Introduction
Nasopharyngeal carcinoma (NPC) is the most common
type of head and neck cancer among the Southeastern Asian
population. Radiotherapy is an important treatment approach for NPC
(1). The recurrence of NPC
following radiotherapy is a major modality of failure in patients
with NPC. Approximately 20% of patients with NPC present with local
failure following initial radiotherapy (2). Recurrence leads to a poor prognosis
in patients with NPC since the sensitivity to radiotherapy is
reduced in the recurrent tumor. Palliative therapeutic modalities
include reirradiation, radioactive seed implantation, chemotherapy
and surgical debulking for locally recurrent NPC. Reirradiation has
gained wide acceptance and offers a chance of long-term tumor
control for patients with local recurrence of NPC (3). However, the tolerance and toxicity of
normal tissues experienced during reirradiation compared with the
initial radiotherapy is a serious challenge.
A new technique of reirradiation, pulsed reduced
dose-rate radiotherapy (PRDR), was developed for the local failure
of tumors following radiotherapy. PRDR delivers a series of 0.2 Gy
pulses separated by 3 min intervals. It has a high local control
rate and is well-tolerated in patients with local recurrence of
breast cancer or glioblastoma (4,5).
Epidermal growth factor receptor (EGFR) is usually overexpressed in
NPC (6). The overexpression of
EGFR in squamous cell carcinoma of the head and neck is associated
with a lower local control following radiotherapy (7). Cetuximab, an anti-EGFR monoclonal
antibody, modulates apoptosis and enhances the effects of radiation
and chemotherapy (8,9). Therefore, in the present study a
patient with a second neck recurrence of NPC received combined
treatment of PRDR and cetuximab following two courses of
radiotherapy and several cycles of chemotherapy.
Case report
A 56-year-old Asian male experienced a firm neck
mass in September 2003. A computed tomography (CT) scan revealed an
enhanced mass in the nasopharynx and several bilateral enhanced
lymph nodes in the neck. A flexible fiberoptic nasopharyngoscope
examination was performed and biopsies from the lesion of the
nasopharynx were obtained. The biopsies were identified as poorly
differentiated squamous cell carcinoma (Type II according to the
WHO). Following a CT scan of the head/neck and thorax and
ultrasound examination of the abdomen, the cancer of the patient
was classified as stage T2N2M0, based on clinical and radiological
evaluation and according to the TNM staging criteria (AJCC 2002)
(10).
The patient received radical conventional
external-beam radiotherapy (EBRT) with 8 MV X-rays and 6–12 MeV
electrons from a linear accelerator. Facial-cervical, preauricular
and cervical tangent fields were applied. A total dose of 70 Gy was
administered to the gross tumor targets and metastatic lymph node
and ≥50 Gy to the bilateral cervical lymphatics. EBRT was delivered
with a daily fraction of 2 Gy, five fractions per week. The patient
was then followed up according to standard medical protocol.
Endoscope and CT examinations revealed the disappearance of the
gross tumor and metastatic lymph nodes at the first follow-up 1
month after radiation. A lymph node 1.5 cm in diameter was found on
the right upper neck of the patient in October 2004. The patient
refused to undergo a PET/CT or MRI scan or a biopsy of the lymph
node. The patient also declined to treat this lymph node, which
grew slowly. A contrast-enhanced CT scan revealed multiple
bilateral metastatic lymph nodes in the neck of the patient in
November 2009 and the greatest dimension of the mass in the right
upper neck was 6 cm. Three-dimensional conformal radiotherapy
(3D-CRT) was performed to treat the recurrent metastatic lymph
nodes in the neck. The dosage of clinic target volume (CTV) was 60
Gy. The reliquus metastatic lymph nodes in the right neck were
surgically resected following reirradiation therapy. Adjuvant
chemotherapy was initiated within 2 weeks of surgery for 4 cycles.
The chemotherapy regimen consisted of paclitaxel, 135
mg/m2 on day 1, and cisplatin, 30 mg/m2/day
on days 1–3 of a 21-day treatment cycle.
In May 2010, a 1.5×2 cm mass in the left upper neck
was found by the patient. A contrast-enhanced CT scan of the neck
revealed that the mass was a recurrent metastatic lymph node. No
other metastastic lesion was found following a CT scan of the
head/neck and thorax and ultrasound examination of the abdomen. The
patient declined to undergo surgical management and received
chemotherapy with cisplatin (90 mg/m2 on day 1) plus
fluorouracil (500 mg/m2/day for 5 days) for 2 cycles.
