Introduction
Neoadjuvant chemotherapy is often used in the
treatment of large, operable, locally advanced breast cancers. This
therapy successfully reduces tumor size in most patients and may
enable them to consider breast-conserving therapy rather than
mastectomy (1–6). In addition, it may permit patients
with large inoperable tumors to undergo mastectomy. This approach
has not provided a survival advantage as compared to postoperative
adjuvant therapy (1,2,4–7).
However, patients achieving a pathological complete response (pCR)
have a substantially improved disease-free survival (DFS) and
overall survival (OS) compared to those with residual disease
(8–13).
Estrogen receptor (ER) status is an important
predictive factor to achieve pCR in neoadjuvant chemotherapy for
operable breast cancer (14–18).
Several reports have demonstrated that patients with ER-negative
tumors (<10% ER-positive tumor cells) are more likely to achieve
pCR than those with ER-positive tumors for neoadjuvant chemotherapy
for operable breast cancer (14,15).
Other studies have reported that patients with ER-absent tumors (0%
ER-positive tumor cells) are more likely to achieve pCR than those
with ER-positive tumors (the presence of any detectable
positive-staining tumor cells) (16–18).
Recently, the definition of hormone receptor
positivity has changed. The cutoff point of 10% for ER or
progesterone receptor (PgR) immunohistochemistry has been the
global standard until recently. In the 2009 St. Gallen consensus
meeting, the panel recommended the inclusion of adjuvant endocrine
therapy for almost all patients whose tumors showed evidence of
endocrine responsiveness (presence of any detectable ER).
Furthermore, the American Society of Clinical Oncology and the
College of American Pathologists (ASCO/CAP) recommended that ER and
PgR assays are considered positive when there are at least 1%
positive tumor nuclei in the sample (19).
How the proportion of ER-positive or PgR-positive
tumor cells affects the response to neoadjuvant chemotherapy for
operable breast cancer remains unclear. The purpose of this study
was to examine the correlation between the proportion of
ER-positive or PgR-positive-staining cells and the
clinico-pathological response to neoadjuvant chemotherapy. We
retrospectively investigated the clinicopathological factors and
responses to neoadjuvant chemotherapy for operable breast cancer at
the Kumamoto City Hospital, Japan. We eliminated human epidermal
growth factor receptor 2 (HER2)-positive breast cancer from our
data as the administration of trastuzumab affects the outcome of
neoadjuvant therapy (20).
Patients and methods
Patients and treatments
From April 2002 to October 2010, 103 patients were
enrolled in this study. The clinical and pathological
characteristics of all patients were obtained from our
institutional medical records. All patients were pathologically
diagnosed with invasive breast cancer by core needle biopsy.
Moreover, they had positive axillary nodes or tumors of sizes ≥3 cm
measured objectively by breast ultrasonography. Prior to starting
chemotherapy, patients had a good performance status and no
metastatic lesions, which was confirmed by chest radiography, bone
scanning, abdominal ultrasonography and whole body computed
tomography. Tumor size was measured and followed up by breast
ultrasonography. Patients undergoing breast-conserving surgery
received radiation therapy for the preserved breast. Patients with
receptor-positive tumors underwent standard hormonal therapy.
Patient characteristics, such as the clinical stage, menopausal
status, histological grade, hormone receptor status, chemotherapy
regimen, and clinical and pathological responses in the breast,
were recorded.
Chemotherapy regimens
From April 2002 to October 2005, 28 patients
received 4 cycles of tri-weekly epirubicin (60 mg/m2)
and docetaxel (60 mg/m2) concurrently. From April 2002
to October 2010, 75 patients received 4 cycles of tri-weekly
5-fluorouracil (500 mg/m2), epirubicin (75 or 100
mg/m2) and cyclophosphamides (500 mg/m2),
followed by 4 cycles of tri-weekly docetaxel (75 mg/m2)
or 12 cycles of weekly paclitaxel (80 mg/m2). The
patients underwent surgery for the following conditions: after
completion of neoadjuvant chemotherapy; when the tumors continued
to be progressive under concurrent therapy of epirubicin and
docetaxel; when the tumors continued progression after taxane was
administered, in cases where therapy with 5-fluorouracil,
epirubicin and cyclophosphamide was ineffective.
Evaluation of the response to
chemotherapy
We evaluated the clinical response of primary breast
cancer and axillary lymph nodes using ultrasonography, according to
the Response Evaluation Criteria in Solid Tumors (21): complete response (CR),
disappearance of all target lesions; partial response (PR), a
decrease of ≥30% in the diameter of the target lesion; progressive
disease (PD), an increase of ≥20% in the diameter of the target
lesion; and stable disease (SD), neither sufficient shrinkage to
qualify for PR nor sufficient increase to qualify for PD.
Pathological response was evaluated according to the
following definition: pCR was defined as the complete disappearance
of cancer cells from the breast stroma. A non-pathological complete
response (non-pCR) was defined as the presence of pathological
residues of cancer cells in the breast stroma. However, the
outcomes of the axillary lymph nodes were not taken into
account.
