Introduction
In the majority of adult leukemic patients it is
possible to achieve a complete remission (CR). In the case of acute
myeloid leukemia (AML) 60–80% of patients reach CR. However, the
majority of these patients subsequently relapse. Currently, there
is an effort to predict relapse by monitoring minimal residual
disease (MRD) and subsequently to begin the treatment of the
patients during their clinical and hematological remission prior to
overt hematological relapse. Treatment of MRD has a greater
probability of success, is less distressing for the patients and
also has a lower cost than the treatment of a probable
hematological relapse. Monitoring of the MRD thus contributes to
the success of the treatment of leukemic patients. The so-called
molecular techniques, based on an analysis of nucleic acids, appear
to be most useful for MRD monitoring. Molecular techniques are
highly specific and in connection with polymerase chain reaction
(PCR) also very sensitive. These techniques enable the detection of
a proliferation of the leukemic clone weeks or several months prior
to the hematological relapse, when the patient is still in
hematological and clinical remission. However, not all leukemic
patients have a specific molecular marker for the detection of MRD.
Intensive effort is therefore being applied to finding new
molecular nonspecific markers for these patients.
The Wilms’ tumor gene (WT1) appears to be highly
promising as a molecular MRD marker in leukemic patients,
particularly those with AML (1).
The WT1 gene was originally identified as a tumor suppressor gene
(2). This gene functions also as a
transcription factor for a number of genes. It inhibits apoptosis
via p53 and Bcl-2 and also inhibits the differentiation of leukemic
cells (3). It is expressed in a
small number of human tissues (4–6) and
in various cancer cells (7)
including leukemia cells (8). WT1
is highly expressed in the cells of the majority leukemic patients
at diagnosis and it appears to participate in leukemogenesis
(9). WT1 expression is markedly
low in cells of normal healthy individuals, with the exception of
the CD34+ hematopoietic progenitors (10). The expression of the WT1 gene in
leukemic cells is up to 10-fold higher than in normal bone marrow
or peripheral blood (PB) cells (11). Consequently, this gene is a
suitable candidate as a marker for monitoring MRD in AML, either in
bone marrow or in PB, particularly in patients without specific
molecular markers (1,10–22).
The appropriate marker for MRD monitoring should be overexpressed
minimally 2 logs higher in diagnostic samples compared to those
from normal healthy individuals. Therefore, estimation of the level
of the WT1 expression could be beneficial for predicting
hematological relapse. As the WT1 gene is often expressed in
patients in permanent remission, it was necessary to establish an
upper remission limit which, when exceeded, signals a high risk of
hematological relapse. In fact, the upper remission limit defines
the molecular relapse level. In this study, we describe an
estimation of the upper remission limit in PB which could prove
beneficial when deciding on an optimal treatment for AML patients.
The exceeding of this level predicts an impending relapse.
Conversely, when WT1 expression does not fall below this level
following induction and/or consolidation therapy, it signals future
hematological relapse (20).
Materials and methods
Patient samples
A total of 161 AML patients were enrolled in our
study, 101 of which were analysed at the time of
diagnosis/presentation. In total, 760 consecutive samples of these
AML patients were analysed during the follow-up. Additionally, 11
patients were not evaluated at the time of diagnosis. However,
these patients relapsed with concurrent/simultaneous rise of WT1
expression. In 49 of the patients consecutive samples were not
available and they were analysed only at diagnosis. The AML
patients were subdivided according to the FAB classification and
the particular FAB subtypes comprise: AML M0 (n=2), AML M1 (n=36),
AML M2 (n=57), AML M3 (n=17), AML M4 (n=25), AML M5 (n=10), AML M6
(n=2) and biphenotypic leukemia (n=1) and 1 patient not defined.
The median age of the AML patients was 51 years at presentation and
the median of the follow-up was 16 months. The WT1 expression was
additionally measured in the PB from 28 healthy donors. A total of
10 million white blood cells from PB were isolated using the red
cell lysis method immediately following collection. The cell
sediment was lysed and mRNA stabilised by guanidine isothiocyanate.
Total RNA was extracted according to a previously described method
(23).
cDNA synthesis
Isolated total RNA (0.5–1 μg) was incubated
with 50 pmol of random hexamers at 65°C for 10 min; subsequently,
120 units of Mu-MLV reverse transcriptase (Promega, Madison, USA),
10 units of RNAse inhibitor RNAsin (Promega) and a mixture of
deoxynucleotide triphosphates (final concentration 2.5 mM each)
were added in a total volume of 10 μl. Synthesis of cDNA was
performed at 42°C for 60 min followed by heating at 95°C for 2
min.
Real-time PCR
The quantitative real-time PCR (RQ-PCR) of WT1 was
performed with the TaqMan probe labeled with fluorescein and Tamra.
The expression of WT1 was related to the expression of the
housekeeping gene ABL. Amplification and data analysis were carried
out in the Rotor-Gene 3000A thermocycler (Corbett Research, Sydney,
Australia). The primers and TaqMan probe specific for the WT1 cDNA
were designed as described previously (24) and synthesized by Invitrogen
(Carlsbad, CA, USA) and Metabion (Munich, Germany). Optimum
reaction conditions for WT1 and ABL amplification were: 1.00 units
FastStart DNA Polymerase (Roche Applied Science, Mannheim,
Germany), 4.0 mM magnesium chloride, 0.5 μM primers: WT1
sense, 5′-ACA ggg TAC gAg AgC gAT AAC CA-3′; WT1 antisense, 5′-CAC
ACg TCg CAC ATC CTg AAT-3′; and 0.2 μM of the specific
fluorogenic probe, 5′-6-FAM-CAA CgC CCA TCC TCT gCg gAg CCC A XTph
where X is Tamra and 200 μM mixture of the particular
deoxyribonucleotide triphosphate in a total volume of 20 μl.
