Introduction
Renal cell carcinoma (RCC) is one of the most lethal
urologic cancers, with a global incidence of approximately 200,000
new cases and a mortality rate of more than 100,000 patients
annually (1). Of all patients with
RCC, 20–30% subsequently experience local or distant recurrence
within 5 years after an initial curative nephrectomy (2,3). RCC
has a dismal prognosis after metastasis has occurred, although
immunotherapy and several molecular-targeted agents lead to
prolonged survival in some patients with 5-year survivals of
<20% (4,5). Therefore, it is important to predict
which patients will develop disease recurrence after surgery for
localized RCC.
Currently, several prognostic models for
non-metastatic RCC, such as the University of California Los
Angeles Integrated Staging System and the stage, size, grade and
necrosis score, are mainly based on clinicopathological parameters
(6–8). The most important conventional
features are pathological tumor-node-metastasis stage, Fuhrman
nuclear grade and Eastern Cooperative Oncology Group performance
status (ECOG PS). Although risk grouping with these features is
possible, significant heterogeneity persists among patients with a
similarly predicted prognosis. In addition to clinicopathological
prognostic features, several molecular and genetic tissue markers
have been investigated as potential prognosticators for RCC,
suggesting that molecular markers may play an important role in
predicting prognosis (4,9,10).
CD44 is a ubiquitous multistructural and
multifunctional cell surface adhesion molecule involved in
cell-cell and cell-matrix interactions. Several isoforms of CD44
have been identified and are the result of alternative
post-transcriptional splicing modifications of 10 exons within a
single gene located on the short arm of chromosome 11. CD44
transmembrane glycoproteins were originally described to mediate
lymphocyte homing to peripheral lymphoid tissues through an
interaction with hyaluronic acid on high endothelial venules
(11–13). The standard form of CD44 is the
main receptor for hyaluronate and has been found to play a vital
role in the hematogenous dissemination of tumor cells in various
human cancers (14). Although CD44
is closely associated with proliferation, metastasis, cancer
recurrence and prognosis, contradictory results have been reported
concerning CD44 overexpression in relation to tumor development and
progression in different tumors at different sites (15). CD44 expression is altered in RCC
and has been suggested as a useful prognostic marker, but its
prognostic role in RCC remains controversial (13,16–23).
In the present study, we examined CD44 expression as a prognostic
marker in tumor specimens from patients with localized clear cell
RCC (CCRCC) and investigated the relationship between CD44
expression and clinicopathological features and patient
survival.
Materials and methods
Patients and tumor samples
We retrospectively investigated 110 consecutive
patients with CCRCC who underwent a radical or partial nephrectomy
for sporadic, localized RCC (pT1-3N0M0) at the Chungnam National
University Hospital, Daejeon, Korea, between 2000 and 2006.
Clinicopathological baseline data were obtained through medical
record review. ECOG performance status was assigned to each patient
at the time of diagnosis. T classification was defined according to
the 2002 American Joint Committee on Cancer criteria and nuclear
grade was determined according to Fuhrman’s grading system. Tumor
samples were collected from tissue blocks used for routine
pathological examination. This study was approved by the local
ethics committee.
Tissue microarray construction
Tissue microarrays were constructed from 110
archival, original, formalin-fixed, paraffin-embedded tissue blocks
of localized CCRCC. A representative tumor area was carefully
selected from a hematoxylin and eosin-stained section of each donor
block. Each case was represented by two cylindrical cores (2-mm
diameter) from a tumor, which was punched using an automated tissue
arrayer (UNITMA, Seoul, Korea). Thus, tissue microarray blocks
containing 220 cylinders were constructed.
Specimen preparation and
immunohistochemistry
Sections (3-μm thick) were cut from recipient
blocks, placed on 3-amino-propyltriethoxysilane-coated slides and
dried at 57°C for 2 h before staining. All procedures were
performed at room temperature, as recommended by the manufacturer.