Pathological examination and
immunohistochemistry
Pathological evaluation was performed on patients at
the Department of Clinical Pathology, Kumamoto City Hospital. Our
pathologists analyzed samples obtained by core needle biopsy prior
to starting chemotherapy, as well as those obtained from surgical
resection. Formalin-fixed, paraffin-embedded tissue blocks were
prepared and stained immunohistochemically for the expression of ER
and PgR, the HER2 receptor, p53 and Ki-67 (22). The slides were incubated with a
diluted anti-ER primary antibody (1:75; Dako, Glostrup, Denmark), a
diluted anti-PgR primary antibody (1:700; Dako), a diluted anti-p53
primary antibody (1:50; Japan Tanner, Osaka, Japan) and a HER2/neu
oncoprotein antibody (Herceptest; Dako). The Dako EnVision system
(Dako EnVision labeled polymer, peroxidase) or the Benchmark XT
system (Ventana Medical System, AZ, USA) were used as the detection
systems for ER, PgR and HER2.
Investigated parameters included tumor size, lymph
node status, histological grade, ER and PgR status, proliferation
index (Ki-67), as well as expression of HER2 and p53. The
proportion of ER-positive and PgR-positive tumor cells was
expressed as a percentage. The positivities of the ER and PgR were
defined as ≥1% according to ASCO/CAP (19). After patient distribution according
to the proportion of receptor-positive tumor cells was examined and
compared to the distribution of patients achieving pCR, the
patients were classified into groups at appropriate cutoff points.
Ki-67 values were expressed as the percentage of positive staining
cells in each case and were classified into two groups based on the
percentage of positive nuclei: >20 and ≤20%. p53 expression was
categorized into three groups: negative (absent or focal staining
with <5% tumor cells), 1+ (heterogeneous or focal staining with
>5% tumor cells) and 2+ (homogeneous and diffuse staining). HER2
overexpression was defined as the strong and diffuse membranous
staining of tumor cells. HER2-2+ staining was tested by
fluorescence in situ hybridization, with a threshold for
positive HER2/CEP17 ratio of >2.0.
Statistical analyses
The influence of tumoral pre-operative baseline
characteristics on the likelihood of achieving pCR was tested using
the Chi-square or Fisher’s exact test. Independent significance of
variables was analyzed using a multivariate logistic regression
model with a step-up procedure. Odds ratios, 95% confidence
intervals (CIs) and p-values were estimated from the final model.
The Kaplan-Meier method was used for the assessment of DFS and OS.
The log-rank test was used to examine the statistical significance
of the differences between groups. Statistical analyses were
performed using the Statistical Package for the Social Sciences
(SPSS) version 18.0 for Windows (SPSS Japan Inc./IBM Company,
Tokyo, Japan).
Results
Patient distribution according to the
proportion of ER-positive or PgR-positive tumor cells
From April 2002 to October 2010, 103 patients
underwent surgery following neoadjuvant chemotherapy for primary
operable breast cancer. Pathological specimens were available in
all cases. Of the 103 patients, 46 (45%) were ER-negative and 57
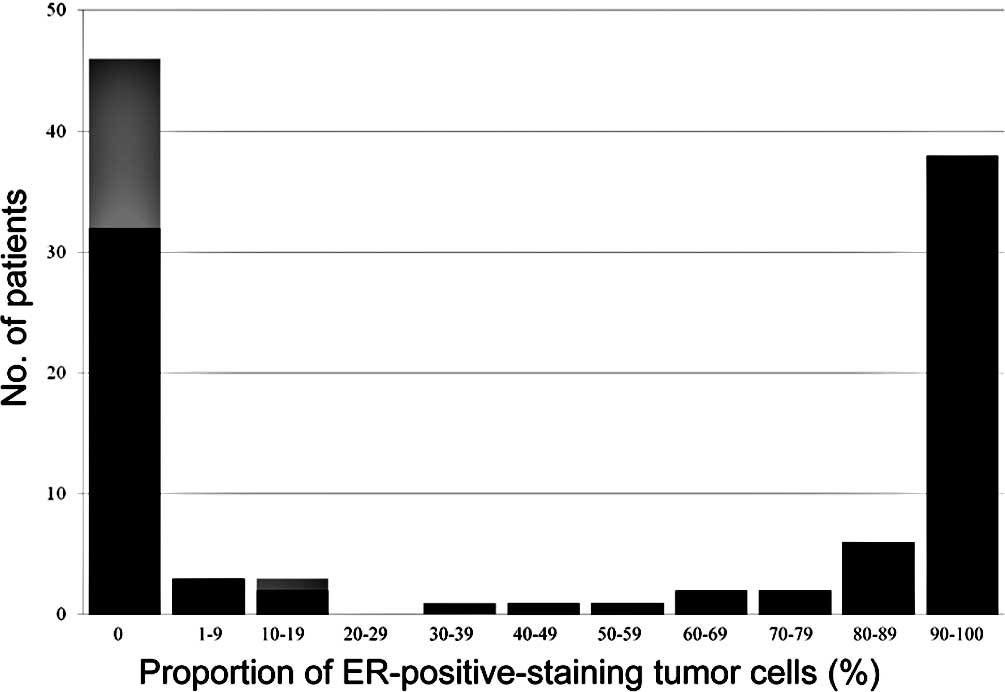
(55%) were ER-positive (Fig. 1).