Cycling was initiated by denaturation at 95°C for 5 min followed by
45 cycles of denaturation at 95°C for 20 sec and
annealing/elongation at 60°C for 60 sec as recommended for the
TaqMan assays. Final results were expressed as the mathematical
formula: Relative WT1 expression = 2CtABL-CtWT1.
Simultaneously, the dilution range standards were quantified in
order to check the efficiency and sensitivity of our RQ-PCR
protocol for quantification of WT1 related to the quantification of
the reference gene ABL. These standards were prepared by cloning
PCR products of WT1 and ABL into a plasmid and the copy number was
subsequently calculated. The two RQ-PCRs, WT1 and ABL, had the same
efficiency. The aforementioned RQ-PCR achieved the
10−4–10−5 sensitivity and it was confirmed
and correlated with the quantification of WT1 expression using the
WT1 ProfileQuant® kit (Ipsogen, Marseille, France), the
protocol recommended by the European LeukemiaNet. The two methods
were found to be markedly significantly correlated
(P<0.001).
Statistical evaluation
Statistical evaluation of our results, i.e.
determination of medians, column statistics and calculation of
significance, was performed by the Prism GraphPfad software. The
nonparametric Mann-Whitney test was used for comparison of
expression WT1 in the particular FAB subtypes. The upper remission
value of WT1 expression was estimated as 3 standard deviations (SD)
above the mean expression in PB cells from patients in permanent
hematological remission.
Results
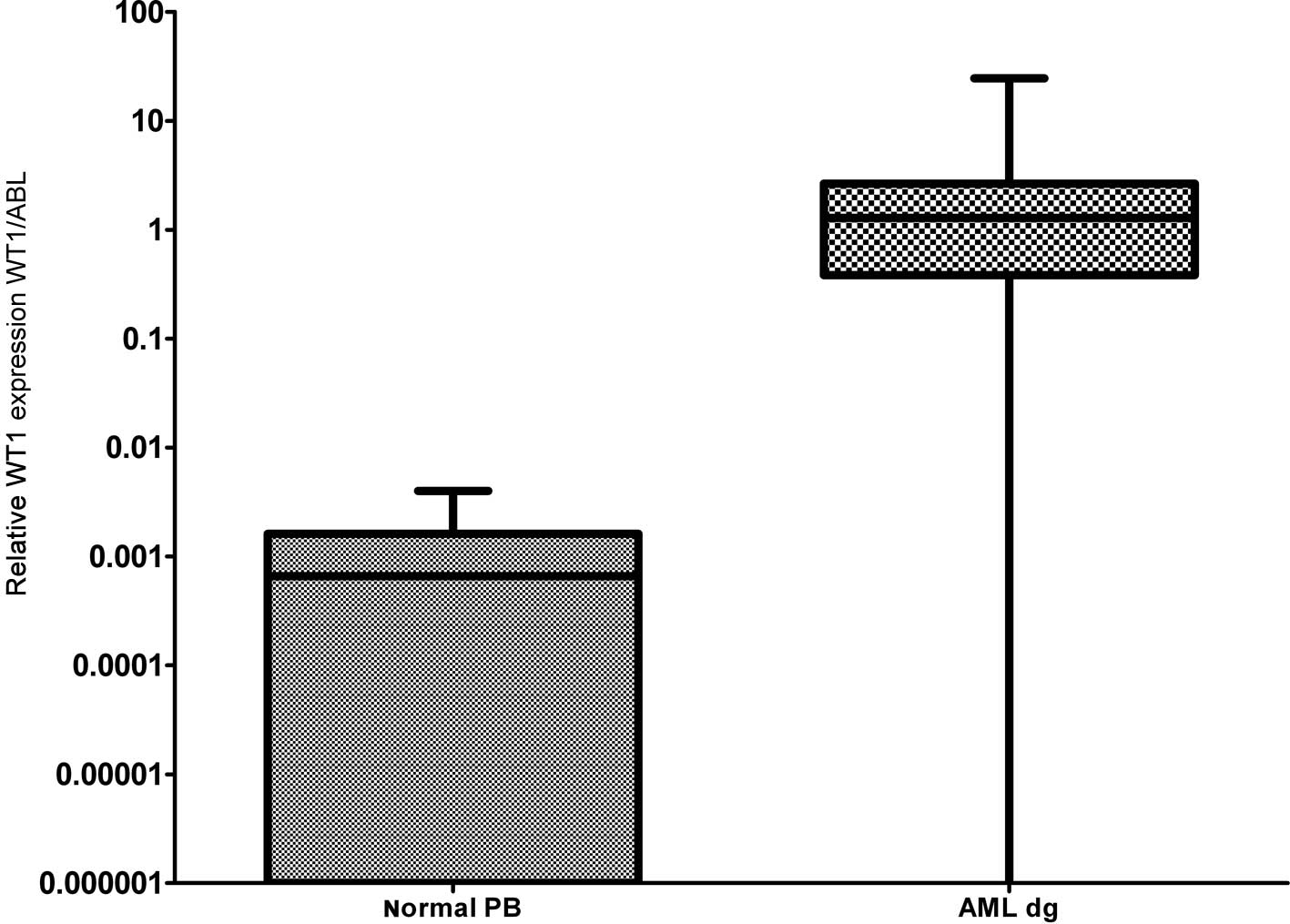
As documented in Fig.