Briefly, sections were dewaxed in xylene and rehydrated in a graded
alcohol series. Sections were then washed in water before antigen
retrieval using a Dako PTLink machine (Dako, Glostrup, Denmark)
with 10 mM sodium citrate buffer (pH 6.0) at 97°C for 20 min. The
sections were then treated with 3% hydrogen peroxide for 10 min to
block endogenous peroxidase and were preincubated with a serum-free
protein block solution (Dako, Carpinteria, CA, USA) for 20 min to
eliminate background staining. Monoclonal mouse CD44 antibody
(NeoMarkers, Fremont, CA, USA) as a primary antibody was diluted
1:800 with background-reducing diluents (Dako, Carpinteria, CA,
USA). After 30 min of incubation in a humidity chamber and a wash
with Tris-buffered saline Tween-20 (TBS-T), the slides were
incubated for 30 min with an EnVision anti-mouse (Dako, Glostrup,
Denmark) polymer. Reaction products were visualized with
diaminobenzidine plus substrate-chromogen solution applied for 5
min. The slides were counterstained with Meyer’s hematoxylin and
mounted. Careful rinses with several changes of phosphate-buffered
saline (PBS) were performed between each stage of the procedure.
Negative controls were prepared by excluding the primary
antibody.
Evaluation of immunohistochemical
staining
The immunohistochemical staining results were
evaluated by two independent pathologists (Jin Man Kim and Zhe Long
Liang), who were blinded to the clinicopathological details of the
patients. Immunohistochemical staining was categorized according to
a scoring method; tumors were classified into four grades based on
staining intensity (0, none; 1, weak; 2, intermediate; and 3,
strong). In the case of heterogeneous staining within a sample, the
respective higher score was chosen if >50% of cells exhibited
that staining intensity. The scores of two tumor cores from each
patient were averaged to obtain a mean score. Cases with staining
intensity scores of 0–2 were placed in the CD44-low expression
group (CD44-LEG), and those with staining intensity scores of 3
were placed in the CD44-high expression group (CD44-HEG).
Statistical analyses
Pearson’s χ2 test was used to assess the
correlation between CD44 expression and clinicopathological
features. Recurrence-free survival (RFS), disease-specific survival
(DSS) and overall survival (OS) were estimated using the
Kaplan-Meier method and the log-rank test. RFS was measured from
the date of nephrectomy to the date of recurrence or death from
CCRCC. DSS was measured from the date of nephrectomy to the date of
death from CCRCC only. OS was measured from the date of surgery to
the date of death from all causes. A Cox’s proportional hazards
model was prepared to analyze the effect of CD44 expression on RFS,
DSS and OS. A P-value <0.05 was considered statistically
significant. All statistical analyses were conducted using SPSS
statistical software (vers. 13.0; SPSS, Inc., Chicago, IL,
USA).
Results
Patient characteristics
The patient characteristics are summarized in
Table I. Median age was 60 years
(range, 30–78 years), and 71.8% of the patients were male. Of the
patients, 44.5% were ECOG 0, whereas 53.6 and 1.8% were ECOG 1 and
2, respectively. Median tumor size was 5 cm (range, 1–15 cm). Of
the patients, 42.7, 15.5 and 41.9% had pT1–pT3 primary tumors,
respectively. Fuhrman nuclear grading demonstrated grade 1–4
lesions in 14.5, 68.2, 14.5 and 2.7% of patients, respectively. In
total, 17.3% of the patients experienced recurrence following
nephrectomy (Table II).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Features | N | (%) |
|---|
| Age, years | | |
| Median | 60 |
| Range | 30–78 |
| Gender | | |
| Male | 79 | 71.8 |
| Female | 31 | 28.2 |
| ECOG PS | | |
| 0 | 49 | 44.5 |
| ≥1 | 61 | 55.5 |
| Tumor size, cm | | |
| Median | 5 |
| Range | 1–15 |
| T stage | | |
| pT1a | 25 | 22.7 |
| pT1b | 22 | 20.0 |
| pT2 | 17 | 15.5 |
| pT3a | 39 | 35.5 |
| pT3b | 7 | 6.4 |
| Fuhrman nuclear
grade | | |
| 1 | 16 | 14.5 |
| 2 | 75 | 68.2 |
| 3 | 16 | 14.5 |
| 4 | 3 | 2.7 |
 | Table II.Associations of the expression of CD44
with clinicopathological parameters. |
Table II.