The mass increased slowly in size and paclitaxel (135
mg/m2 on day 1) and cisplatin (30 mg/m2/day
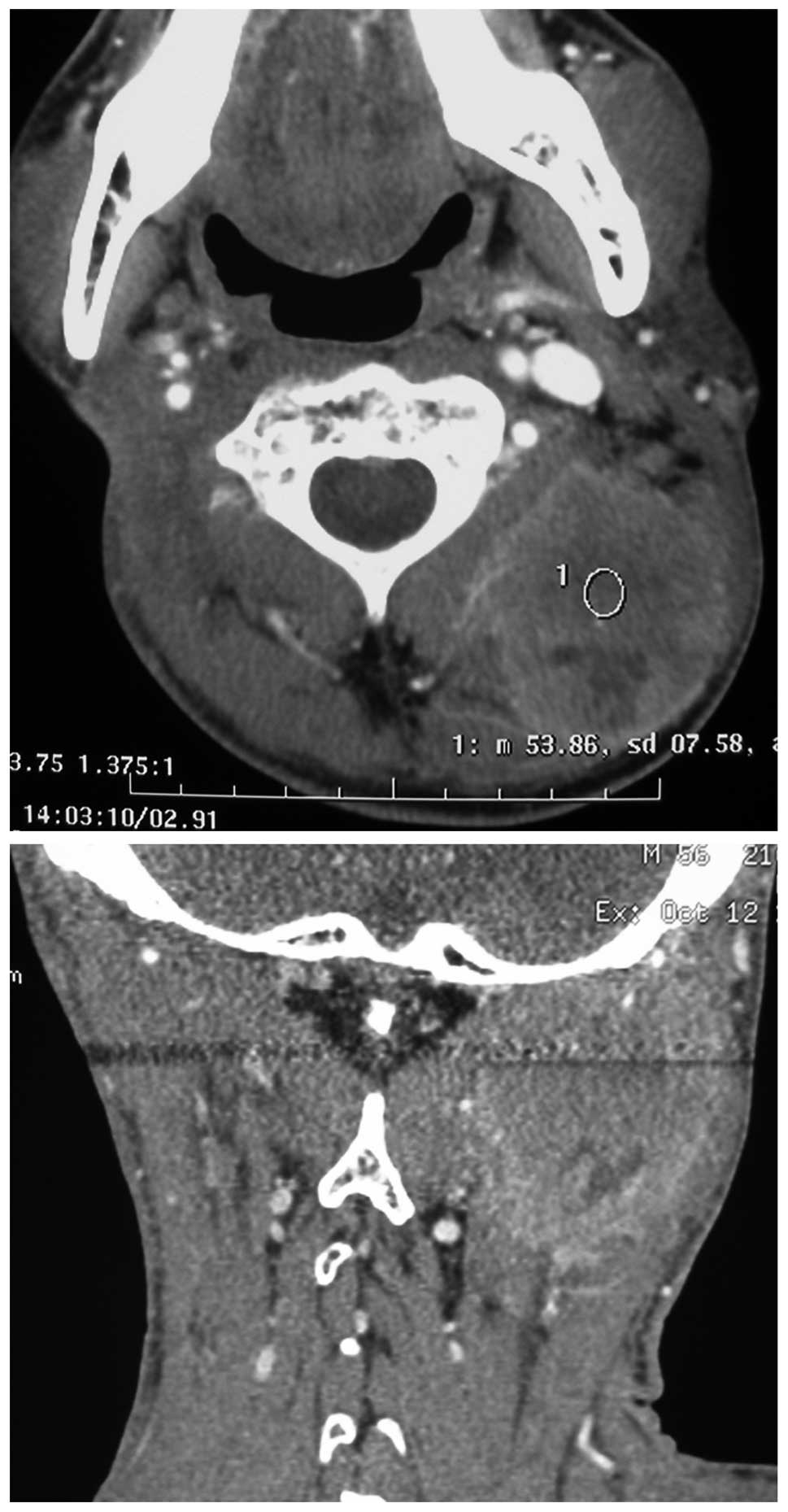
on day 1–3) were used for 2 cycles. The lesion progressed and the
greatest dimension of the mass was 5.5 cm (Fig. 1). The clinical consensus was to
proceed with PRDR and concurrent cetuximab following an evaluation
of the medical oncology and the choice of the patient. PRDR was
performed using 3D-CRT and each fraction was delivered with 0.2 Gy
pulses separated by 3 min intervals, creating a dose-rate of 0.0667
Gy/min (7,8,18).