1 more than 90% of AML patients have a two-orders-of-magnitude
overexpression of the WT1 gene compared to median expression in
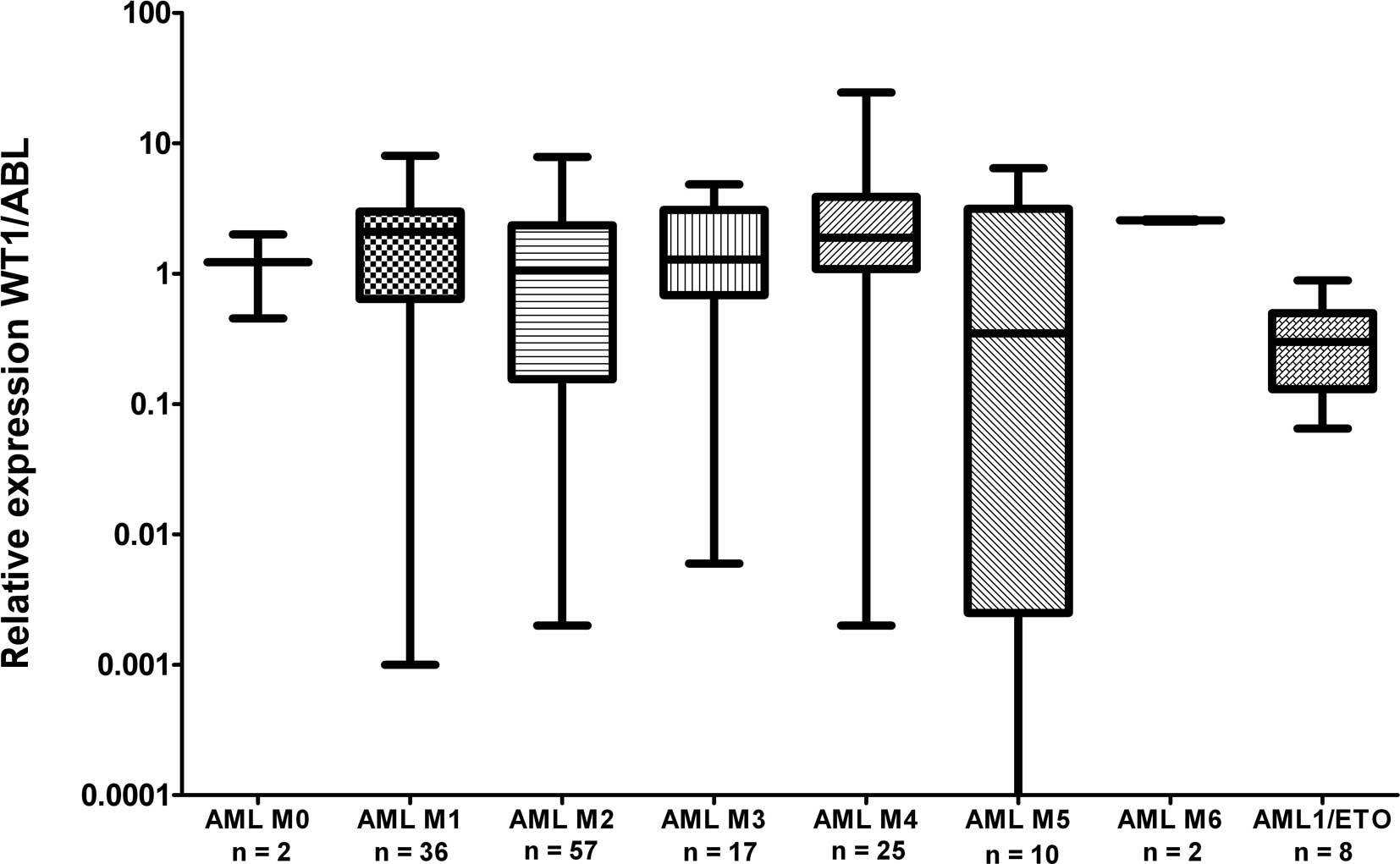
normal PB. WT1 expression was almost uniform among the particular
FAB subgroups with the exception of the AML M5 patients, whose WT1
expression varied in a broad range; this observation is in
accordance with a previous study (25). We have only found a statistically
significantly lower expression in AML1/ETO-positive patients at
diagnosis compared to the rest of AML patients at diagnosis
(P=0.0089) (Fig. 2), in accordance
with the results from another study (15). Due to the significant
overexpression of WT1 in more than 90% of AML patients, its
decrease in patients in remission and its increase prior to a
hematological relapse, WT1 expression could be used as a marker of
MRD. The main objection to the utility of the WT1 expression as a
marker of MRD is its expression in the PB of healthy normal
individuals and AML patients in permanent hematological remission.