Associations of the expression of CD44
with clinicopathological parameters.
| Features | CD44 expression
| P-value |
|---|
LEG (n=92)
| HEG (n=18)
|
|---|
| N | (%) | N | (%) |
|---|
| Age, years | | | | | |
| ≤70 | 79 | 85.9 | 12 | 82.7 | 0.81 |
| >70 | 13 | 14.1 | 6 | 17.3 | |
| Gender | | | | | |
| Male | 64 | 69.6 | 15 | 83.3 | 0.390 |
| Female | 28 | 30.4 | 3 | 16.7 | |
| ECOG PS | | | | | |
| 0 | 38 | 41.3 | 11 | 61.1 | 0.194 |
| ≥1 | 54 | 58.7 | 7 | 38.9 | |
| Tumor size, cm | | | | | |
| ≤10 | 87 | 94.6 | 16 | 88.9 | 0.321 |
| >10 | 5 | 5.4 | 2 | 11.1 | |
| T stage | | | | | |
| T1 | 43 | 46.7 | 5 | 27.8 | 0.195 |
| T2/3 | 49 | 53.3 | 13 | 72.2 | |
| Fuhrman nuclear
grade | | | | | |
| G1 | 15 | 16.3 | 1 | 5.6 | 0.014 |
| G2 | 65 | 70.7 | 10 | 55.6 | |
| G3 | 10 | 10.9 | 6 | 33.3 | |
| G4 | 2 | 2.2 | 1 | 5.6 | |
| Recurrence | | | | | |
| No | 84 | 91.3 | 7 | 38.9 | <0.001 |
| Yes | 8 | 8.7 | 11 | 61.1 | |
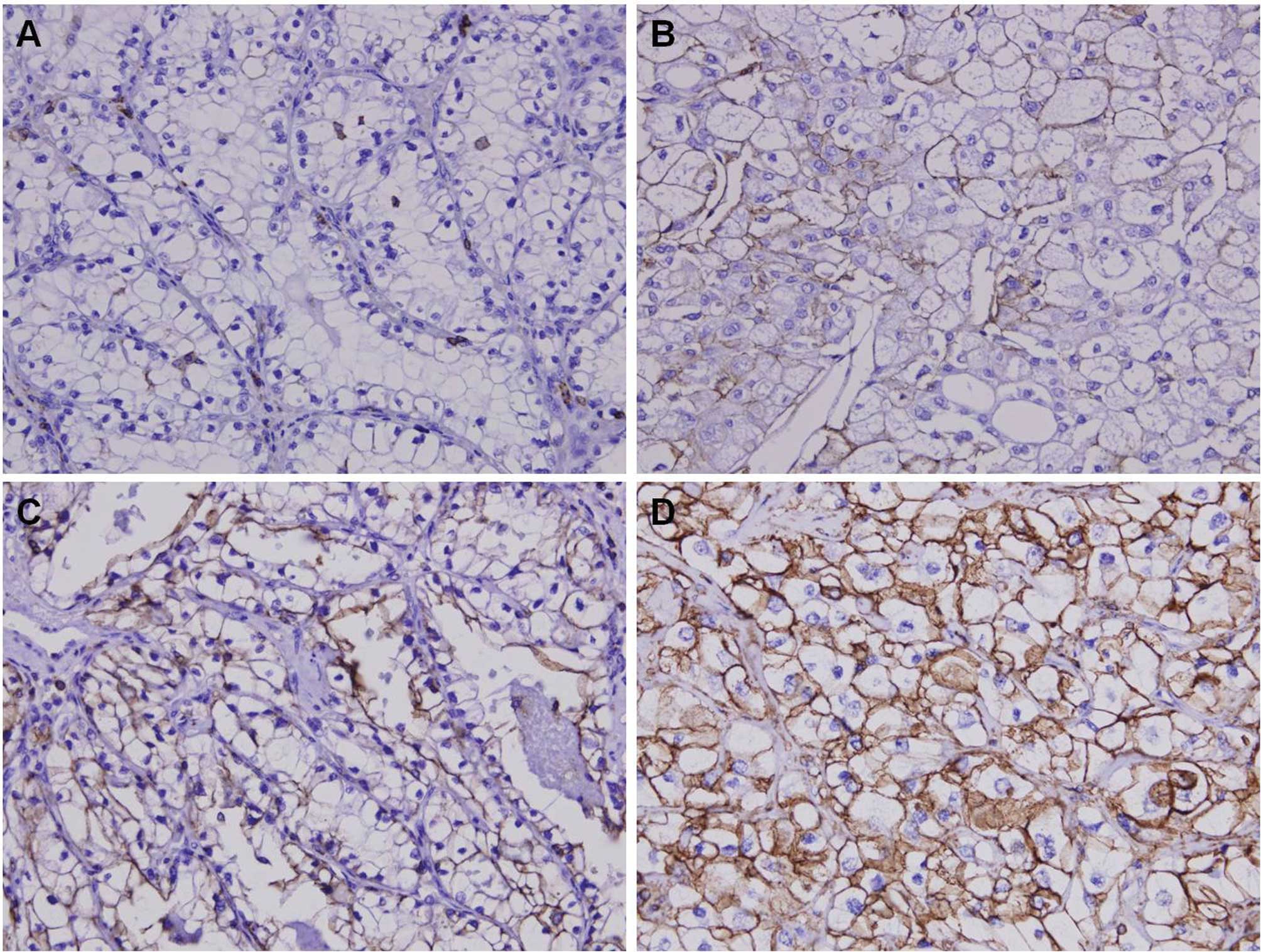
Immunohistochemical analysis for CD44
expression in CCRCC
We analyzed the expression patterns of the CD44
protein using immunohistochemistry from the tissue micro-arrays
(TMAs) of 110 patients with CCRCC. CD44 was diversely stained
mainly in the membrane and cytoplasm of CCRCC cells (Fig. 1). Next, we assessed CD44 expression
levels by determining the positively stained tumor cells using the
staining intensity score (0, +1, +2 and +3). Eighteen cases (16.4%)
showed +3 staining intensity (CD44-HEG), whereas 92 cases (83.6%)
