Introduction
Cancer biomarkers play multiple roles in oncology.
They can have prognostic functions by providing information on
outcome and patient tractability. Somatic mutations in KRAS are
considered a predictive marker of response to therapy in colorectal
cancer (CRC), due to their association with clinical resistance to
cetuximab and panitumumab, chimeric monoclonal antibodies acting as
epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs) (1,2). The analysis of KRAS mutations has
been approved by the US Food and Drug Administration and European
Medicines Agency as a diagnostic tool with which to select
colorectal cancer patients eligible to be treated with EGFR-TKIs
(1,3).
Although KRAS mutations are detectable in freshly
frozen specimens and formalin-fixed paraffin-embedded (FFPE)
specimens, some specimens cannot be accessed in a clinical setting,
particularly for patients who have had recurrence of tumor and
metastasis. However, shed tumor DNA circulating in patient plasma
may provide a viable alternative for the identification of tumor
mutations and other alterations (4). As a large quantity of wild-type
background DNA circulates in the plasma, mutations cannot be
sequenced using conventional methods.
Co-amplification at lower denaturation
temperature-polymerase chain reaction (COLD-PCR), a novel form of
PCR that amplifies minority alleles selectively from mixtures of
wild-type and mutation-containing sequences, provides a general
platform to improve the sensitivity of DNA variation detection
technologies (5,6). DNA genotyping with mutation-specific
TaqMan® probes (Applied Biosystems) is broadly used in
the detection of single-nucleotide polymorphisms but is less
frequently used for somatic mutations due to its limited
selectivity for low-level mutations (7). Minor-groove binder-based TaqMan
probes (TaqMan-MGB) have a higher specificity than common TaqMan
probes. We investigated whether combining COLD-PCR with the
TaqMan-MGB probe genotyping method is able to determine KRAS
mutations in plasma from patients with CRC, and we ascertained
whether plasma can be used for mutation analysis instead of tissue
specimens.
Materials and methods
Plasma sample collection and DNA
extraction
We collected plasma from 62 patients with CRC prior
to treatment between the years 2009 and 2010. This was carried out
as follows. Whole blood specimens were collected in ethylene
diaminetetraacetic acid tubes and centrifuged at 3000 rpm for 10
min. Plasma was stored at −80°C until DNA extraction. DNA was
extracted from 0.5 to 1.0 ml of plasma using the QIAamp DNA Blood
mini kit (Qiagen) following the manufacturer’s instructions.
Estimated final DNA concentrations generally ranged from 1 to 8
ng/μl, with an average of 2.2 ng/μl.
Tissue specimen collection and DNA
extraction
CRC tissue specimens (n=62) paired with plasma were
obtained surgically, and were confirmed by the Department of
Pathology. Tissue was stored at −80°C prior to use. DNA was
extracted by phenol-chloroform, as follows. We added lysate and
protease K, and then placed the specimens overnight in a 60°C
waterbath. DNA was extracted by phenol/chloroform/isopentanol the
following day. Following salt washing, the sediment was dissolved
in quantity-sufficient Tris-EDTA buffer and then preserved at −20°C
for use (8).
Determination of the critical
denaturation temperature (Tc) for COLD-PCR
We determined Tc experimentally for each amplicon as
previously described. In order to determine Tc, the melting
temperature (Tm) of the amplicon was first identified. A real-time
PCR of the target amplicon was performed in a PCR machine in the
presence of 0.1X LCGreen Plus dye using conventional thermocycling
conditions, followed by a melting curve analysis (9). A set of COLD-PCR reactions were then
carried out at graded temperatures below the Tm from 79 to 82°C to
identify the optimal critical denaturation temperature. We found
that 81.0°C was the the optimal Tc for the fast COLD/direct
sequencing, and 81.0 and 80°C for the first and second round
COLD-PCR/TaqMan-MGB probe.
Regular PCR and COLD-PCR amplification
and direct sequencing for KRAS
Mutations in KRAS on codon 12 (GGT>GAT) were
amplified by regular PCR and COLD-PCR following extraction of DNA
from the tissue specimens, using the following forward and reverse
primers: KRAS-F, 5′-AAG GCC TGC TGA AAA TGA CTG-3′ and KRAS-R
5′-GGT CCT GCA CCA GTA ATA TGC A-3′ (10). PCR reaction was performed using 25
μl total volume consisting of 20 ng genomic DNA, a final
concentration of 1X PCR buffer (10 mmol/l Tris-HCl, pH 8.3; 50
mmol/l KCl), 2.5 mmol/l MgCl solution, 0.2 mmol/l each dNTP, 0.5
μmol/l each primer and 1.25 U FastStart Taq Gold DNA polymerase.
