokine receptor
CXCR4Br J Cancer95210217200610.1038/sj.bjc.660325116819541
|
|
23.
|
ZL XiangZC ZengZY TangChemokine receptor
CXCR4 expression in hepatocellular carcinoma patients increases the
risk of bone metastases and poor survivalBMC
Cancer9176200910.1186/1471-2407-9-17619508713
|
|
24.
|
G ValentinP HaasD GilmourThe chemokine
SDF1a coordinates tissue migration through the spatially restricted
activation of Cxcr7 and Cxcr4bCurr
Biol1710261031200710.1016/j.cub.2007.05.02017570670
|
|
25.
|
Z MiaoKE LukerBC SummersCXCR7 (RDC1)
promotes breast and lung tumor growth in vivo and is expressed on
tumor-associated vasculatureProc Natl Acad Sci
USA1041573515740200710.1073/pnas.061044410417898181
|
|
26.
|
SW JonesSM BrockbankML MobbsThe orphan
G-protein coupled receptor RDC1: evidence for a role in chondrocyte
hypertrophy and articular cartilage matrix turnoverOsteoarthritis
Cartilage14597608200610.1016/j.joca.2006.01.00716647866
|
|
27.
|
S RajagopalJ KimS AhnBeta-arrestin - but
not G protein-mediated signaling by the ‘decoy’ receptor CXCR7Proc
Natl Acad Sci USA1076286322010
|