showed +2 (21 cases), +1 (16 cases) or 0 (55 cases) staining
intensity (CD44-LEG).
Correlation between CD44 expression and
clinicopathological features
We next investigated the correlation between CD44
expression and various clinicopathological parameters. The results
are summarized in Table II. No
significant difference in age, gender, ECOG PS or tumor size was
detected. However, CD44-HEG tumors tended to have a higher T stage
(pT2 and pT3) compared with CD44-LEG tumors (72.2 vs. 53.3%).
Furthermore, the CD44-HEG group was significantly associated with a
higher nuclear grade (P=0.014) and tumor recurrence (P<0.001)
than those in the CD44-LEG group.
Correlation between CD44 expression and
survival
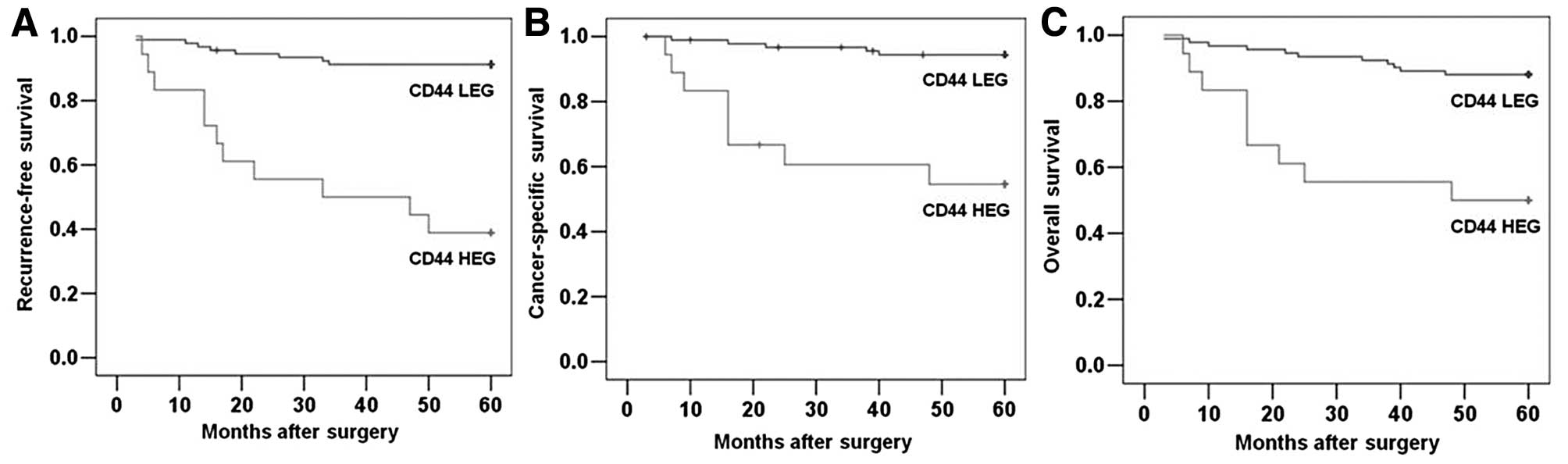
To further investigate the clinical usefulness of
CD44 expression in CCRCC, we compared RFS, DSS and OS based on CD44
expression. The 5-year RFS, DSS and OS rates were 82.7, 88.2 and
81.8%, respectively, for the entire study population. The survival
curves according to CD44 expression are depicted in Fig. 2. The 5-year RFS rates for the
CD44-HEG and CD44-LEG groups were 38.9 and 91.3%, respectively
(Fig. 2A; P<0.001), and the
5-year DSS rates for the CD44-HEG and CD44-LEG groups were 55.6 and
94.6%, respectively (Fig. 2B;
P<0.001). The rates of 5-year OS were 50.0 and 88.0%,
respectively (Fig. 2C;
P<0.001). These results clearly show the significant effect of
CD44 expression on clinical outcome in patients with localized
CCRCC.