The regular PCR cycling conditions were as follows: 95°C for 3 min
followed by 35 cycles of 95°C for 30 sec, 58°C for 20 sec, 72°C for
20 sec, and a final extension of 72°C for 8 min. The COLD-PCR
cycling conditions were as follows: 95°C for 3 min followed by 10
cycles of 95°C for 15 sec, 58°C for 20 sec, 72°C for 20 sec, 40
cycles of 81°C for 1 sec, 58°C for 20 sec, 72°C for 20 sec and a
final extension of 72°C for 8 min. The reactions were performed in
a Bio-Rad PCR instrument. The size of the product was 166 bp. The
PCR products were subjected to sequence analysis.
A nested COLD-PCR/TaqMan-MGB probe to
detect KRAS mutations (GGT>GAT) in plasma and tumors
KRAS gene mutation (GGT>GAT) was detected in the
plasma and tumor using nested COLD-PCR for 62 CRC patients. The
first fast COLD-PCR amplification was performed using the same
reaction system as the sequencing. The reaction conditions were as
follows: predegeneration at 95°C for 3 min, 10 cycles of
degeneration at 95°C for 15 sec, renaturation at 58°C for 20 sec
and an extension at 72°C for 20 sec, 40 cycles of degeneration at
81°C for 1 sec, renaturation at 58°C for 20 sec and extension at
72°C for 20 sec and a final extension of 5 min at 72°C in a Bio-Rad
PCR instrument. The PCR products were diluted 1000-fold as a
template for the second amplification. The second fast COLD-PCR
reactions were performed in the presence of 0.2 μmol/l of a
TaqMan-MGB probe (5′-FAMTTGGAGCTGATGGC-BHQ-MGB-3′) that fully
matched the mutation-containing sequence. The final concentrations
of the other reagents were as follows: 1X PCR buffer (10 mmol/l
Tris-HCl, pH 8.3; 50 mmol/l KCl), 2.0 mmol/l MgCl solution, 0.2
mmol/l each dNTP, 0.5 μmol/l each primer (forward primer:
5′-TGCTGAAAATGACTGAATATAAACTTGT G-3′, reverse primer:
GCTGTATCGTCAAGGCACTCTTG) and 1.25 U FastStart Taq Gold DNA
polymerase. The size of the second COLD-PCR amplicon was 75 bp, and
the cycling conditions were as follows: 95°C for 180 sec; 10 cycles
at 95°C for 15 sec and 67.5°C for 50 sec; and 50 cycles at 80°C for
1 sec and 67.5°C (fluorescence reading on) for 60 sec in an ABI
StepOne™ instrument. Experiments were repeated at least three times
independently.
Statistical Analysis
All statistical analyses in the study were conducted
using SPSS 13.0 software. The relationship between KRAS mutations
in the plasma and matched tumor tissue, as well as KRAS mutation
sequencing in the tumor tissue between regular PCR and COLD-PCR,
were analyzed using the χ2 test, with a P-value of
<0.05 as a bilateral indicator of a statistically significant
difference.
