Introduction
Gastric cancer is the second most common cause of
cancer-related deaths worldwide, particularly in China, and the
incidence is increasing yearly (1–3).
Gastric adenocarcinoma accounts for the majority of gastric cancer
cases. Despite substantial advances in treatment and effort in
research over the past few decades, the outcome of gastric cancer
remains unsatisfactory, and the overall 5-year survival rate of
advanced gastric adenocarcinoma patients is low. Therefore,
improvement in the therapy of gastric cancer now depends on
improving our understanding of the complex molecular mechanisms
governing the progression and aggressiveness of the disease.
Invasion and metastasis are major prognostic factors for advanced
gastric cancer (4). In addition to
surgery, adjuvant chemotherapy is used to negate the effects of
invasion and metastasis in gastric adenocarcinoma, but the survival
benefit is only marginal. Thus, understanding the mechanism of
invasion and metastasis is critical to develop new treatment
strategies that contribute to improving the survival of patients
with advanced gastric adenocarcinoma (5).
The aggressive nature of human gastric carcinoma is
dependent on a number of events, including cell degradation of the
basement membrane, cell migration through surrounding tissues,
intravasation into lymphatic or blood vessels, cancer cells exiting
from these vessels, cell survival and proliferation (5,6).
Chemokine receptors are believed to be involved in these
complicated processes. Chemokine receptors are divided into various
families (7,8): CXC chemokine receptors, CC chemokine
receptors, CX3C chemokine receptors and XC chemokine receptors,
which correspond to the 4 distinct subfamilies of chemokines that
they bind. Chemokine receptors are G protein-coupled receptors
containing 7 transmembrane domains that are found predominantly on
the surface of leukocytes. Previous studies have found that certain
chemokine receptors are expressed in certain tumor cells, which,
under the action of chemotactic substances, show directed
chemotaxis and play a significant role in tumor angiogenesis,
invasion and metastasis (9–18).
Therefore, the association between chemokine receptors and tumor
cell growth, progression, invasion and metastasis has attracted
significant attention. Identification of such chemokine receptors
not only leads to a better understanding of the carcinogenesis and
progression of gastric adenocarcinoma, but also provides new
strategies for developing targeted agents that specifically
suppress the process.
CXCR1 is a receptor for interleukin 8 (IL-8), which
binds to CXCR1 with high affinity and transduces the signal through
a G-protein-activated second messenger system. CXCR1 is mainly
expressed in neutrophils and is originally characterized by its
ability to induce chemotaxis of leukocytes. CXCR1 has been shown to
act on multiple cell types. Knockout studies in mice have indicated
that this protein inhibits embryonic oligodendrocyte precursor
migration in developing spinal cord. Moreover, it was found that
CXCR1 overexpresses in many solid tumors, which shows a close
correlation with drug-resistance, invasion, and metastasis
(11,19–23).
Although CXCR1 has been studied in several cancer types and a small
number of studies have examined the role of CXCR1 in gastric
adenocarcinoma specifically (24–29),
the precise functional role of CXCR1 in gastric adenocarcinoma
progression remains controversial and unclear. In our study, we
investigated the level of CXCR1 protein expression in primary and
sporadic gastric adenocarcinoma as well as in its corresponding
non-neoplastic mucosa, and preliminarily discussed the clinical
implications of our findings.
Materials and methods
Patients and specimens
Our study was conducted on 83 primary and sporadic
gastric adenocarcinoma tissue samples and their corresponding
non-neoplastic mucosa specimens retrieved from the archives at the
Department of Pathology of Xiang-ya Hospital of Central South
Univesrsity between 2008 and 2010. All patients provided informed
consent, and the protocol followed the ethical guidelines of the
Declaration of Helsinki. None of the patients received chemotherapy
or radiation therapy prior to tumor resection. Tissue blocks of
non-neoplastic mucosa (>5 cm from the edge of the tumor) were
obtained. Tumors stage was classified according to the AJCC staging
system. Patient data and the histopathological characteristics of
the tumors are shown in Table
I.
 | Table I.Patient data and tumor
characteristics. |
Table I.