Univariate analyses were performed to assess the
clinical significance of various parameters that might influence
tumor recurrence and survival in patients with CCRCC. As summarized
in Table III, Fuhrman nuclear
grade (P=0.010) and CD44 expression (P<0.001) were significant
risk factors affecting the RFS of patients with CCRCC. Age
(P=0.026), Fuhrman nuclear grade (P=0.006) and CD44 expression
(P<0.001) were also significant risk factors for DSS.
Furthermore, age (P=0.021), Fuhrman nuclear grade (P=0.028) and
CD44 expression (P<0.001) were significant risk factors for OS.
Multivariate analyses were performed using Cox’s proportional
hazards model to determine the independent prognostic effects of
these factors. These analyses showed that CD44 expression [hazard
ratio (HR), 9.204; 95% confidence interval (CI), 3.196–26.506;
P<0.001] was an independent risk factor predicting RFS in
patients with CCRCC (Table IV).
CD44 expression remained an independent prognostic factor for DSS
and OS (P=0.002 and 0.008, respectively; Table IV). Taken together, our findings
indicate that CD44 overexpression could be a useful marker to
predict tumor recurrence and survival in patients with localized
CCRCC.
 | Table III.Univariate analyses of the
association of prognosis with clinicopathological parameters and
CD44 expression in patients with renal cell carcinoma. |
Table III.
Univariate analyses of the
association of prognosis with clinicopathological parameters and
CD44 expression in patients with renal cell carcinoma.
| Variables | HR | 95% CI | P-value |
|---|
| Recurrence-free
survival | | | |
| Age (>70/≤70
years) | 1.908 | 0.686–5.302 | 0.215 |
| Gender
(male/female) | 2.333 | 0.680–8.009 | 0.178 |
| ECOG PS
(≥1/0) | 0.873 | 0.355–2.149 | 0.768 |
| Tumor size
(>10/≤10 cm) | 3.405 | 0.990–11.714 | 0.052 |
| Pathologic T
stage (T2–3/T1) | 2.430 | 0.875–6.750 | 0.088 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 3.433 | 1.350–8.727 | 0.010 |
| CD44 expression
(high/low) | 9.669 | 3.869–24.162 | <0.001 |
| Disease-specific
survival | | | |
| Age (>70/≤70
years) | 3.571 | 1.165–10.947 | 0.026 |
| Gender
(male/female) | 4.928 | 0.641–37.908 | 0.125 |
| ECOG PS
(≥1/0) | 0.911 | 0.306–2.710 | 0.867 |
| Tumor size
(>10/≤10 cm) | 2.995 | 0.663–13.520 | 0.154 |
| Pathologic T
stage (T2–3/T1) | 2.855 | 0.785–10.377 | 0.111 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 4.683 | 1.572–13.954 | 0.006 |
| CD44 expression
(high/low) | 10.421 | 3.393–32.002 | <0.001 |
| Overall
survival | | | |
| Age (>70/≤70
years) | 2.925 | 1.178–7.265 | 0.021 |
| Gender
(male/female) | 1.328 | 0.487–3.627 | 0.579 |
| ECOG PS
(≥1/0) | 0.855 | 0.363–2.012 | 0.719 |
| Tumor size
(>10/≤10 cm) | 1.754 | 0.408–7.535 | 0.450 |
| Pathologic T
stage (T2–3/T1) | 1.720 | 0.694–4.262 | 0.242 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 2.767 | 1.115–6.863 | 0.028 |
| CD44 expression
(high/low) | 5.509 | 2.274–13.348 | <0.001 |
 | Table IV.Multivariate analyses of the
association of prognosis with clinicopathological parameters and
CD44 expression in patients with renal cell carcinoma. |
Table IV.