Results
Comparison of KRAS mutation detection
using regular PCR/sequencing and COLD-PCR/sequencing on tissue
specimens
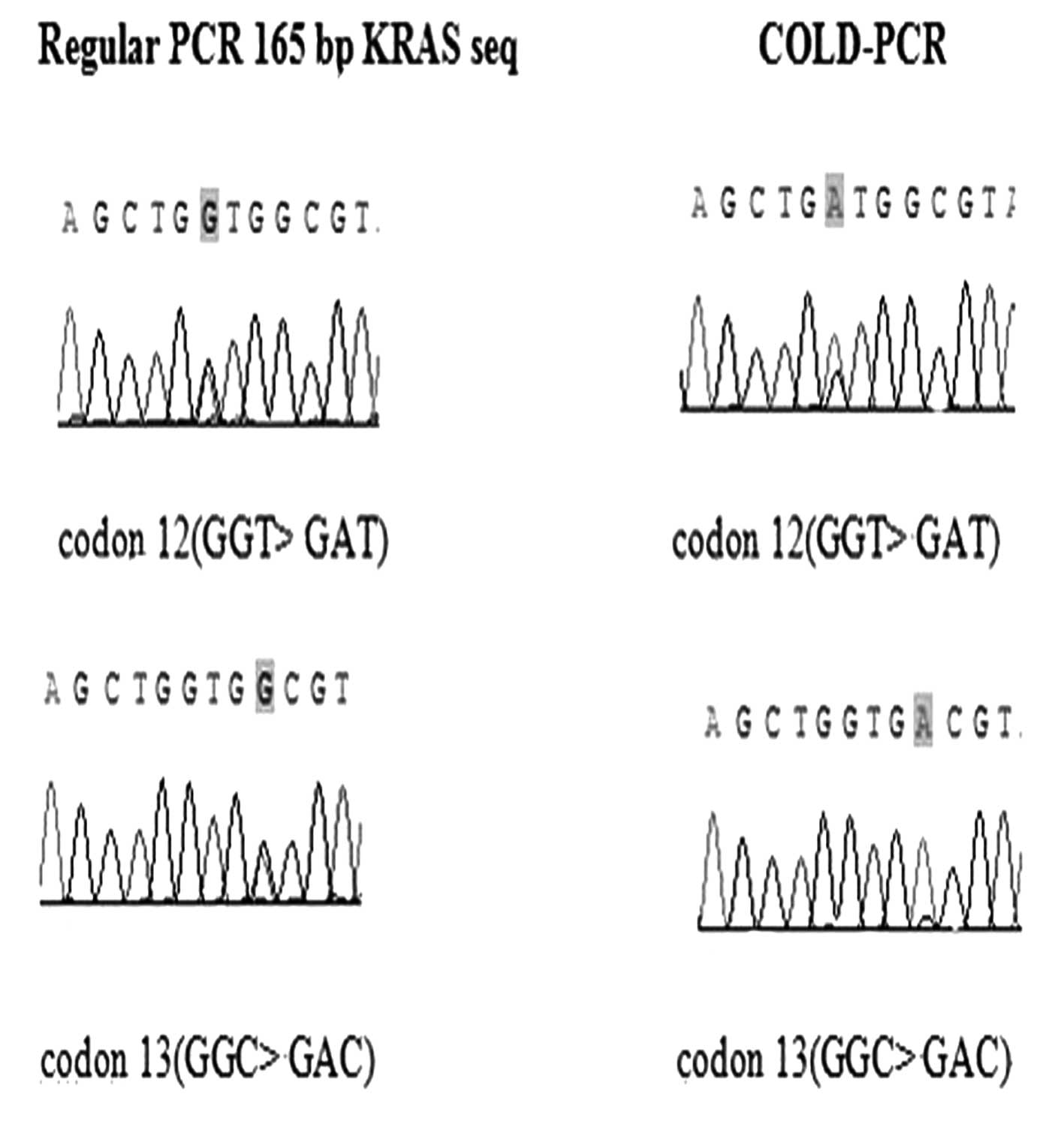
KRAS mutations were analyzed in the 62 specimens by
direct sequencing. Twelve (19.4%) tissue specimens were considered
to have the KRAS mutation (GGT>GAT) by regular PCR and COLD-PCR.
The wild-type sample did not contain mutations, as detected by
either method of amplification. A more pronounced mutation
enhancement was obtained by COLD-PCR than by regular PCR for the
same tissue specimens (Fig.
1).
KRAS mutations in plasma are correlated
with mutations detected in the matched tumors
Thirteen (21.0%) KRAS mutations (GGT>GAT) were
detected from the 62 plasma specimens whereas 12 (19.4%) KRAS
mutations were found in the paired tumor tissues. Notably, four
(6.5%) patients with plasma DNA mutations had no detectable KRAS
mutation in the corresponding tumor DNA specimens, and three (4.8%)
patients with KRAS mutations in tumor DNA specimens had no
detectable KRAS mutation in the corresponding plasma. The
consistency of KRAS mutations between plasma and tumors was 75%
(9/12), which indicated a high correlation between the mutations
detected in plasma DNA and the corresponding tumor DNA (P<0.001;
correlation index, k=0.649). The correlation between mutations
detected in the plasma and tumors is summarized in Table I.
 | Table I.Correlation of the KRAS mutation
(GGT>GAT) in plasma and matched tumor tissues. |
Table I.