It was therefore necessary to establish a molecular relapse border
for WT1 expression. The impending hematological relapse could be
signaled by exceeding this WT1 level, defined as the molecular
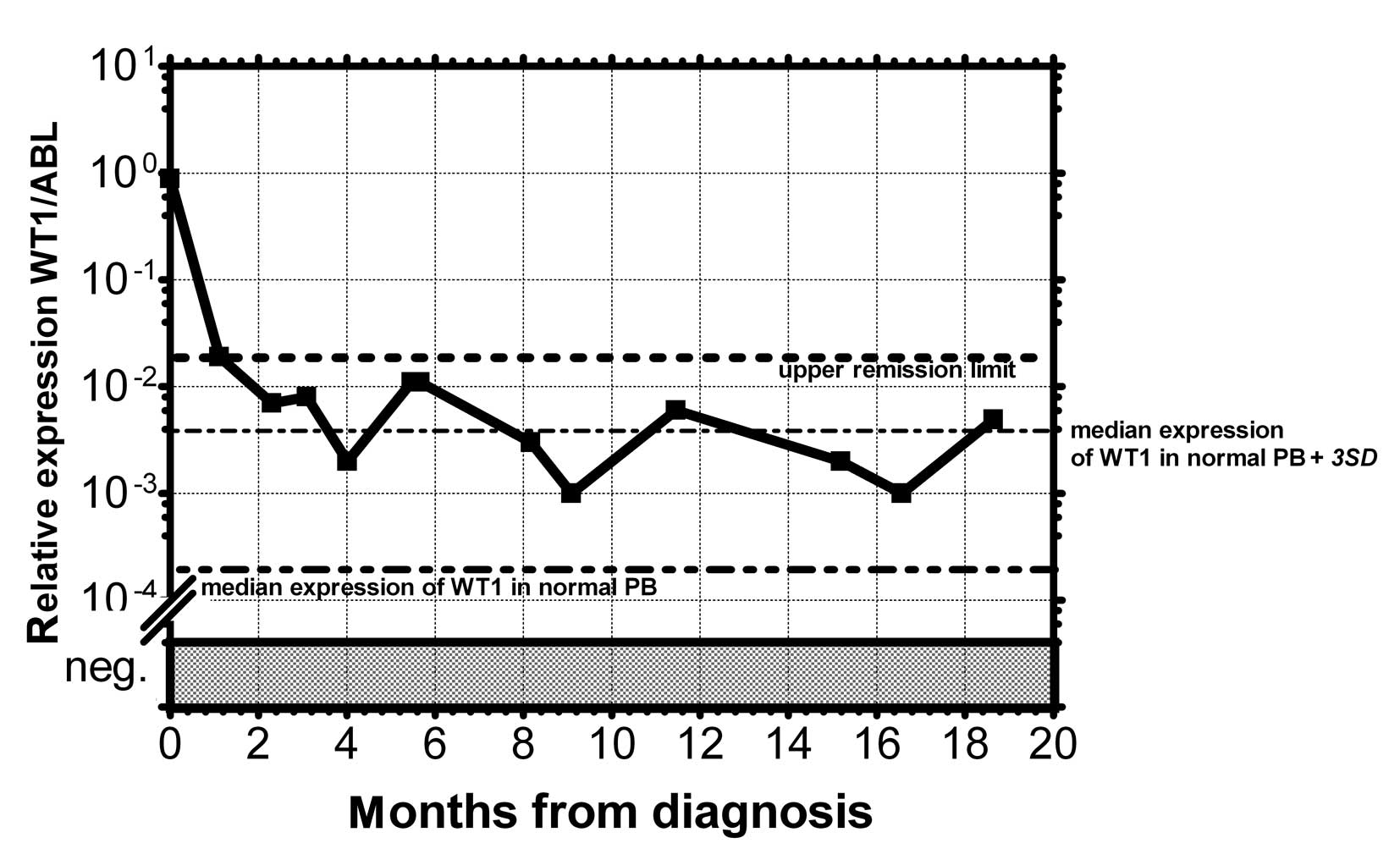
relapse level. At the beginning we took the mean level of WT1 of
healthy persons plus 3 SD. However, the WT1 level in patients in
permanent remission exceeded this level in certain cases without
subsequent hematological relapse. Therefore we took the mean WT1
level of patients in permanent remission plus 3 SD, as documented
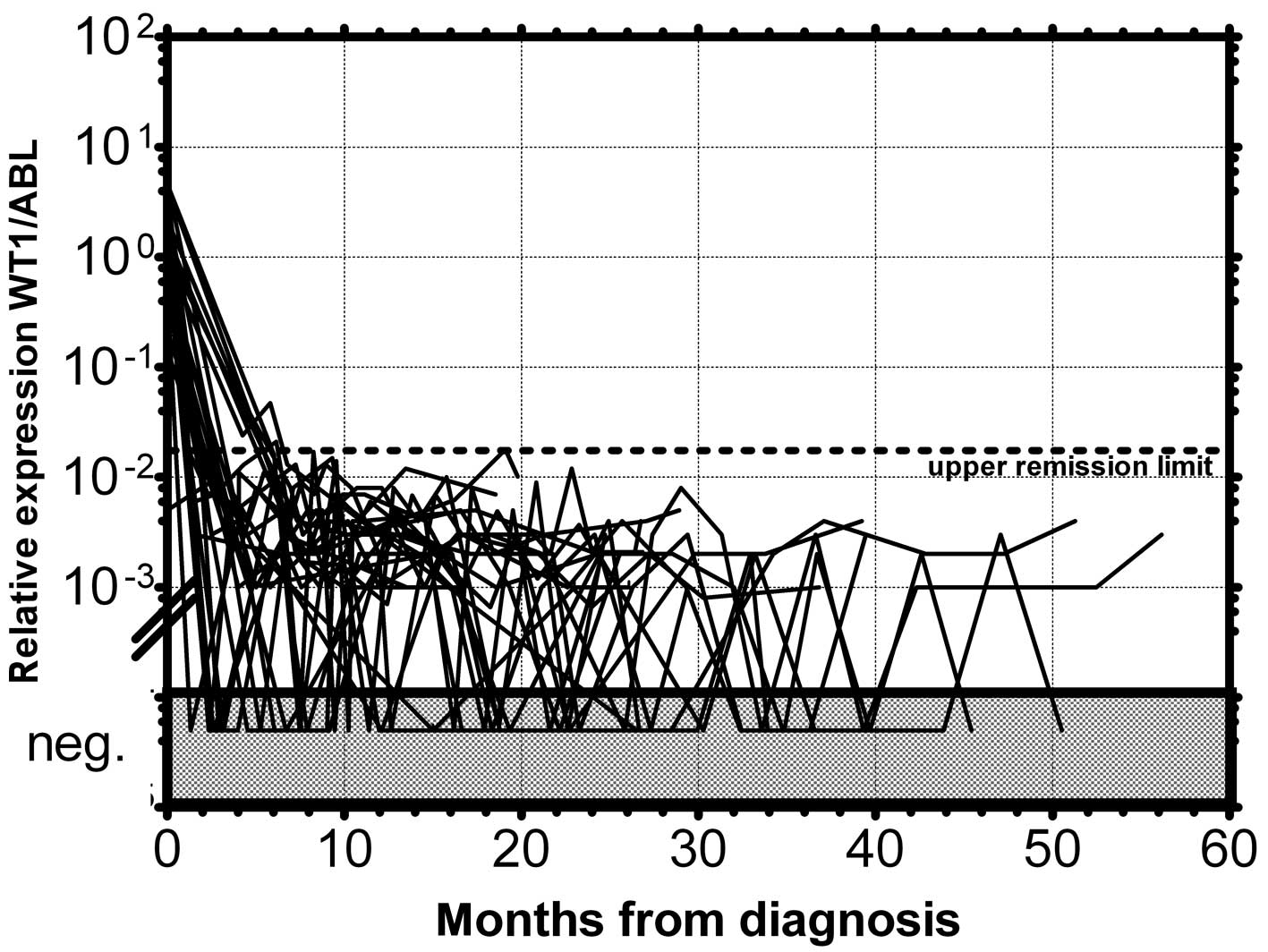