Multivariate analyses of the
association of prognosis with clinicopathological parameters and
CD44 expression in patients with renal cell carcinoma.
| Variables | HR | 95% CI | P-value |
|---|
| Recurrence-free
survival | | | |
| Age (>70/≤70
years) | 0.909 | 0.293–2.819 | 0.869 |
| Gender
(male/female) | 2.057 | 0.523–8.088 | 0.302 |
| ECOG PS
(≥1/0) | 1.657 | 0.593–4.635 | 0.336 |
| Tumor size
(>10/≤10 cm) | 3.150 | 0.729–13.613 | 0.125 |
| Pathologic T
stage (T2–3/T1) | 1.786 | 0.604–5.276 | 0.294 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 1.212 | 0.410–3.580 | 0.728 |
| CD44 expression
(high/low) | 9.204 | 3.196–26.506 | <0.001 |
| Disease-specific
survival | | | |
| Age (>70/≤70
years) | 1.976 | 0.519–7.526 | 0.318 |
| Gender
(male/female) | 3.401 | 0.385–30.004 | 0.271 |
| ECOG PS
(≥1/0) | 2.089 | 0.567–7.699 | 0.268 |
| Tumor size
(>10/≤10 cm) | 1.901 | 0.313–11.552 | 0.485 |
| Pathologic T
stage (T2–3/T1) | 2.098 | 0.533–8.256 | 0.289 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 1.955 | 0.535–7.142 | 0.311 |
| CD44 expression
(high/low) | 7.927 | 2.113–29.737 | 0.002 |
| Overall
survival | | | |
| Age (>70/≤70
years) | 2.641 | 0.938–7.436 | 0.066 |
| Gender
(male/female) | 1.139 | 0.347–3.741 | 0.830 |
| ECOG PS
(≥1/0) | 1.347 | 0.499–3.636 | 0.556 |
| Tumor size
(>10/≤10 cm) | 1.029 | 0.203–5.210 | 0.937 |
| Pathologic T
stage (T2–3/T1) | 1.280 | 0.488–3.355 | 0.616 |
| Fuhrman nuclear
grade (G3–4/G1–2) | 2.216 | 0.739–6.113 | 0.162 |
| CD44 expression
(high/low) | 4.001 | 1.440–11.116 | 0.008 |
Discussion
To our knowledge, this is the largest study to
identify CD44 expression as an independent poor prognostic marker
for RFS, DSS and OS in patients who have undergone nephrectomy for
localized CCRCC. Patients with high CD44 expression were at 9.2-,
7.9- and 4.0-fold increased risk for poor RFS, DSS and OS,
respectively. These results may have important clinical
implications for risk stratification, planning of meticulous
post-surgical surveillance and the development of adjuvant clinical
trials.
CD44 proteins have been implicated in several
cellular functions, including cell-cell and cell-matrix adhesion,
migration and tumor metastasis. Cell adhesion and migration are
critical steps in malignancy, and hyaluronic acid, which is the
main component of the extracellular matrix, can activate the CD44
receptor, resulting in adhesion, invasion, and extravasation for
metastasis. CD44 also interacts with soluble extracellular proteins
and acts as a platform to harbor growth factors and matrix
metalloproteinases. The binding of heparin-binding growth factor to
heparan sulfate proteoglycans is prerequisite to the activation of
the high-affinity epidermal growth factor receptor 4 tyrosine
kinase, which signals cell survival (24,25).
CD44 ablation in some types of murine and human cancers
significantly reduces tumor induction (26–28)
Antibody blockade of CD44 results in a significant reduction in the
invasive capacity of tumor cells in patients with RCC (19). However, the mechanism of such
pathogenesis has not been clearly elucidated.