Of the 57 patients with ER-positive tumors, 38 (67%) had tumors
with ≥90% ER-positive cells (Fig.
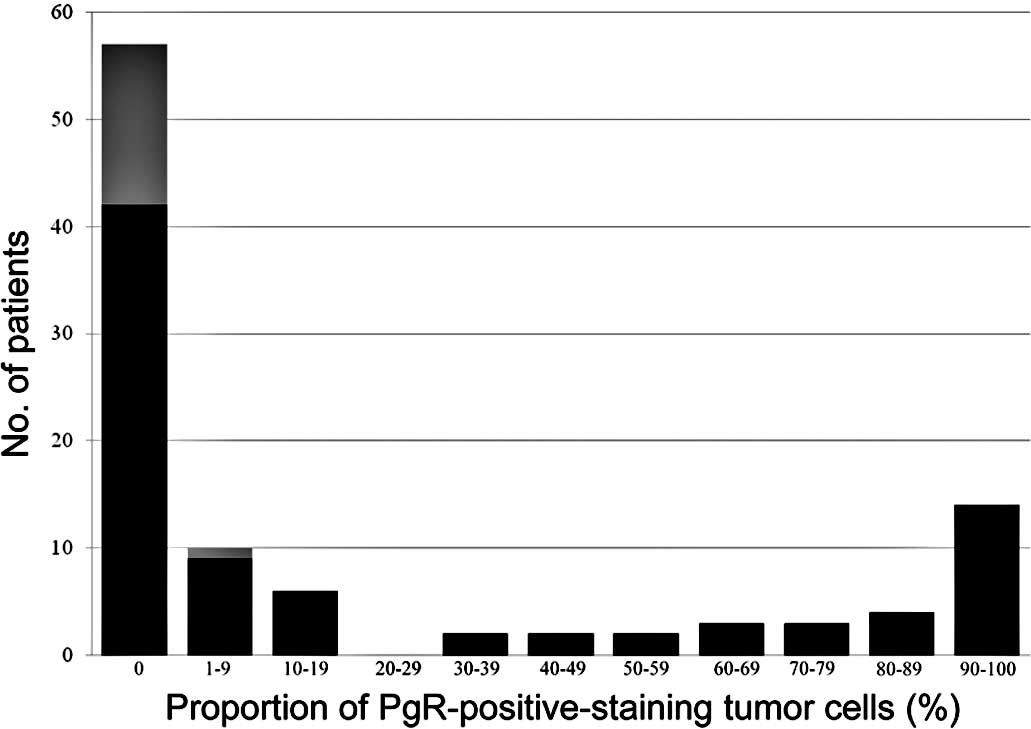
1). Of the 103 patients, 57 (55%) were PgR-negative and 46
(45%) were PgR-positive (Fig.
2).
Response to neoadjuvant chemotherapy
Pathologically, 16 (16%) patients had no residual
disease after chemotherapy. Of 16 patients with pCR in the breast,
3 had positive lymph nodes. Fourteen (30%) of the 46 patients with
ER-negative tumors achieved pCR and 15 (26%) of the 57 patients
with PgR-negative tumors achieved pCR following neoadjuvant
chemotherapy (Table I). Although
one patient with 30% ER-positive cells achieved pCR, none of the
patients with >30% ER-positive cells or >1% PgR-positive
cells achieved pCR (Figs. 1 and
2).
 | Table IUnivariate analysis of factors
predicting a pCR according to baseline factors. |
Table I
Univariate analysis of factors
predicting a pCR according to baseline factors.
| Baseline
factor | pCR, no. (%) | Total | p-value |
|---|
| Total | 16 (16) | 103 | |
| ER | | | |
| Negative | 14 (30) | 46 | 0.001 |
| 1–29% | 1 (17) | 6 | |
| ≥30% | 1 (2) | 51 | |
| PgR | | | |
| Negative | 15 (26) | 57 | 0.0001 |
| Positive | 1 (2) | 46 | |
| Clinical tumor
size | | | |
| T1 | 1 (14) | 7 | 0.73 |
| T2 | 10 (16) | 63 | |
| T3 | 5 (19) | 27 | |
| T4 | 0 (0) | 6 | |
| Nodal Status | | | |
| Negative | 6 (19) | 32 | 0.37 |
| Positive | 10 (14) | 71 | |
| Histological
grade | | | |
| Grade 1 | 2 (8) | 26 | 0.09 |
| Grade 2 | 6 (13) | 48 | |
| Grade 3 | 8 (28) | 29 | |
| Ki-67 | | | |
| <20% | 0 (0) | 11 | 0.14 |
| ≥20% | 16 (17) | 92 | |
| p53 | | | |
| 0 | 2 (15) | 13 | 0.14 |
| 1 | 3 (8) | 40 | |
| 2 | 11 (23) | 48 | |
| Menopausal
status | | | |
|
Pre-menopause | 7 (12) | 60 | 0.16 |
|
Postmenopause | 9 (21) | 43 | |
| Regimen | | | |
| ET | 1 (4) | 28 | 0.03 |
| FEC-T | 15 (20) | 75 | |
All groups demonstrated a good clinical response to
neoadjuvant chemotherapy regardless of their ER status (Table II). Out of the 46 patients with
ER-negative tumors following neoadjuvant chemotherapy, 16 (35%)
achieved CR and 22 (48%) achieved PR. Out of the 57 patients with
ER-positive tumors following neoadjuvant chemotherapy, 8 (14%)
achieved CR and 40 (70%) achieved PR; a significant difference was
observed between them.