Correlation of the KRAS mutation
(GGT>GAT) in plasma and matched tumor tissues.
| Tissue
|
|---|
| KRAS− | KRAS+ | Total |
|---|
| Plasma | | | |
|
KRAS− | 46 | 3 | 49 |
|
KRAS+ | 4 | 9 | 13 |
| Total | 50 | 12 | 62 |
Discussion
Several studies have already reported the presence
of free circulating DNA in the plasma of cancer patients,
exhibiting the same characteristics as primary tumor DNA such as
oncogene expression, tumor-suppressor gene mutations,
microsatellite and epigenetic alterations (11). Although the mechanism leading to
the presence of free tumor DNA in the plasma of cancer patients is
still not fully understood, it may be caused by the lysis of
circulating cancer cells or by DNA leakage resulting from tumor
necrosis or apoptosis (12). A
number of studies have investigated the association of gene
mutations between plasma or serum-free DNA and tumor tissues, and
their clinical significance in cancer (13,14).
In our study, we found 13 of 62 (21.0%) patients
with CRC had the KRAS mutation (GGT>GAT) in their plasma using
the nested COLD-PCR/TaqMan-MGB probe, and 12 (19.4%) KRAS mutations
were found in the paired tumor tissues by direct sequencing and the
nested COLD-PCR/TaqMan-MGB probe, which was a positive correlation.
A high consistency of the KRAS mutation between the plasma and
paired tissue DNA was observed, which is consistent with studies by
Yung et al and Maheswaran et al (15,16).
Notably, four (6.5%) patients with plasma DNA mutations had no
detectable KRAS mutation in the corresponding tumor DNA specimens,
which may be attributed to the heterogeneity of the tumor cells.
Only small samples of tumor tissue were used in this experiment,
which may have been the portions without KRAS mutations in the
tumor cells. In contrast, DNA in the plasma was released from
different parts of the tumor, so in the plasma KRAS mutations could
be detected. Three (4.8%) patients with DNA mutations in the tumor
specimens had no detectable KRAS mutation in the corresponding
plasma, which is possibly explained by the lower tumor cell content
in some of the tumors contributing to the lack of detectable
mutations in plasma. That the tumor areas carrying mutations shed
less DNA than the other parts of the tumors into plasma may also be
a reason for the lack of detectable mutations in the plasma.
Compared with regular PCR, the enhancement using
COLD-PCR enabled clear detection of the mutation by direct
sequencing (Fig. 1) and the
TaqMan-MGB probe, which is consistent with studies by Mancini et
al and Zuo et al (17,18).
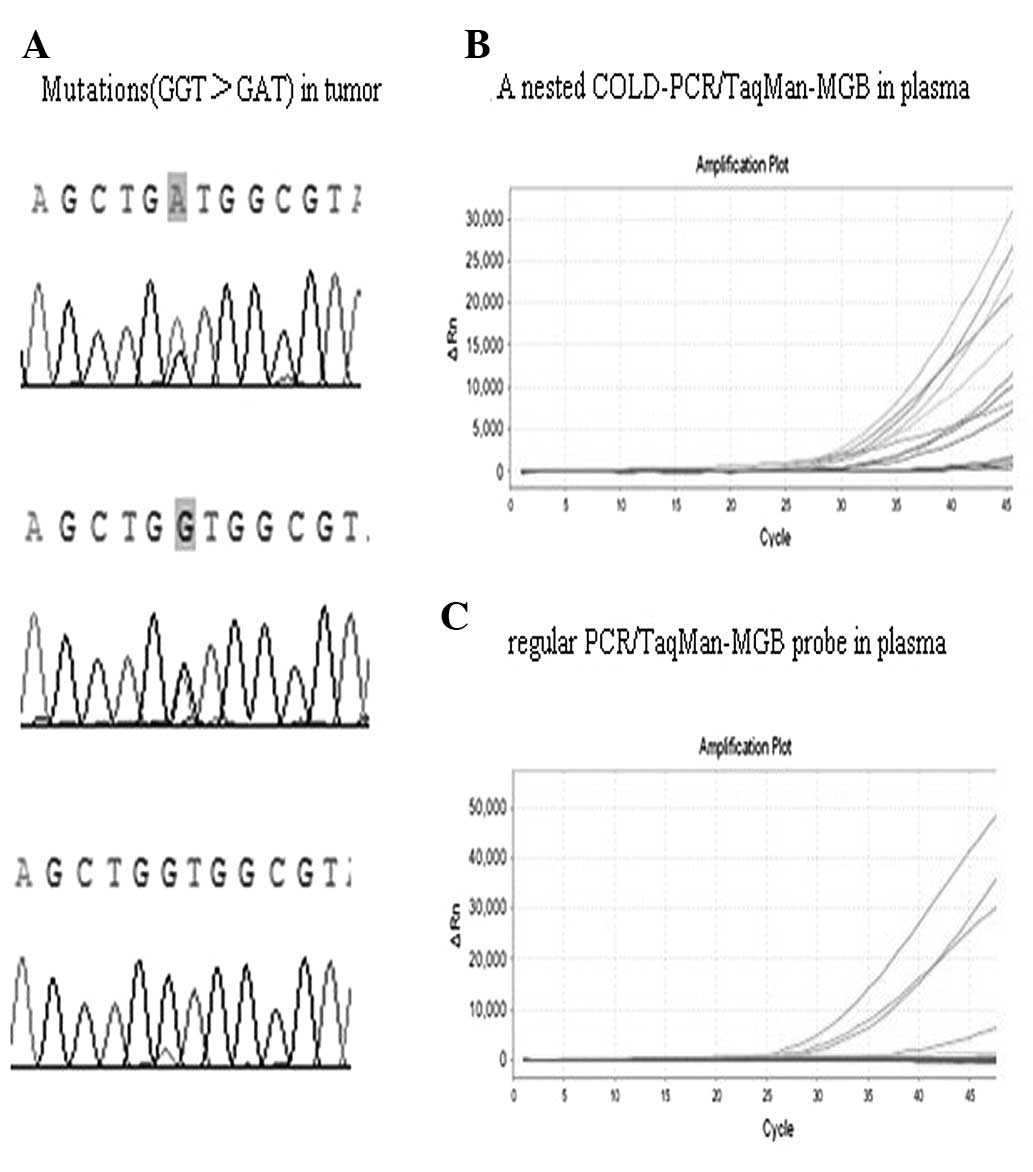
We also demonstrated that the KRAS mutation in the plasma DNA was