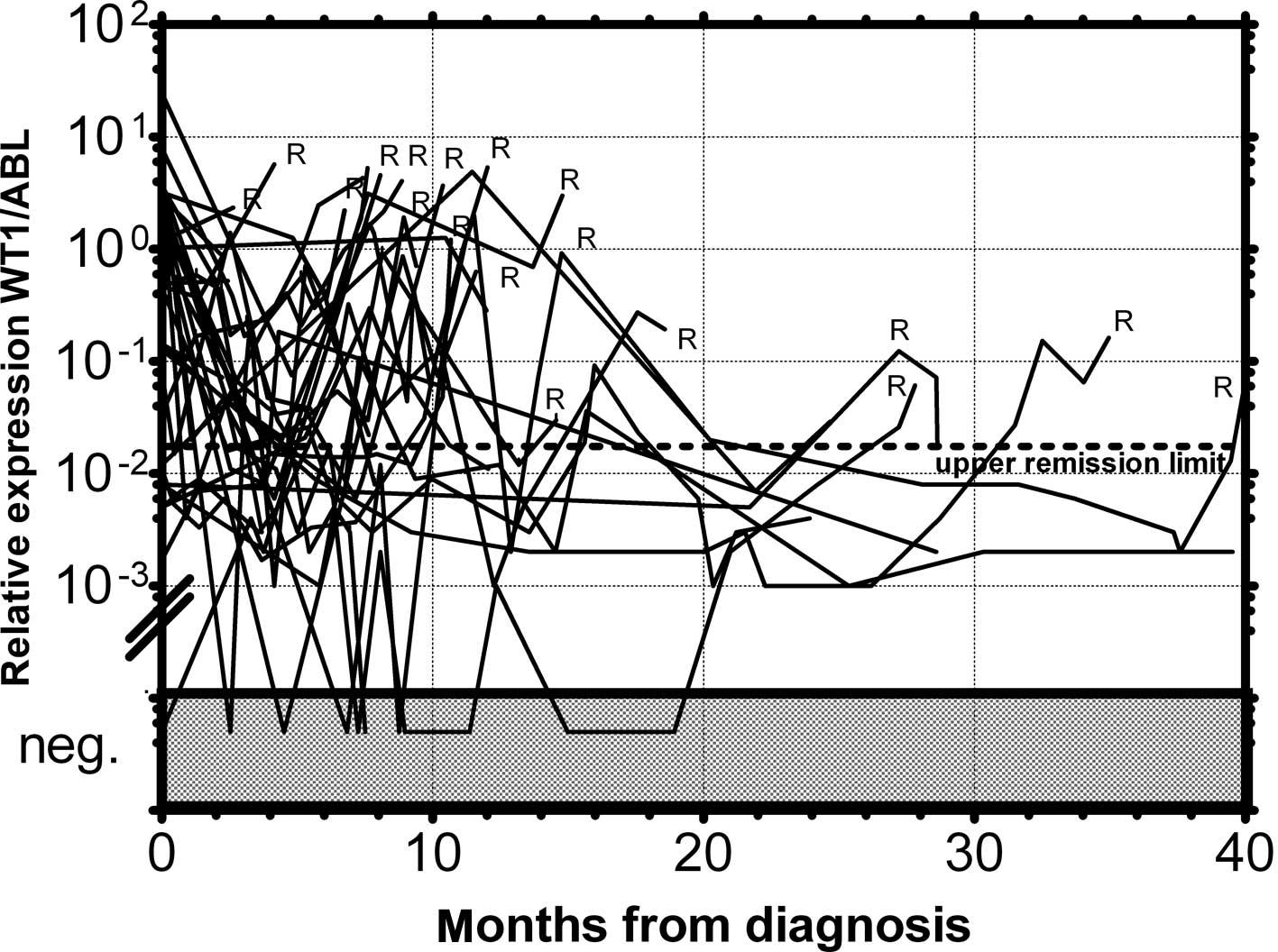
in 1 patient in permanent remission (Fig. 3). None of the monitored patients in
permanent remission (n=73) exceeded this upper remission level
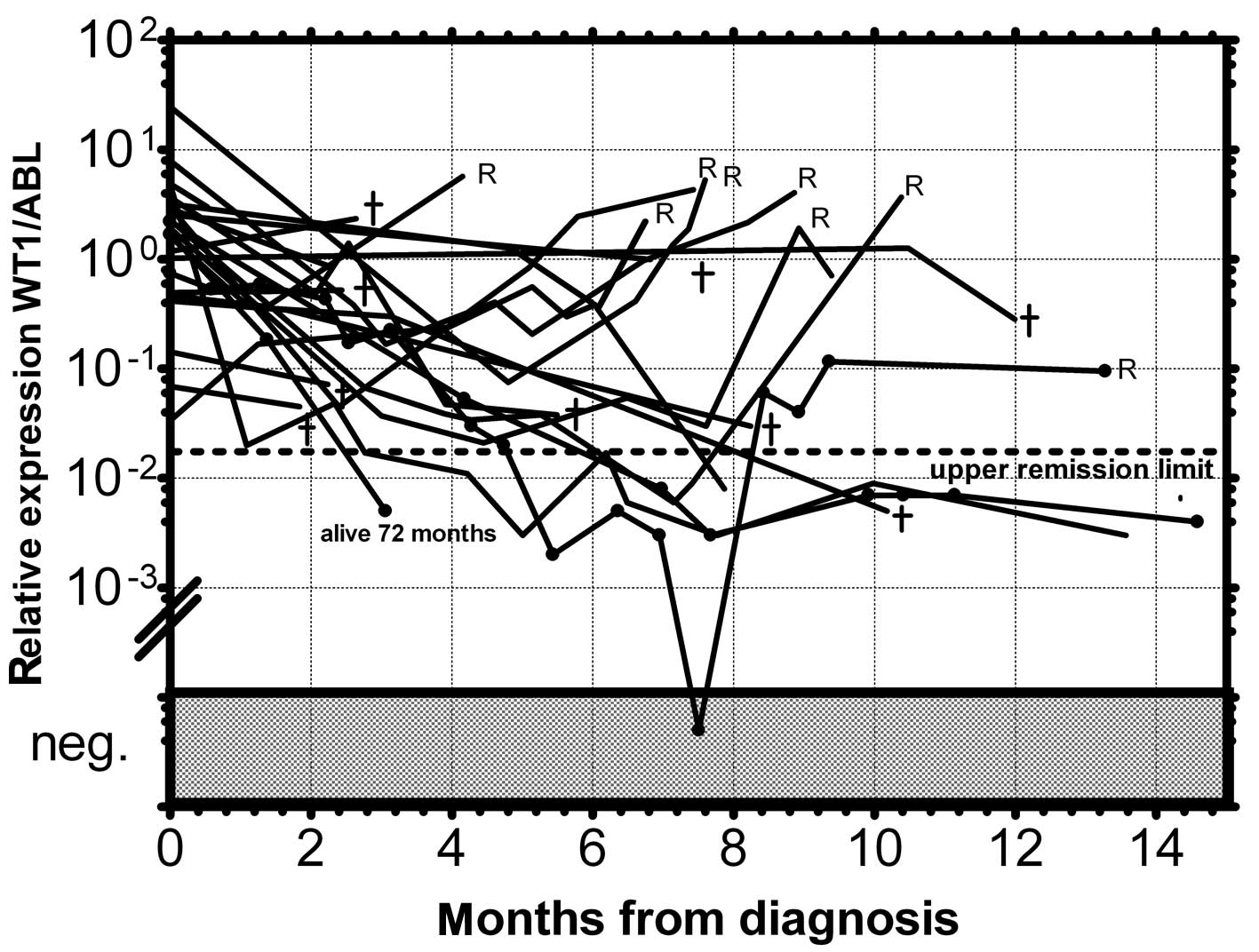
(Fig. 4). Conversely, the relapsed
patients (n=39) crossed this upper remission level prior to relapse
(median prediction, 1.76 months prior to overt relapse; range
0.29–2.57) and 32 of them succumbed to their disease (Fig. 5). Therefore, we would suggest
crossing this value as the molecular relapse in AML patients. In
our experimental setting, the upper remission level was estimated
to be the relative (WT1/ABL) expression WT1 = 0.02. Also in
accordance with a previous study (20), the majority of our patients (21/24)
whose WT1 level did not fall below our upper remission level
following induction and/or consolidation therapy relapsed and the
majority of them subsequently succumbed to their disease (Fig. 6). We therefore propose that our
upper remission limit can be considered as the molecular relapse
level and patients whose WT1 expression exceeds this level should
be monitored more carefully and, if possible, the treatment of the
impending relapse should be begun in advance.