Patient data and tumor
characteristics.
| Characteristic | n (%) |
|---|
| Total | 83 (100) |
| Gender | |
| Male | 61 (73.5) |
| Female | 22 (26.5) |
| Median age, years
(range) | 55 (31–79) |
| TNM stage | |
| T stage | |
| T1 | 5 (6.0) |
| T2 | 17 (20.5) |
| T3 | 44 (53.0) |
| T4 | 17 (20.5) |
| N stage | |
| N0 | 26 (31.3) |
| N1 | 24 (28.9) |
| N2 | 18 (21.7) |
| N3 | 15 (18.1) |
| Overall stage | |
| IA, IB | 10 (12.0) |
| II | 39 (47.0) |
| IIIA | 17 (20.5) |
| IIIB, IIIC,
IV | 17 (20.5) |
|
Differentiation | |
| Good | 21 (25.3) |
| Moderate | 24 (28.9) |
| Poor | 38 (45.8) |
Detection of CXCR1 protein in
specimens
Immunohistochemical staining for CXCR1 was performed
on formalin-fixed and paraffin-embedded material using standard
procedures. Sections (4 μm thick) were deparaffinized in
turpentine and rehydrated in a series of graded alcohol. Microwave
antigen retrieval was performed in citrate buffer (0.01 M, pH 6.0)
for 2x10 min at 450 W. After cooling to room temperature, the
specimens were rinsed three times for 3 min with phosphate-buffered
saline. Endogenous peroxidase was blocked by pre-incubation of the
slides with 3% hydrogen peroxide (H2O2), and
non-specific binding was blocked with non-immune goat serum.
Blocked sections were incubated in anti-CXCR1 antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight, and the
antibody was used at a dilution of 1:100. The subsequent reaction
was performed using the S-P kit (ZhongshanGoldenBridge
Biotechnology Co., Beijing, China) according to the manufacturer’s
instructions. Finally, the immunoreaction was developed using
diaminobenzidine (DAB) and counterstained with hematoxylin.
IgG2b-stained sections were used as negative controls, and sections
from tonsil were used as positive controls. Reddish-brown granules
on the membrane and in the cytoplasm of tumor cells or in that of
corresponding non-neoplastic mucosa epithelial cells indicated
positive immunoreactivity. The intensity of immunostaining in tumor
tissue was scored using corresponding non-neoplastic mucosa tissue
as an internal control. Tumor tissue was considered to have strong
expression if it showed stronger intensity than that of the
corresponding nonneoplastic mucosa tissue. If the staining
intensity was similar to that in the corresponding non-neoplastic
mucosa tissue, we considered the sample to have weak expression. If
the intensity was weaker than in corresponding non-neoplastic
mucosa tissue, the samples were considered to have no expression.
The samples were evaluated by two pathologists who were blinded to
the patients’ clinical data (5).
Detection of microvascular density in
specimens
Immunohistochemical staining using monoclonal
antibody to CD34 (Santa Cruz) was performed as described above to
measure microvascular density (MVD) in the tumor tissue and
corresponding non-neoplastic mucosa. Stained vessels were counted
under high-power microscopic fields. The average number of vessels
counted in the best-visualized area was recorded for each case
(30).
Statistical analysis
Statistical analysis was performed using the
Spearman correlation, when appropriate, to analyze the significance
of the correlation between CXCR1 protein expression and tumor data,
such as cancer cell differentiation, T stage, N stage, overall
stage and MVD. Multivariate logistic regression analysis was
performed to determine factors associated with tumor stage. The
SPSS13.0 software system was used and a P-value <0.05 was
considered to indicate statistical significance.