not detectable using the regular PCR/TaqMan-MGB probe, since the
level of the KRAS mutation in the plasma DNA was extremely low;
only four samples that may have released a large quantity of mutant
DNA into plasma appeared on the positive amplification curve
(Fig. 2). We applied a nested
COLD-PCR/TaqMan-MGB probe to detect KRAS mutations in the plasma.
The first round of COLD-PCR increased the concentration of mutant
alleles, and the second round of COLD-PCR further increased the
concentration of mutant alleles detected by the TaqMan-MGB probe
(Fig. 2). We found that the Tc
played an important role in the enhancement of mutations during
COLD-PCR. Using a Tc lower than 81°C further enhanced the relative
proportion of mutant alleles for direct sequencing, however, this
reduced PCR efficiency, made it more difficult to interpret the
amplification curve when the Tc was lower than 80°C for the second
nested COLD-PCR/TaqMan-MGB assay.
We know from previous studies that COLD-PCR/HRM is a
convenient and sensitive method for identifying mutations (1,19),
however, the specialist equipment required puts it beyond the reach
of many hospitals. The COLD-PCR/TaqMan-MGB probe approach can be
carried out using a real-time quantitative PCR instrument, which is
relatively simple and cost-effective, so it may be widely used to
detect point mutations in tissue and plasma, and plasma DNA
analysis provides a noninvasive means of assessing KRAS mutations.
We used fast-COLD-PCR during the experiment, since the method is
rapid and the results are quickly obtainable (2 h for the nested
COLD-PCR/TaqMan-MGB assay).
Although this method has numerous advantages, it
also has limitations in that the results are false-negative when
there are tumors with multiple mutations (codon 12 and 13
mutations). We detected KRAS mutations in the plasma during the
experiment, but the experimental sample of 62 cases was limited,
which may have resulted in bias. As such, further studies with a
greater sample size and multi-point detection are required to
further validate our results. In conclusion, KRAS mutations in
plasma DNA correlated with the mutation status in the matched tumor
tissues of patients with CRC. Our study provides evidence to
suggest that plasma DNA may be used as a potential sample for KRAS
mutation analysis in CRC using the COLD-PCR/TaqMan-MGB probe,
particularly when tissue specimens are uable to be obtained. The
COLD-PCR/TaqMan-MGB probe is a convenient, sensitive and
cost-effective method for the detection of mutations and may have
broad application for detecting mutations in a wide range of
clinical settings.