The patient received a total dose of 70 Gy using 35 daily fractions
of 2.0 Gy from 15 October 2010 to 2 December 2010. Cetuximab was
administered at an initial dose of 400 mg/m2 and was
subsequently administered at a weekly dose of 250 mg/m2
concurrently with radiotherapy (11).
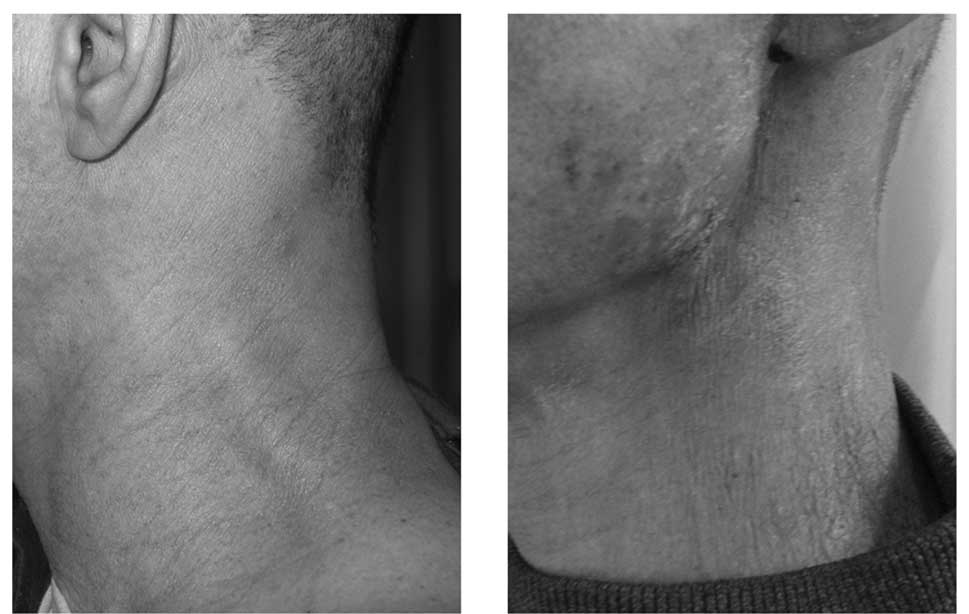
The side effects of reirradiation and cetuximab were
well-tolerated by the patient. At the third week of radiotherapy,
papulo-pustular skin lesions developed in the face of the patient.
The patient experienced grade 1 skin toxicity in response to
cetuximab and grade 2 pharyngeal mucositis during radiotherapy.
Notably, the patient experienced only grade 1 acute skin toxicity
induced by irradiation in the reirradiated field and no significant
late toxicity (Fig. 2). In
February 2011, a contrast-enhanced CT scan of the neck of the
patient revealed the complete response of the recurrent lymph node
in the left neck following treatment with PRDR plus cetuximab
(Fig. 3).
Discussion
Curative approaches to recurrent NPC include surgery
with or without radiotherapy and definitive radiation-based
therapy. Recurrent disease is often not resectable, or patients
decline surgical resection due to concerns about quality of life.
Reirradiation is an important approach for the treatment of
patients with recurrent head and neck carcinoma. However,
reirradiation is difficult due to normal tissue tolerances. Normal
tissues that receive reirradiation may experience more severe acute
and late radiation complications compared with the initial course.
It is difficult to achieve the correct balance between tumor
control and the severe toxicity of normal tissue. Reirradiation
using conventional techniques often has modest palliative and
survival benefits.
A new irradiation technique, PRDR, was developed to
treat recurrent malignant lesions. It appears to be a highly
effective modality on irradiated recurrences and a low toxicity
method to reirradiate normal tissues. Due to the different repair
capacities of normal tissues and tumor cells, below standard
dose-rates are preferable to protect normal tissue, while PRDR
produced almost identical toxicity to cancer cells as conventional
radiotherapy. The mechanism by which PRDR kills tumor cells may be
low dose hyperradiosensitivity (LDHRS). LDHRS, which is increased
radiosensitivity to doses <0.3–0.5 Gy, has been demonstrated in
numerous tumor cells (12,13). On the other hand, the reduced
dose-rate and a fixed time interval reduce toxicity and improve the
sublethal damage repair of normal tissue. It allows normal tissues
to repair during each sub-fraction and is an effective method with
low toxicity to treat recurrent breast cancer and glioblastoma
(4,5).