Results
Association between CXCR1 overexpression
and late-stage tumors
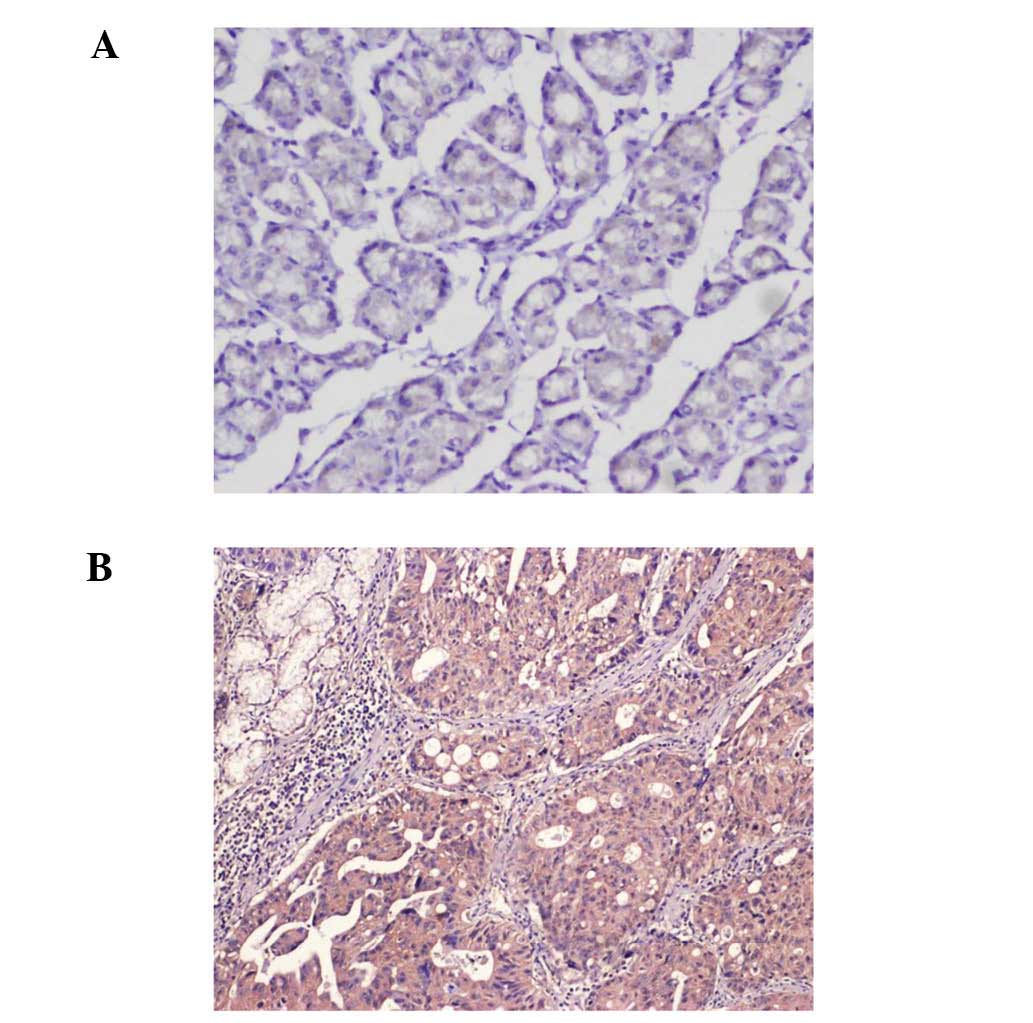
Non-neoplastic gastric mucosal epithelium expressed
CXCR1 in a heterogeneous manner. In all cases, immuno-reactivity
was observed in the membrane and in the cytoplasm of the tumor
cells (Fig. 1). Demographic
characteristics and tumor status were analyzed according to CXCR1
expression levels (Table II). In
this study, we grouped the tumor stages as follows: IA and IB as I,
II as II, IIIA as III, and IIIB, IIIC and IV as IV. As tumor
expression of CXCR1 increased, so did the overall tumor stage
(P<0.05). Of 13 tumors with strong CXCR1 expression, 11 (84.6%)
were stage IV, but only 1 (4.2%) of 24 tumors and 5 (10.9%) of 46
tumors with no or weak CXCR1 expression, respectively, were stage
IV. N stage positively correlated with CXCR1 expression, as 1
(4.2%) of 24 tumors with no expression and 4 (8.7%) of 46 tumors
with weak expression were at the N3 stage, compared to 10 (76.9%)
of 13 tumors with strong expression (P<0.05). CXCR1 expression
also correlated with T stage (P<0.05). However, we observed no
correlation between cancer cell differentiation and CXCR1
expression.
 | Table II.CXCR1 expression and tumor
status. |
Table II.