References
|
1
|
Solassol J, Ramos J, Crapez E, et al: KRAS
mutation detection in paired frozen and formalin-fixed
paraffin-embedded (FFPE) colorectal cancer tissues. Int J Mol Sci.
12:3191–3204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raymond E, Faivre S and Armand JP:
Epidermal growth factor receptor tyrosine kinase as a target for
anticancer therapy. Drugs. 60:15–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JS, Webber EM, Senger CA, Holmes RS
and Whitlock EP: Systematic review of pharmacogenetic testing for
predicting clinical benefit to anti-EGFR therapy in metastatic
colorectal cancer. Am J Cancer Res. 1:650–662. 2011.PubMed/NCBI
|
|
4
|
Mack PC, Holland WS, Burich RA, et al:
EGFR mutations detected in plasma are associated with patient
outcomes in erlotinib plus docetaxel-treated non-small cell lung
cancer. J Thorac Oncol. 4:1466–1472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Wang L, Mamon H, Kulke MH, Berbeco R
and Makrigiorgos GM: Replacing PCR with COLD-PCR enriches variant
DNA sequences and redefines the sensitivity of genetic testing. Nat
Med. 14:579–584. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milbury CA, Li J, Liu P and Makrigiorgos
GM: COLD-PCR: improving the sensitivity of molecular diagnostics
assays. Expert Rev Mol Diagn. 11:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Wang L, Jänne PA and Makrigiorgos
GM: Coamplification at lower denaturation temperature-PCR increases
mutation-detection selectivity of TaqMan-based real-time PCR. Clin
Chem. 5:748–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, An T, Wang J, et al: Potential
clinical significance of a plasma-based KRAS mutation analysis in
patients with advanced non-small cell lung cancer. Clin Cancer Res.
16:1324–1330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Milbury CA, Li C and Makrigiorgos
GM: Two-round coamplification at lower denaturation temperature-PCR
(COLD-PCR)-based sanger sequencing identifies a novel spectrum of
low-level mutations in lung adenocarcinoma. Hum Mutat.
30:1583–1590. 2009. View Article : Google Scholar
|
|
10
|
Pritchard CC, Akagi L, Reddy PL, Joseph L
and Tait JF: COLD-PCR enhanced melting curve analysis improves
diagnostic accuracy for KRAS mutations in colorectal carcinoma. BMC
Clin Pathol. 26:62010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boni L, Cassinotti E, Canziani M, Dionigi
G, Rovera F and Dionigi R: Free circulating DNA as possible tumour
marker in colorectal cancer. Surg Oncol. 1:29–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bazan V, Bruno L, Augello C, et al:
Molecular detection of TP53, Ki-Ras and p16INK4A promoter
methylation in plasma of patients with colorectal cancer and its
association with prognosis. Results of a 3-year GOIM (Gruppo
Oncologico dell’Italia Meridionale) prospective study. Ann Oncol.
7:84–90. 2006.PubMed/NCBI
|
|
13
|
Kimura T, Holland WS, Kawaguchi T, et al:
Mutant DNA in plasma of lung cancer patients: potential for
monitoring response to therapy. Ann NY Acad Sci. 1022:55–60. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trombino S, Neri M, Puntoni R, et al:
Mutations in K-ras codon 12 detected in plasma DNA are not an
indicator of disease in patients with non-small cell lung cancer.
Clin Chem. 51:1313–1314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yung TK, Chan KC, Mok TS, Tong J, To KF
and Lo YM: Single-molecule detection of epidermal growth factor
receptor mutations in plasma by microfluidics digital PCR in
non-small cell lung cancer patients. Clin Cancer Res. 15:2076–2084.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maheswaran S, Sequist LV, Nagrath S, et
al: Detection of mutations in EGFR in circulating lung-cancer
cells. N Engl J Med. 359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mancini I, Santucci C, Sestini R, et al:
The use of COLD-PCR and high-resolution melting analysis improves
the limit of detection of KRAS and BRAF mutations in colorectal
cancer. J Mol Diagn. 12:705–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuo Z, Chen SS, Chandra PK, et al:
Application of COLD-PCR for improved detection of KRAS mutations in
clinical samples. Mod Pathol. 22:1023–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Milbury CA, Li J and Makrigiorgos GM:
COLD-PCR-enhanced high-resolution melting enables rapid and
selective identification of low-level unknown mutations. Clin Chem.
55:2130–2143. 2009. View Article : Google Scholar : PubMed/NCBI
|