Several CD44 isoforms are the products of
alternative splicing, resulting in considerable heterogeneity in
CD44 expression among different tissues (29). The clinical significance of CD44
and its variants in various cancers is the subject of ongoing
debate, due to contradictory findings regarding its prognostic and
predictive value (15,29). The significance of CD44 isoforms in
RCC progression and metastasis is also controversial, and most
studies have examined small, heterogeneous cohorts of patients with
varying demographic parameters, such as histology and tumor extent
(13,16–19,21–23).
Tawfik et al (13) and
Lucin et al (20) reported
that CD44 shows no independent prognostic value for the prediction
of patient survival. Bamias et al (16) also indicated that CD44 expression
tended to correlate with T stage, but found no association between
CD44 expression and survival. Additionally, Matusan et al
(23) reported that upregulation
of the CD44s and v6 isoforms, although found in a considerable
number of papillary RCCs, appears to have no prognostic value in
this type of renal cancer. However, Paradis et al (18) demonstrated that CD44 is an
independent prognostic factor for OS and DFS, suggesting that it
could be a useful prognostic parameter in conventional RCC,
although they analyzed only 66 cases. Gilcrease et al
(21) also reported that CD44
expression correlated with progression or recurrence in only 25
patients with CCRCC. Rioux-Leclercq et al (19) showed that CD44 expression appeared
to be a powerful marker for identifying patients with an adverse
prognosis, although the study included 73 patients with differing
histology and tumor extent. In the present study, we focused on
patients with localized CCRCC who underwent curative surgery
because this population was homogeneous. The ability to predict
which patients will develop recurrence after surgery is valuable;
the identification of a new molecular marker is thus greatly
needed. Our results showed that CD44 expression tended to be
correlated with T stage and was significantly associated with
Fuhrman nuclear grade, comparable with other reports (30,31).
Furthermore, univariate and multivariate analyses showed that CD44
expression was significantly associated with RFS, DSS and OS,
suggesting that it might be a useful prognostic marker independent
of other factors.
Our study had some limitations. First, this study
was based on a retrospective analysis, although all patients in our
study population had CCRCC and were followed up for at least 5
years. Second, the number of patients was relatively small,
although this study was the largest providing evidence that CD44
expression is inversely related to survival in patients with CCRCC
who underwent curative surgery. Thus, a well-designed prospective
study with a large number of patients is needed.
In conclusion, our results indicate that CD44
expression was associated with the progression of CCRCC and was an
independent poor prognostic factor for tumor recurrence and
survival, suggesting that CD44 may serve as a useful molecular
marker.
Acknowledgements
This research was supported by the
National Research Foundation of Korea (NRF) grant funded by the
Korea government (MEST) (no. 2011-0006229).
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Crispen PL, Boorjian SA, Lohse CM,
Leibovich BC and Kwon ED: Predicting disease progression after
nephrectomy for localized renal cell carcinoma: the utility of
prognostic models and molecular biomarkers. Cancer. 113:450–460.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zisman A, Pantuck AJ, Wieder J, et al:
Risk group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Klatte T, Seligson DB, Leppert JT, et al:
The chemokine receptor CXCR3 is an independent prognostic factor in
patients with localized clear cell renal cell carcinoma. J Urol.
179:61–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: An outcome prediction model for patients
with clear cell renal cell carcinoma treated with radical
nephrectomy based on tumor stage, size, grade and necrosis: the
SSIGN score. J Urol. 168:2395–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Patard JJ, Kim HL, Lam JS, et al: Use of
the University of California Los Angeles integrated staging system
to predict survival in renal cell carcinoma: an international
multicenter study. J Clin Oncol. 22:3316–3322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zisman A, Pantuck AJ, Dorey F, et al:
Improved prognostication of renal cell carcinoma using an
integrated staging system. J Clin Oncol. 19:1649–1657.
2001.PubMed/NCBI
|
|
9.
|
Lam JS, Klatte T, Kim HL, et al:
Prognostic factors and selection for clinical studies of patients
with kidney cancer. Crit Rev Oncol Hematol. 65:235–262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Volpe A and Patard JJ: Prognostic factors
in renal cell carcinoma. World J Urol. 28:319–327. 2010. View Article : Google Scholar
|
|
11.
|
Marhaba R and Zoller M: CD44 in cancer
progression: adhesion, migration and growth regulation. J Mol
Histol. 35:211–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tawfik OW, Kramer B, Shideler B, Danley M,
Kimler BF and Holzbeierlein J: Prognostic significance of CD44,
platelet-derived growth factor receptor alpha, and cyclooxygenase 2
expression in renal cell carcinoma. Arch Pathol Lab Med.