CXCR1 expression and tumor
status.
| Characteristic | No expression
(n=24), n (%) | Weak expression
(n=46), n (%) | Strong expression
(n=13), n (%) | P-value |
|---|
| Male:female | 18:6 | 34:12 | 9:4 | P>0.05 |
| Age (years) | 55.6±24.6 | 52.3±20.0 | 56.0±27.3 | P>0.05 |
| Cancer cell
differentiation | | | | P>0.05 |
| Good | 7 (29.2) | 11 (23.9) | 3 (23.1) | |
| Moderate | 5 (20.8) | 15 (32.6) | 4 (30.8) | |
| Poor | 12 (50.0) | 20 (43.5) | 6 (46.1) | |
| T stage | | | | P<0.05a |
| T1 | 4 (16.7) | 1 (2.2) | 0 (0.0) | |
| T2 | 7 (29.2) | 9 (19.6) | 1 (7.7) | |
| T3 | 11 (45.8) | 25 (54.3) | 8 (61.5) | |
| T4 | 2 (8.3) | 11 (23.9) | 4 (30.8) | |
| N stage | | | | P<0.05a |
| N0 | 13 (54.2) | 12 (26.1) | 1 (7.7) | |
| N1 | 8 (33.3) | 16 (34.8) | 0 (0.0) | |
| N2 | 2 (8.3) | 14 (30.4) | 2 (15.4) | |
| N3 | 1 (4.2) | 4 (8.7) | 10 (76.9) | |
| Overall stage | | | | P<0.05a |
| IA, IB | 7 (29.2) | 3 (6.5) | 0 (0.0) | |
| II | 14 (58.3) | 24 (52.2) | 1 (7.7) | |
| IIIA | 2 (8.3) | 14 (30.4) | 1 (7.7) | |
| IIIB, IIIC and
IV | 1 (4.2) | 5 (10.9) | 11 (84.6) | |
No correlation between CXCR1 expression
and MVD
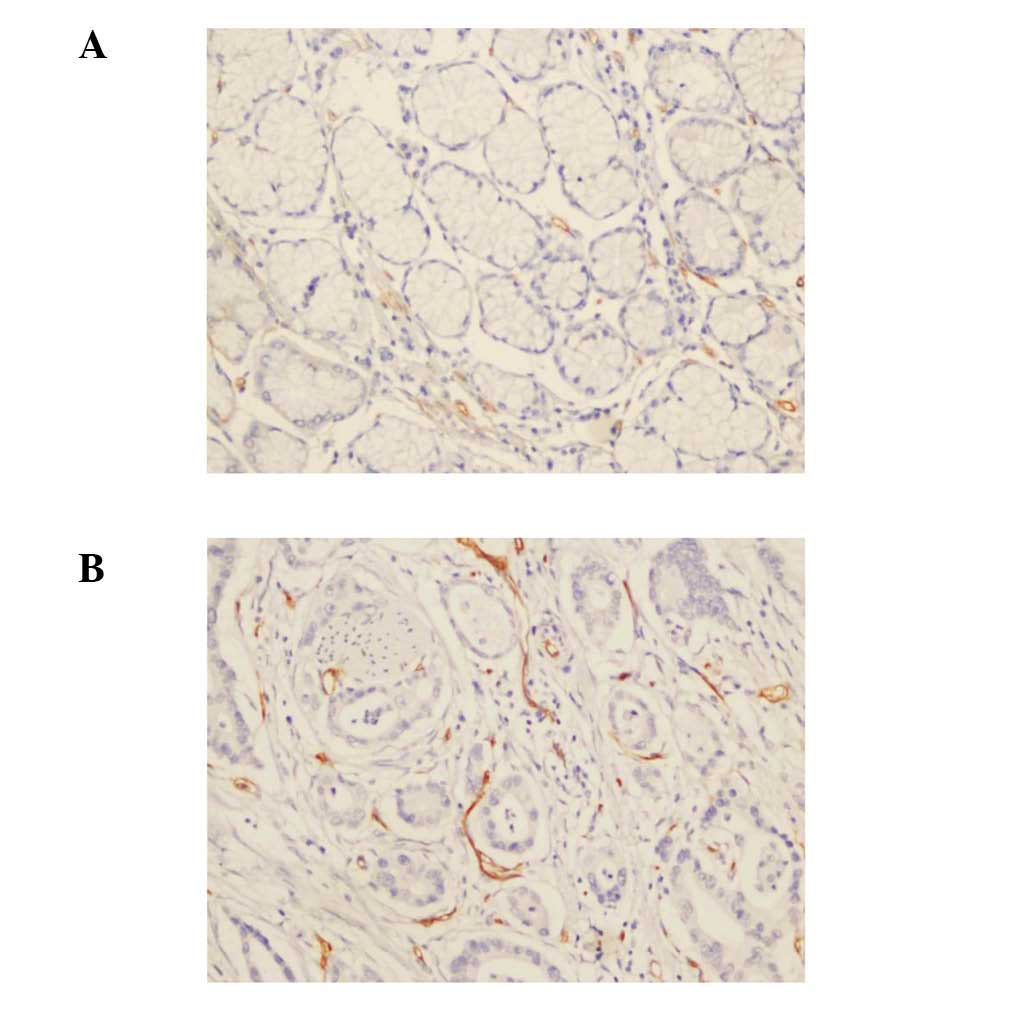
MVD was calculated as the number of vessels per
high-power microscopic field (Fig.
2). According to the statistical analysis, MVD correlated with
N stage and overall tumor stage (P<0.05), but not with T stage
or CXCR1 expression (Table III).
MVD for stages I, II, III and IV was 12.0±11.5, 14.8±12.0,
20.5±13.7, and 17.4±15.1, respectively. MVD positively correlated
with overall stage (P<0.05). The average MVD was 14.5±12.8 for
tumors with no CXCR1 expression, 16.5±14.7 for tumors with weak
CXCR1 expression, and 17.8±12.2 for tumors with strong expression.
MVD tended to be higher in tumors with higher T stage and marked
CXCR1 expression, but the correlation with T stage and CXCR1