 | Table IIClinical response for neoadjuvant
chemotherapy in accordance with ER status. |
Table II
Clinical response for neoadjuvant
chemotherapy in accordance with ER status.
| CR, no. (%) | PR, no. (%) | NC, no. (%) | PD, no. (%) | Total | p-value |
|---|
| ER-negative | 16 (35) | 22 (48) | 7 (15) | 1 (2) | 46 | 0.045 |
| ER-positive | 8 (14) | 40 (70) | 9 (16) | 0 (0) | 57 | |
Predictive factors for pCR
In univariate analysis, pCR was associated with ER
status (p=0.001), PgR status (p=0.0001) and chemotherapy regimens
(p=0.03) (Table I). No significant
difference in the pCR rate was observed according to menopausal
status, clinical tumor size, nodal status, histological grade and
Ki-67 or p53 expression (Table
I).
A multivariate analysis was performed using the ER
status, PgR status and chemotherapy regimens. ER and PgR status
correlated with pCR following neoadjuvant chemotherapy using the
step-up procedure. Patients with ER-negative tumors were 18.6 times
more likely to achieve pCR than those with ≥30% ER-positive tumor
cells (p= 0.006; 95% CI 2.3–149.9) (Table III).
 | Table IIIMultvariate analysis to identify the
baseline factors predicting a pCR. |
Table III
Multvariate analysis to identify the
baseline factors predicting a pCR.
| Odds ratio | 95% CI | p-value |
|---|
| ER status | | | |
|
Negative/≥30% | 18.6 | 2.3–149.9 | 0.006 |
| 1–29%/≥30% | 12.8 | 0.6–256.9 | 0.100 |
| PgR status | | | |
|
Negative/positive | 14.6 | 1.8–116.4 | 0.020 |
| Regimen | | | |
| FEC-D/ET | 4.8 | 0.6–41.70 | 0.160 |
Survival