131:261–267. 2007.PubMed/NCBI
|
|
14.
|
Liao HX, Lee DM, Levesque MC and Haynes
BF: N-terminal and central regions of the human CD44 extracellular
domain participate in cell surface hyaluronan binding. J Immunol.
155:3938–3945. 1995.PubMed/NCBI
|
|
15.
|
Lim SD, Young AN, Paner GP and Amin MB:
Prognostic role of CD44 cell adhesion molecule expression in
primary and metastatic renal cell carcinoma: a clinicopathologic
study of 125 cases. Virchows Arch. 452:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bamias A, Chorti M, Deliveliotis C, et al:
Prognostic significance of CA 125, CD44, and epithelial membrane
antigen in renal cell carcinoma. Urology. 62:368–373. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wu ST, Sun GH, Hsieh DS, Chen A, Chen HI,
Chang SY and Yu D: Correlation of CD44v5 expression with
invasiveness and prognosis in renal cell carcinoma. J Formos Med
Assoc. 102:229–233. 2003.PubMed/NCBI
|
|
18.
|
Paradis V, Ferlicot S, Ghannam E, et al:
CD44 is an independent prognostic factor in conventional renal cell
carcinomas. J Urol. 16:1984–1987. 1999. View Article : Google Scholar
|
|
19.
|
Rioux-Leclercq N, Epstein JI, Bansard JY,
et al: Clinical significance of cell proliferation, microvessel
density, and CD44 adhesion molecule expression in renal cell
carcinoma. Hum Pathol. 32:1209–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lucin K, Matusan K, Dordević G and Stipić
D: Prognostic significance of CD44 molecule in renal cell
carcinoma. Croat Med J. 45:703–708. 2004.PubMed/NCBI
|
|
21.
|
Gilcrease MZ, Guzman-Paz M, Niehans G,
Cherwitz D, McCarthy JB and Albores-Saavedra J: Correlation of
CD44S expression in renal clear cell carcinomas with subsequent
tumor progression or recurrence. Cancer. 86:2320–2326. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Heider KH, Ratschek M, Zatloukal K and
Adolf GR: Expression of CD44 isoforms in human renal cell
carcinomas. Virchows Arch. 428:267–273. 1996.PubMed/NCBI
|
|
23.
|
Matusan K, Dordevic G, Mozetic V and Lucin
K: Expression of osteopontin and CD44 molecule in papillary renal
cell tumors. Pathol Oncol Res. 11:108–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee SM, Lee KE, Chang HJ, et al:
Prognostic significance of CD44s expression in biliary tract
cancers. Ann Surg Oncol. 15:1155–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zeilstra J, Joosten SP, Dokter M, Verwiel
E, Spaargaren M and Pals ST: Deletion of the WNT target and cancer
stem cell marker CD44 in Apc(Min/+) mice attenuates
intestinal tumorigenesis. Cancer Res. 68:3655–3661. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F and
Dick JE: Targeting of CD44 eradicates human acute myeloid leukemic
stem cells. Nat Med. 12:1167–1174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Krause DS, Lazarides K, von Andrian UH and
van Etten RA: Requirement for CD44 in homing and engraftment of
BCR-ABL-expressing leukemic stem cells. Nat Med. 12:1175–1180.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Huh JW, Kim HR, Kim YJ, Lee JH, Park YS,
Cho SH and Joo JK: Expression of standard CD44 in human colorectal
carcinoma: association with prognosis. Pathol Int. 59:241–246.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Terpe HJ, Störkel S, Zimmer U, Anquez V,
Fischer C, Pantel K and Günthert U: Expression of CD44 isoforms in
renal cell tumors. Positive correlation to tumor differentiation.
Am J Pathol. 148:453–463. 1996.PubMed/NCBI
|
|
31.
|
Zolota V, Tsamandas AC, Melachrinou M,
Batistatou A and Scopa C: Expression of CD44 protein in renal cell
carcinomas: association with p53 expression. Urol Oncol. 7:13–17.
2002. View Article : Google Scholar : PubMed/NCBI
|