expression levels was not linear (P>0.05).
 | Table III.Microvascular density (MVD) and tumor
status. |
Table III.
Microvascular density (MVD) and tumor
status.
| Characteristic | MVD | P-value |
|---|
| CXCR1
expression | | P>0.05 |
| No
expression | 14.5±12.8 | |
| Weak
expression | 16.5±14.7 | |
| Strong
expression | 17.8±12.2 | |
| T stage | | P>0.05 |
| T1 | 11.0±7.2 | |
| T2 | 14.4±11.5 | |
| T3 | 17.3±14.6 | |
| T4 | 16.5±14.1 | |
| N stage | | P<0.05a |
| N0 | 13.7±11.8 | |
| N1 | 16.3±14.3 | |
| N2 | 18.0±14.0 | |
| N3 | 18.0±14.8 | |
| Overall stage | | P<0.05a |
| IA, IB | 12.0±11.5 | |
| II | 14.8±12.0 | |
| IIIA | 20.5±13.7 | |
| IIIB, IIIC and
IV | 17.4±15.1 | |
Factors associated with tumor stage
N2- and N3-stage tumors were considered high-N-stage
tumors. T3- and T4-stage tumors served as high-T-stage tumors.
Based on univariate analysis, cancer cell differentiation, T stage,
MVD, and CXCR1 levels were significantly associated with high N
stage, and cancer cell differentiation, N stage, MVD, and CXCR1
levels were significantly associated with high T stage. However,
multivariate logistic regression analysis with cancer cell
differentiation, T stage, MVD, and CXCR1 levels showed that CXCR1
was the only factor significantly associated with high N stage
(Table IV), but CXCR1 was not a
factor significantly associated with high T stage. Poorly
differentiated cancer cells were associated with high N stage, but
this finding was not statistically significant. Strong CXCR1
expression had a 52.3- and 6.6-fold higher risk for high N stage
compared to no and weak CXCR1 expression, respectively (P<0.05
and P>0.05, respectively). Multivariate analysis showed 78.8%
sensitivity and 90.0% specificity for predicting high N stage.
 | Table IV.Results of the multivariate analysis
regarding high N stage. |
Table IV.