At a median follow-up of 40.5 months, the 5-year DFS
in all patients was 60% and the 5-year OS was 89%. One patient who
achieved pCR following neoadjuvant chemotherapy relapsed and died
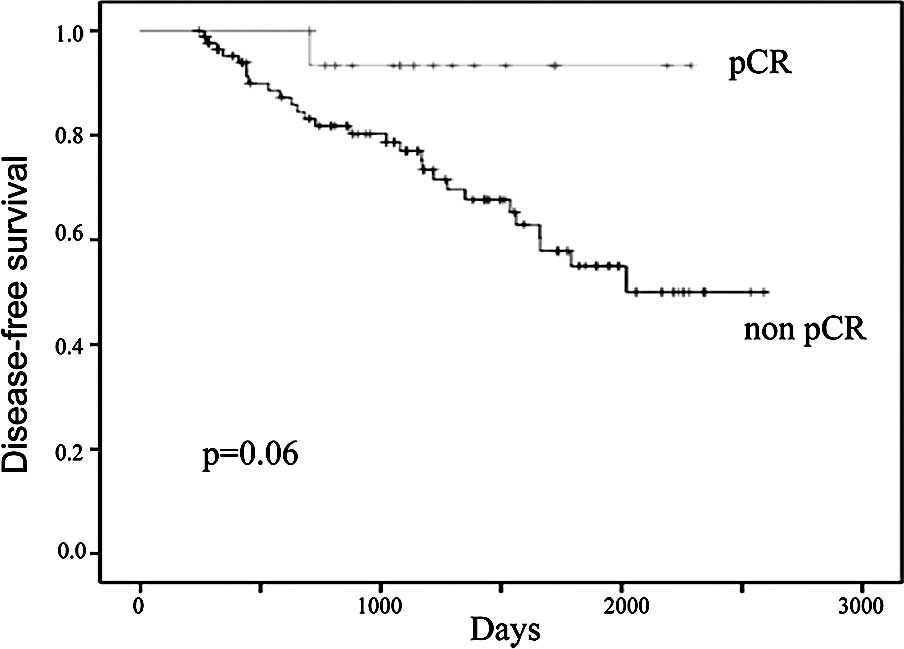
during the follow-up period. The 5-year DFS in patients who
achieved and in those who did not achieve pCR following neoadjuvant
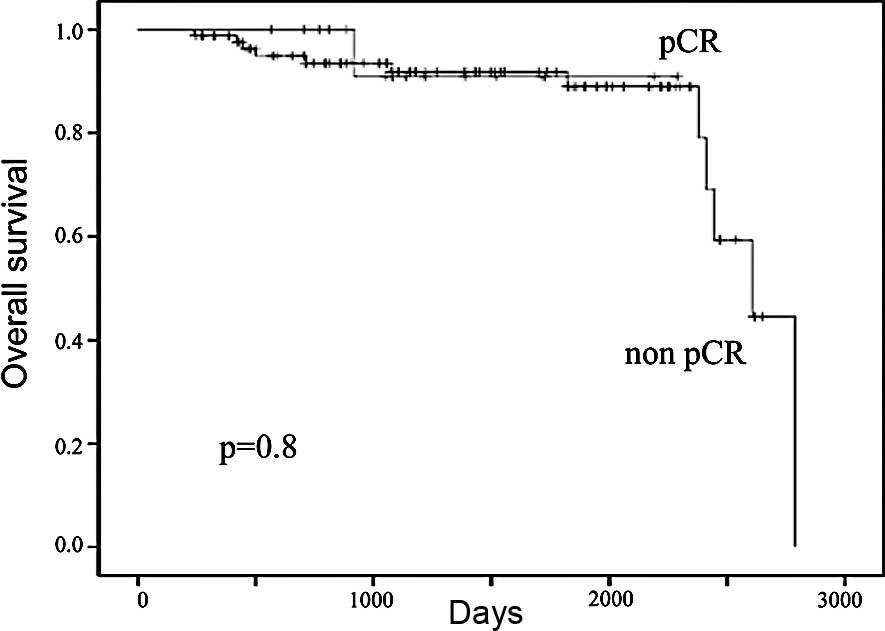
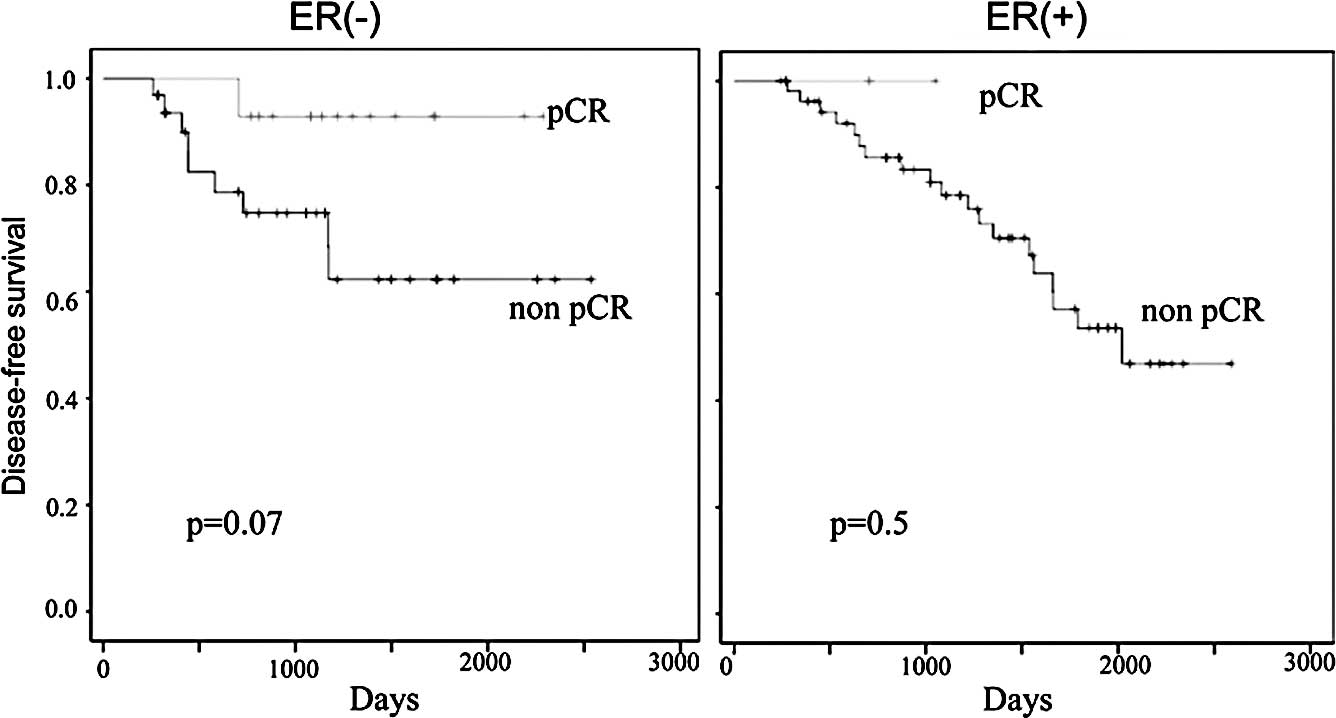
chemotherapy was 55 and 91%, respectively (Fig. 3). The 5-year OS in patients who
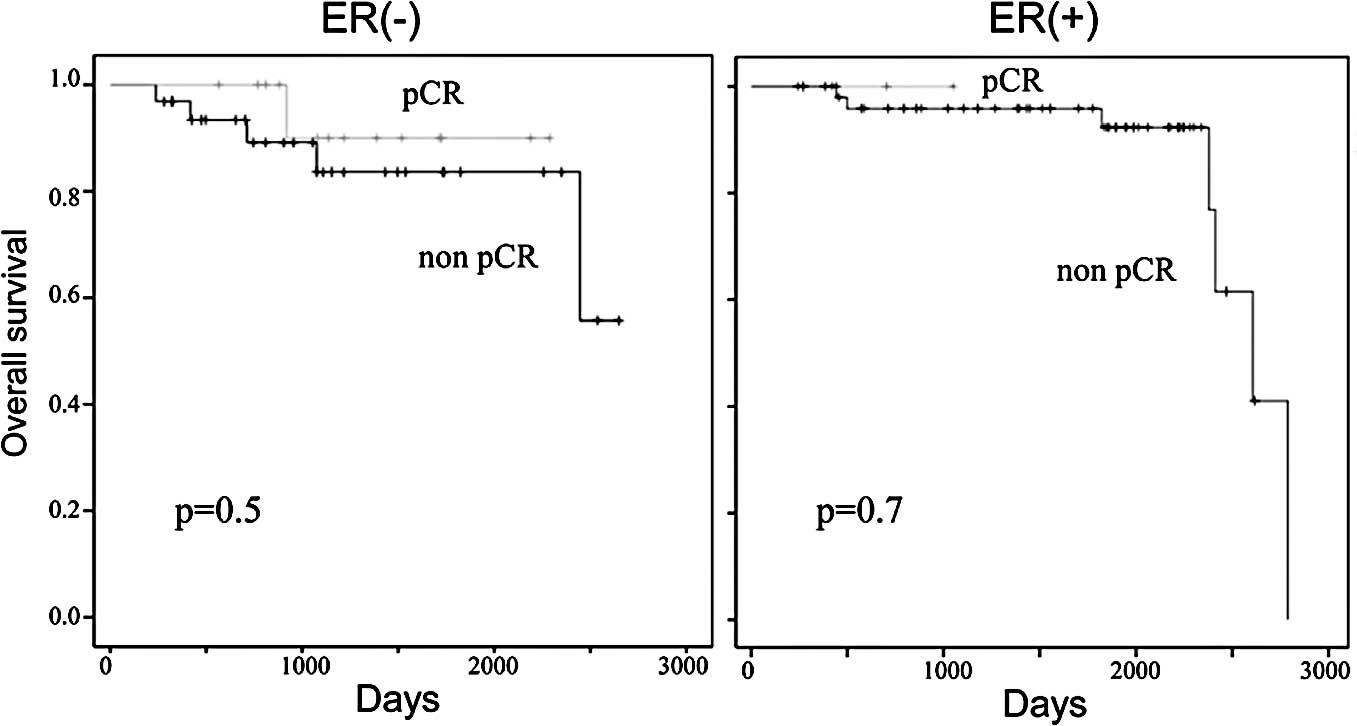
achieved and in those who did not achieve pCR following neoadjuvant
chemotherapy was 89 and 93%, respectively (Fig. 4). There were trends in improvement
in the DFS of patients who achieved pCR, however, they did not
reach statistically significant values (p=0.06; Fig. 3). When patients with ER-negative
and ER-positive tumors were examined separately, the patients who
achieved pCR showed an improved DFS and OS compared to those with
residual diseases, but no significant differences were observed
between them (Figs. 5 and 6).
Discussion
We retrospectively investigated several pathological
factors to examine the correlation between the proportion of
ER-positive or PgR-positive tumor cells and the clinicopathological
response to neoadjuvant chemotherapy for HER2-negative operable
breast cancer. In multivariate analysis, ER- or PgR-negativity was
a significant predictive factor to achieve pCR. Patients with more
than 30% ER-positive tumor cells or more than 1% PgR-positive tumor
cells did not achieve pCR. Most patients achieved a favorable