As squamous cell carcinoma of the head and neck
commonly has a high level of EGFR expression, treatment with
cetuximab shows the clearest benefit to locally advanced head and
neck cancer compared with radiotherapy alone when it is combined
with radiotherapy (14). We
treated a patient with recurrent NPC with PRDR and concurrent
cetuximab. The treatment of the recurrent neck lesion in this
patient achieved complete regional control and low toxicity to
normal tissue around the lesion. The combination of radiation and
concurrent cetuximab has been reported to enhance skin toxicity
compared with radiotherapy alone in patients with head and neck
cancer (15,16). However, only CTC grade 1
radiodermatitis occurred in this patient. Certain studies have
reported a lack of cetuximab-induced skin rash in the previously
radiated field. The mechanism of this phenomenon is uncertain
(17,18). It may be related to microvascular
injury which reduces the delivery of cetuximab, a reduced number of
EGFR-expressing cells or a reduced receptor sensitivity following
radiation.
In conclusion, the treatment of PRDR with concurrent
cetuximab is well-tolerated and is a promising therapeutic option
for patients with recurrent head and neck carcinoma following
radiotherapy, although the mechanism by which it functions remains
unclear.
References
|
1.
|
Shen C, Gao Y, Xu T, et al: Carcinoma of
the nasopharynx in young patients: a single institution experience.
Clin Oncol (R Coll Radiol). 21:617–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yi JL, Gao L, Huang XD, et al:
Nasopharyngeal carcinoma treated by radical radiotherapy alone:
ten-year experience of a single institution. Int J Radiat Oncol
Biol Phys. 65:161–168. 2006.PubMed/NCBI
|
|
3.
|
Sher DJ, Haddad RI, Norris CM, et al:
Efficacy and toxicity of reirradiation using intensity-modulated
radiotherapy for recurrent or second primary head and neck cancer.
Cancer. 116:4761–4768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Adkison JB, Tomé W, Seo S, et al:
Reirradiation of large-volume recurrent glioma with pulsed
reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys.
79:835–841. 2011.PubMed/NCBI
|
|
5.
|
Richards GM, Tomé WA, Robins HI, et al:
Pulsed reduced dose-rate radiotherapy: a novel locoregional
retreatment strategy for breast cancer recurrence in the previously
irradiated chest wall, axilla, or supraclavicular region. Breast
Cancer Res Treat. 114:307–313. 2009. View Article : Google Scholar
|
|
6.
|
Chung CH, Ely K, McGavran L, et al:
Increased epidermal growth factor receptor gene copy number is
associated with poor prognosis in head and neck squamous cell
carcinomas. J Clin Oncol. 24:4170–4176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zimmermann M, Zouhair A, Azria D and
Ozsahin M: The epidermal growth factor recepter (EGFR) in head and
neck cancer: its role and treatment implications. Radiat Oncol.
1:11–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Greene FL, wPage DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morros M: AJCC Cancer Staging Manual. 6th
ed. Springer-Verlag; New York: 2002, View Article : Google Scholar
|
|
11.
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Harney J, Short SC, Shah N, et al: Low
dose hyperradiosensitivity in metastatic tumors. Int J Radiat Oncol
Biol Phys. 59:1190–1195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lin PS and Wu A: Not all 2 Gray radiation
prescriptions are equivalent: cytotoxic effect depends on delivery
sequences of partial fractionated doses. Int J Radiat Oncol Biol
Phys. 63:536–544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Specenier P and Vermorken JB: Cetuximab in
the treatment of squamous cell carcinoma of the head and neck.
Expert Rev Anticancer Ther. 11:511–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Walsh L, Gillham C, Dunne M, et al:
Toxicity of cetuximab versus cisplatin concurrent with radiotherapy
in locally advanced head and neck squamous cell cancer (LAHNSCC).
Radiother Oncol. 98:38–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pryor DI, Porceddu SV, Burmeister BH, et
al: Enhanced toxicity with concurrent cetuximab and radiotherapy in
head and neck cancer. Radiother Oncol. 90:172–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bossi P, Liberatoscioli C, Bergamini C, et
al: Previously irradiated areas spared from skin toxicity induced
by cetuximab in six patients: implications for the administration
of EGFR inhibitors in previously irradiated patients. Ann Oncol.
18:601–602. 2007. View Article : Google Scholar
|
|
18.
|
Kanakamedala MR, Packianathan S and
Vijayakumar S: Lack of cetuximab induced skin toxicity in a
previously irradiated field: case report and review of the
literature. Radial Oncol. 5:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|