Results of the multivariate analysis
regarding high N stage.
| Characteristic | P-value | Exp (B) | 95% CI for Exp
(B) |
|---|
| T stage (1, 2 vs.
3, 4) | 0.799 | 1.230 | (0.250–6.042) |
| Cancer cell
differentiation (good vs. poor) | 0.896 | 0.895 | (0.169–4.729) |
| MVD | 0.716 | | |
| CXCR1
expression | | | |
| No
expression | 0.002a | 0.019 | (0.001–0.246) |
| Weak
expression | 0.113 | 0.151 | (0.015–1.564) |
| Strong
expression | | 1 | |
Discussion
Currently, despite advances in early diagnosis and
treatment that have improved the survival of patients with gastric
adenocarcinoma, this malignancy has retained a high mortality rate
(5). To further improve survival,
treatments based on a better understanding of cancer progression
are necessary (31). CXCR1
protein, a receptor for interleukin 8 (IL-8), is a member of the
G-protein-coupled receptor family, which binds to IL-8 with high
affinity and transduces the signal through a G-protein-activated
second messenger system. Previous studies have found that CXCR1
expression shows a close correlation with drug-resistance, invasion
and metastasis in a number of solid tumors (11,19–23,32).
Taken together, these observations indicate that CXCR1 may play a
role in the development and progression of certain tumors by
interacting with IL-8.
To investigate whether CXCR1 is associated with the
invasion and metastasis of gastric adenocarcinoma and performs
certain biological functions, we examined the expression of CXCR1
protein in primary gastric carcinoma and its corresponding
non-neoplastic mucosa using immunohistochemistry. Our study showed
that the expression level of CXCR1 was higher in primary gastric
adenocarcinoma than in its corresponding non-neoplastic mucosa in
certain cases. Our experimental results revealed a marked
association between overexpression of CXCR1 and late gastric
adenocarcinoma stage. CXCR1 expression was significantly associated
with high N stage, as demonstrated using multivariate analysis.
Tumors with strong CXCR1 expression exhibited higher risk of high N
stage compared to no and weak CXCR1 expression. CXCR1 expression
was also correlated with T stage and overall stage. These findings
suggest that CXCR1 may be involved in gastric adenocarcinoma
invasion and metastasis, and the association between strong CXCR1
expression and late-stage gastric adenocarcinoma may contribute to
its association with high N stage. A number of studies have
postulated an association between CXCR1 expression and cancer cell
invasion and metastasis in certain cancer types (11,19–23);
our findings further support this hypothesis.
It is believed that through various mechanisms,
chemokine receptors play multiple roles in the development and
progression of a number of tumor types (32–36).
CXCR1 regulates cell motility and angiogenesis and cell migration
and invasion, in which various intracellular pathways are involved,
and motility can be activated by chemokine receptors (37–42).
CXCR1 and its related pathways may become potential targets for
cancer treatment, thus it is important to clarify the mechanistic
roles of CXCR1 and whether CXCR1 plays a major role in cancer
progression. IL-8 binding to CXCR1 is a strong neutrophil
attractant. In non-cancerous conditions, neutrophils recruited by
IL-8 binding to CXCR1 cause tissue damage. One study has suggested
that increasing amounts of tumor-infiltrating neutrophils in
advanced gastric cancer are associated with reduced mortality
(43). However, neutrophils can
either eliminate tumor cell populations or contribute to their
invasive potential (44,45). Neutrophils may enable tumor cells
to migrate through the extracellular matrix, helping them to enter
the vasculature (46). Based on
our results, neutrophil recruitment by CXCR1 binding to its ligand
IL-8 may aid gastric adenocarcinoma cells in metastasizing to lymph
nodes. Although neutrophil infiltration was not analyzed and the
role of neutrophil in tumors is controversial, this may be another
hypothesis supporting our results. Another possibility is that
overexpression of CXCR1 in gastric adenocarcinoma cell binding to
its ligand IL-8 results in tumor cell migration. It has been
reported that Helicobacter infection is associated with
chemokine IL-8 and its receptor CXCR1 (47,48),
and moreover that it is associated with gastric cancer; however, we
are currently unable to conclude that Helicobacter infection
contributed to gastric cancer via CXCR1.