clinical response to neoadjuvant chemotherapy for operable breast
cancer regardless of the proportion of ER-positive or PgR-positive
tumor cells.
Our results are in accordance with the findings of
Colleoni et al that ER-absent tumors were more predictive
than ER-low tumors (1–9%) in achieving pCR following neoadjuvant
chemotherapy for breast cancer. They demonstrated that patients
with ER-absent and PgR-absent tumors were 12 times more likely to
achieve pCR than those with ER-positive and PgR-positive tumors or
ER-low tumors (16–18). In addition, they described that
approximately two-thirds of ER-negative patients belonged to the
ER-absent group, and one-third to the ER-low group. Moreover, the
pCR rate of ER-absent patients was 19.4%, but the pCR rate of
ER-low patients was 2.8%. On the other hand, other studies related
to the predictive factors of neoadjuvant chemotherapy demonstrated
that patients with ER-negative tumors were more likely to achieve
pCR than those with ER-positive tumors. However, these studies did
not distinguish ER-absent patients from ER-low patients among the
ER-negative breast cancer patients (5,9,14,15).
In addition, our results suggest that the proportion
of ER-positive or PgR-positive tumor cells is a predictive factor
for non-pCR in neoadjuvant chemotherapy. Patients with more than
30% of ER-positive tumor cells or more than 1% of PgR-positive
tumor cells did not achieve pCR in this study. These data may be
critical to achieve pCR in neoadjuvant chemotherapy for operable
breast cancer.