Discussion
The expression of WT1 gene is considerably increased
in the vast majority of AML patients. There were no significant
diferences in WT1 expression observed among particular FAB subtypes
with one exception: The AML1/ETO-positive patients displayed lower
expression of WT1 compared to the rest of the AML patients at
diagnosis. This observation is in accordance with a previous study
(15). Additionally, the AML M5
patients exhibited a broad range of WT1 expression, as has also
been shown in a previous study (25). The quantitation of the WT1 gene
expression in PB made it possible to detect MRD in AML patients
regardless of the presence or absence of tumor-specific DNA
markers. The only disadvantage of the WT1 gene as a marker of MRD
in AML patients is its expression in leukocytes of normal healthy
persons and AML patients in permanent remission. Therefore, for
practical use of measuring the WT1 expression as the MRD marker, it
was necessary to determine the level of WT1 expression that, when
exceeded, signals a high risk of relapse. This upper normal limit
was originally established in a previous study (26). Additionally, the critical remission
level was also defined previously (14). At the begining of our study, we
attempted to similarly define the molecular relapse level as a mean
expresion of WT1 in normal PB plus 3 SD. As certain patients
crossed this level without subsequent relapse, the level estimated
by this approach was not applicable for monitoring MRD in our
patients. The WT1 level in our remission patients was higher in
some cases than in normal healthy individuals and therefore we used
the mean of WT1 expression plus 3 SD in AML patients who did not
subsequently relapse and were in permanent remission. According to
our results, crossing this level signalises a high risk of relapse
and almost all (95%) of these patients subsequently relapsed.
Therefore this level, determined on the basis of patients in
permanent remission, could be taken as the border of molecular
relapse. As has been shown by previously (20,26),
an insufficient decrease of WT1 expression that does not bring its
level below the upper normal level after induction and/or
consolidation therapy, signalises the future hematological relapse.
This was also found in 88% (21/24) of our patients who did not fall
below our upper remission limit. As previously described, the
determination of WT1 expression following cytoreductive treatment
allows for the identification of patients with markedly poor
prognosis (14,21), i.e., those whose WT1 expression did
not reach normal level, i.e., below the upper remission limit. An
alternative approach could be an absolute decrease of the WT1 level
following the initial treatment. Consequently, the decrease in the
WT1 level by fewer than 2 orders of magnitude also signals the high
risk of relapse (15,26,27).
The WT1 level following induction and consolidation
is therefore a significant prognostic factor of patient outcome.
Patients whose WT1 expression was below the upper remission limit
in their follow-up samples remained in complete remission. Our
results document the utility of monitoring MRD in AML patients by
quantitative estimation of the expression of the WT1 gene.
Acknowledgements
The authors thank Ms. Eva Kohoutová
and Ms. Olga Nosková for their skilled technical assistance. The
study was supported by grants IGA MZ CR NS10632-3/2009 and
IHBT00023736.
Abbreviations:
|
WT1
|
Wilm’s tumor gene;
|
|
AML
|
acute myeloid leukemia;
|
|
MRD
|
minimal residual disease
|
References
|
1.
|
D CilloniE GottardiM FavaUsefulness of
quantitative assessment of the WT1 gene transcript as a marker for
minimal residual disease
detectionBlood102773774200310.1182/blood-2003-03-098012835231
|
|
2.
|
KM CallT GlaserCY ItoIsolation and
characterization of a zinc finger polypeptide gene at the human
chromosome-11 Wilms’ tumor locusCell6050952019902154335
|
|
3.
|
V ScharnhorstAJ van der EbAG JochemsenWT1
proteins: functions in growth and
differentiationGene273141161200110.1016/S0378-1119(01)00593-511595161
|
|
4.
|
AL MenkeA SchedlWT1 and glomerular
functionSemin Cell Dev
Biol14233240200310.1016/S1084-9521(03)00026-014627122
|
|
5.
|
GB SilbersteinHK VanP StricklandCT Roberts
JrCW DanielAltered expression of the WT1 Wilms tumor suppressor
gene in human breast cancerProc Natl Acad Sci
USA9481328137199710.1073/pnas.94.15.81329223327
|
|
6.
|
A MakrigiannakisK AminG CoukosJL TillyC
CoutifarisRegulated expression and potential roles of p53 and
Wilms’ tumor suppressor gene (WT1) during follicular development in
the human ovaryJ Clin Endocrinol Metab854494592000
|
|
7.
|
S NakatsukaY OjiT
HoriuchiImmunohistochemical detection of WT1 protein in a variety
of cancer cellsMod Pathol19804814200616547468
|
|
8.