In pre-operative strategies for HER2-negative breast
cancer, the proportion of ER-positive tumor cells is extremely
important. If we distinguish ER-positive tumors into those with
values of ER-positive cells less than 30% and those having values
greater than or equal to 30%, HER2-negative breast cancer consists
of three groups: triple-negative breast cancers (TNBCs), ER
low-positive tumors and ER highly positive tumors. Chemotherapy is
the only treatment for TNBCs. It aims to reduce tumor size and
prevent mastectomy, prevent tumor recurrence after surgery or
obtain information regarding chemosensitivity in vivo.
Chemotherapy is also a potent strategy for ER low-positive tumors.
If the aim of neoadjuvant chemotherapy is to reduce tumor size and
avoid mastectomy, the treatment must be useful for ER low-positive
tumors despite the extremely low pCR rate. This is because
neoadjuvant chemotherapy successfully reduces clinical tumor size
(Table I). Hormonal therapy may be
unsuccessful in treating ER low-positive breast cancer. However,
postoperative additional hormonal therapy for ER low-positive
breast cancer may prevent tumor recurrence as a more favorable
prognosis for tumors with 1% positive-staining tumor cells when
treated with tamoxifen has been reported in one study (23). In addition, neoadjuvant
chemotherapy can be used for ER highly positive breast cancer as
the treatment effectively reduced clinical tumor size avoiding
mastectomy (Table II). Patients
with values of ER-positive cells greater than or equal to 90% also
achieved a good clinical response to neoadjuvant chemotherapy (data
not shown). However, neoadjuvant hormonal therapy may be preferred
if it reduces tumor size and mastectomy is avoided without
cytotoxic chemotherapy. Moreover, surgery followed by adjuvant
hormonal therapy may be a preferable alternative for treating
operable breast cancer. This is because most patients with highly
endocrine-responsive tumors do not require adjuvant
chemotherapy.
The survival data revealed in this study were
different from those of other studies. pCR following neoadjuvant
chemotherapy was not statistically associated with improved DFS in
our study; however, other studies have demonstrated a statistical
difference (14). There was only a
slight trend in improvement in the DFS of patients who achieved pCR
in our study. Based on the DFS curve according to pCR, the
difference may reach a significant value with an increase in the
number of patients. In addition, we did not find a statistical
difference in DFS or OS according to ER status, although other
studies showed that patients with ER-negative tumors achieved a
statistically worse DFS or OS (14). Conversely, our patients with
ER-negative tumors showed a better prognosis probably as patients
with HER2-positive tumors were not included among the ER-negative
patients. In the event the data from other studies included
HER2-positive patients treated with neoadjuvant chemotherapy
without trastuzumab, HER2 disease may have lowered the survival
curves.
It is difficult to explain why patients with
ER-positive tumors rarely achieved pCR. To understand this
phenomenon, we hypothesize that each ER-positive tumor cell is
insensitive to cytotoxic chemotherapy. In other words, cytotoxic
chemotherapy is effective only for ER-negative tumor cells. Our
results showed that all postmenopausal patients achieving pCR were
ER-negative (data not shown). In postmenopausal patients, the
effectiveness of chemotherapy must be purely cytotoxic. Of all the
pre-menopausal patients, the majority of patients with pCR were
ER-negative, and only 2 patients with pCR were ER-positive (15 and
30%). It is known that chemotherapy not only has a cytotoxic
effect, but also a hormonal effect in pre-menopausal patients. This
indicates that the ovarian function suppression induced by
chemotherapy possibly encouraged the achievement of pCR in
pre-menopausal ER-positive patients with few positive tumor
cells.
We demonstrated a predictive significance of the
proportion of ER-positive or PgR-positive tumor cells in
neoadjuvant chemotherapy for operable HER2-negative breast cancer.
ER- or PgR-negativity is a significant predictive factor to achieve
pCR in multivariate analysis. Conversely, patients with more than
30% ER-positive tumor cells or more than 1% PgR-positive tumor
cells may not achieve pCR. However, pre-menopausal patients with ER
low-positive tumors may achieve pCR. In conclusion, most patients
achieve favorable clinical responses to neoadjuvant chemotherapy
for operable breast cancer regardless of the proportion of
ER-positive or PgR-positive tumor cells.
Acknowledgements
The authors thank the staff members of
the Department of Clinical Pathology at our hospital for the
technical support.
References
|
1.
|
JA Van der HageCJ van de VeldeJP JulienM
Tubiana-HulinC VanderveldenL DuchateauPreoperative chemotherapy in
primary operable breast cancer: results from the European
Organization for Research and Treatment of Cancer trial 10902J Clin
Oncol19422442372001
|
|
2.
|
HD BearS AndersonA BrownThe effect on
tumor response of adding sequential preoperative docetaxel to
preoperative doxorubicin and cyclophosphamide: preliminary results
from the National Surgical Adjuvant Breast and Bowel Project
Protocol B-27J Clin Oncol2141654174200310.1200/JCO.2003.12.005
|
|
3.
|
B FisherJ BryantN WolmarkEffect of
preoperative chemotherapy on the outcome of women with operable
breast cancerJ Clin Oncol162672268519989704717
|
|
4.
|
A MakrisTJ PowlesSE AshleyA reduction in
the requirements for mastectomy in a randomized trial of
neoadjuvant chemoendocrine therapy in primary breast cancerAnn
Oncol911791184199810.1023/A:10084007069499862047
|
|
5.