|
H MiwaM BeranGF SaundersExpression of the
Wilms-Tumor Gene (Wt1) in human
leukemiasLeukemia640540919921317488
|
|
9.
|
HD MenssenHJ RenklU RodeckPresence of
Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in
the majority of human acute leukemiasLeukemia9106010671995
|
|
10.
|
K InoueH OgawaT YamagamiLong-term
follow-up of minimal residual disease in leukemia patients by
monitoring WT1 (Wilms tumor gene) expression
levelsBlood882267227819968822948
|
|
11.
|
H SugiyamaWilms tumor gene (WT1) as a new
marker for the detection of minimal residual disease in
leukemiaLeuk Lymphoma30556119989669676
|
|
12.
|
J TrkaM KalinovaO HrusakReal-time
quantitative PCR detection of WT1 gene expression in children with
AML: prognostic significance, correlation with disease status and
residual disease detection by flow
cytometryLeukemia1613811389200210.1038/sj.leu.240251212094264
|
|
13.
|
H OgawaH TamakiK IkegameThe usefulness of
monitoring WT1 gene transcripts for the prediction and management
of relapse following allogeneic stem cell transplantation in acute
type
leukemiaBlood10116981704200310.1182/blood-2002-06-183112406915
|
|
14.
|
M GargH MooreK TobalJAL YinPrognostic
significance of quantitative analysis of WT1 gene transcripts by
competitive reverse transcription polymerase chain reaction in
acute leukaemiaBr J
Haematol1234959200310.1046/j.1365-2141.2003.04552.x
|
|
15.
|
M OstergaardLH OlesenH HasleE KjeldsenP
HoklandWT1 gene expression: an excellent tool for monitoring
minimal residual disease in 70% of acute myeloid leukaemia patients
- results from a single-centre studyBr J
Haematol125590600200415147374
|
|
16.
|
M WeisserW KernS RauhutC SchochW
HiddemannT HaferlachS SchnittgerPrognostic impact of RT-PCR-based
quantification of WT1 gene expression during MRD monitoring of
acute myeloid
leukemiaLeukemia1914161423200510.1038/sj.leu.240380915920493
|
|
17.
|
D CilloniE GottardiD De
MicheliQuantitative assessment of WT1 expression by real time
quantitative PCR may be a useful tool for monitoring minimal
residual disease in acute leukemia
patientsLeukemia1621152121200210.1038/sj.leu.240267512357365
|
|
18.
|
D OsborneL FrostK TobalJAL YinElevated
levels of WT1 transcripts in bone marrow harvests are associated
with a high relapse risk in patients autografted for acute myeloid
leukaemiaBone Marrow
Transplant366770200510.1038/sj.bmt.170499215908982
|
|
19.
|
A CandoniM TiribelliE
ToffolettiQuantitative assessment of WT1 gene expression after
allogeneic stem cell transplantation is a useful tool for
monitoring minimal residual disease in acute myeloid leukemiaEur J
Haematol826168200910.1111/j.1600-0609.2008.01158.x
|
|
20.
|
D CilloniF MessaF ArrugaEarly prediction
of treatment outcome in acute myeloid leukemia by measurement of
WT1 transcript levels in peripheral blood samples collected after
chemotherapyHaematologica93921924200810.3324/haematol.12165
|
|
21.
|
HB OmmenCG NyvoldK BraendstrupRelapse
prediction in acute myeloid leukaemia patients in complete
remission using WT1 as a molecular marker: development of a
mathematical model to predict time from molecular to clinical
relapse and define optimal sampling intervalsBr J
Haematol141782791200810.1111/j.1365-2141.2008.07132.x
|
|
22.
|
Y SakamotoY MariyaS SasakiWT1 mRNA level
in peripheral blood is a sensitive biomarker for monitoring minimal
residual disease in acute myeloid leukemiaTohoku J Exp
Med219169176200910.1620/tjem.219.16919776535
|
|
23.
|
P ChomczynskiN SacchiSingle-step method of
RNA isolation by acid guanidinium thiocyanate-phenol-chloroform
extractionAnal
Biochem162156159198710.1016/0003-2697(87)90021-22440339
|
|
24.
|
J PolákJ MarkovaJ SchwarzJ MaaloufovaZ
VolkovaJ CermakC Haskovec[The use of quantitative assessment of
Wilms tumour gene 1 for monitoring of residual disease in acute
myeloid leukemia patients]Cas Lek Cesk14536422006
|
|
25.
|
KA KreuzerA SaborowskiJ LupbergerC
AppeltIK NaP Le CoutreCA SchmidtFluorescent 5′-exonuclease assay
for the absolute quantification of Wilms’ tumour gene (WT1) mRNA:
implications for monitoring human leukaemiasBr J
Haematol1143133182001
|
|
26.
|
D CilloniA RennevilleF HermitteReal-time
quantitative polymerase chain reaction detection of minimal
residual disease by standardized WT1 assay to enhance risk
stratification in acute myeloid leukemia: a European LeukemiaNet
studyJ Clin Oncol2751955201200910.1200/JCO.2009.22.4865
|
|
27.
|
G GianfaldoniF MannelliV PonzianiG LongoS
BenciniA BosiAM VannucchiEarly reduction of WT1 transcripts during
induction chemotherapy predicts for longer disease free and overall
survival in acute myeloid
leukemiaHaematologica95833836201010.3324/haematol.2009.011908
|