|
L MauriacG MacGroganA AvrilNeoadjuvant
chemotherapy for operable breast carcinoma larger than 3 cm: a
unicentre randomized trial with a 124-month median
follow-upInstitut Bergonie Bordeaux Groupe Sein (IBBGS) Ann
Oncol1047521999
|
|
6.
|
R RouzierCM PerouWF SymmansBreast cancer
molecular subtypes respond differently to preoperative
chemotherapyClin Cancer
Res1156785685200510.1158/1078-0432.CCR-04-242116115903
|
|
7.
|
P RastogiSJ AndersonHD BearPreoperative
chemotherapy: updates of National Surgical Adjuvant Breast and
Bowel Project Protocols B-18 and B-27J Clin
Oncol26778785200810.1200/JCO.2007.15.023518258986
|
|
8.
|
E ThomasFA HolmesTL SmithThe use of
alternate, non-cross-resistant adjuvant chemotherapy on the basis
of pathologic response to a neoadjuvant doxorubicin-based regimen
in women with operable breast cancer: long-term results from a
prospective randomized trialJ Clin
Oncol2222942302200410.1200/JCO.2004.05.207
|
|
9.
|
G BonadonnaP ValagussaC BrambillaPrimary
chemotherapy in operable breast cancer: eight-year experience at
the Milan Cancer InstituteJ Clin Oncol169310019989440728
|
|
10.
|
HM KuererLA NewmanTL SmithClinical course
of breast cancer patients with complete pathologic primary tumor
and axillary lymph node response to doxorubicin-based neoadjuvant
chemotherapyJ Clin Oncol174604691999
|
|
11.
|
A EltahirSD HeysAW HutcheonTreatment of
large and locally advanced breast cancers using neoadjuvant
chemotherapyAm J
Surg175127132199810.1016/S0002-9610(97)00279-19515529
|
|
12.
|
LA CareyR MetzgerEC DeesAmerican Joint
Committee on Cancer tumor-node-metastasis stage after neoadjuvant
chemotherapy and breast cancer outcomeJ Natl Cancer
Inst9711371142200510.1093/jnci/dji20616077072
|
|
13.
|
BT HennessyGN HortobagyiR RouzierOutcome
after pathologic complete eradication of cytologically proven
breast cancer axillary node metastases following primary
chemotherapyJ Clin Oncol2393049311200510.1200/JCO.2005.02.5023
|
|
14.
|
AE RingIE SmithS AshleyLG FulfordSR
LakhaniOestrogen receptor status, pathological complete response
and prognosis in patients receiving neoadjuvant chemotherapy for
early breast cancerBr J
Cancer9120122017200410.1038/sj.bjc.6602235
|
|
15.
|
RJ BurcombeA MakrisPI RichmanEvaluation of
ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant
anthracycline chemotherapy for operable breast cancerBr J
Cancer92147155200510.1038/sj.bjc.660225615611798
|
|
16.
|
M ColleoniV BagnardiN RotmenszIncreasing
steroid hormone receptor expression defines breast cancer subtypes
non-responsive to preoperative chemotherapyBreast Cancer Res
Treat116359369200910.1007/s10549-008-0223-y
|
|
17.
|
M ColleoniG VialeD ZahriehExpression of
ER, PgR, HER1, HER2, and response: a study of preoperative
chemotherapyAnn Oncol19465472200810.1093/annonc/mdm50917986623
|
|
18.
|
M ColleoniG VialeD ZahriehChemotherapy is
more effective in patients with breast cancer not expressing
steroid hormone receptors: a study of preoperative treatmentClin
Cancer Res1066226628200410.1158/1078-0432.CCR-04-038015475452
|
|
19.
|
ME HammondDF HayesM DowsettAmerican
Society of Clinical Oncology/College of American Pathologists
guideline recommendations for immunohistochemical testing of
estrogen and progesterone receptors in breast cancerJ Clin
Oncol2827842795201010.1200/JCO.2009.25.6529
|
|
20.
|
AU BuzdarNK IbrahimD FrancisSignificantly
higher pathologic complete remission rate after neoadjuvant therapy
with trastuzumab, paclitaxel, and epirubicin chemotherapy: results
of a randomized trial in human epidermal growth factor receptor
2-positive operable breast cancerJ Clin
Oncol2336763685200510.1200/JCO.2005.07.032
|
|
21.
|
EA EisenhauerP TherasseJ BogaertsNew
response evaluation criteria in solid tumours: revised RECIST
guideline (version 1.1)Eur J
Cancer45228247200910.1016/j.ejca.2008.10.026
|
|
22.
|
K KaiN ArimaH MiyayamaY YamamotoH IwaseR
NishimuraPathological lymph node involvement at surgery is a
significant predictive factor of recurrence in locally advanced
breast cancer treated with concomitant epirubicin-docetaxel
neoadjuvant chemotherapy: a cohort studyBreast
Cancer164248200910.1007/s12282-008-0055-y
|
|
23.
|
JM HarveyGM ClarkCK OsborneDC
AllredEstrogen receptor status by immunohistochemistry is superior
to the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancerJ Clin Oncol17147414811999
|