A promising recent study found that CXCR1 expression
subdivides cancer stem cell populations. The IL-8/CXCR1 axis may be
involved in the regulation of cancer stem cell proliferation,
self-renewal and drug-resistance, which leads to tumor cell
invasion and metastasis (22).
Further studies are warranted to determine whether this finding is
applicable to gastric adenocarcinoma.
Studies on malignant melanoma and breast cancer
suggest that expression of CXCR1 in vivo and in vitro
is associated with poor prognosis; these studies indicate that
CXCR1 is associated with tumor growth and enhanced angiogenesis
(22,23,49,50).
Angiogenesis is another essential step for tumor growth and
metastasis, and expression of CXCR1 and VEGF can provide a positive
feedback loop (51); this
hypothesis is supported by our immunohistochemistry results. In our
study, CXCR1 expression and microvessel count were evaluated on 83
sporadic gastric adenocarcinoma tissue sections to observe a
correlation between CXCR1 expression and MVD within a certain area
of the tumor. Notably, tumor samples with strong CXCR1 expression
had a high MVD, but the correlation between CXCR1 expression and
MVD was not linear. Furthermore, there may be more than one pathway
regulating angiogenesis (52).
However, we cannot exclude the possibility that CXCR1 promoted
tumor cell survival by supplying blood vessels to late-stage
gastric adenocarcinoma.
In conclusion, this is, to our knowledge, the first
relatively clear report that overexpression of CXCR1 is associated
with advanced gastric adenocarcinoma stage, specifically high N
stage. Through multiple mechanisms, CXCR1 may be involved in the
invasion and metastasis of gastric adenocarcinoma cells and
late-stage gastric adenocarcinoma progression. Therefore, the novel
expression and function of CXCR1 not only adds to our knowledge of
CXCR1, but also elucidates the pathogenesis of gastric
adenocarcinoma. Further studies are required to confirm and
understand this observation and to determine whether CXCR1 may
serve as a new and promising therapeutic target for gastric
adenocarcinoma treatment.
Acknowledgements
This work was partially supported by
the Hunan Provincial Innovation Foundation for Postgraduate study
(No. CX2011B046), the Graduate Degree Thesis Innovation Foundation
of Central South University (No.2009ssxt062), the Science and
Technology Program Foundation of Changsha City (Nos. K1005005-31
and K1106041-31), the Open-End Fund for the Valuable and Precision
Instruments of Central South University, Key Program of Natural
Science Fund of Hunan Province (No. 09JJ3040) and the National
Natural Science Fund of China (No. 81001080).
References
|
1
|
Alberts SR, Cervantes A and van de Velde
CJ: Gastric cancer: epidemiology, pathology and treatment. Ann
Oncol. 14(Suppl 2): 31–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
3
|
Lu JB, Sun XB, Dai DX, et al: Epidemiology
of gastroenterologic cancer in Henan Province, China. World J
Gastroenterol. 9:2400–2403. 2003.PubMed/NCBI
|
|
4
|
Hyung WJ, Noh SH, Yoo CH, et al:
Prognostic significance of metastatic lymph node ratio in T3
gastric cancer. World J Surg. 26:323–329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JY, Park KH, Bang S, et al: CXCL5
overexpression is associated with late stage gastric cancer. J
Cancer Res Clin Oncol. 133:835–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar
|
|
7
|
Murphy PM, Baggiolini M, Charo IF, et al:
International union of pharmacology. XXII Nomenclature for
chemokine receptors. Pharmacol Rev. 52:145–176. 2000.PubMed/NCBI
|
|
8
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossi D and Zlotnik A: The biology of
chemokines and their receptors. Annu Rev Immunol. 18:217–243. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johrer K, Zelle-Rieser C, Perathoner A, et
al: Up-regulation of functional chemokine receptor CCR3 in human
renal cell carcinoma. Clin Cancer Res. 11:2459–2465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scotton CJ, Wilson JL, Milliken D, Stamp G
and Balkwill FR: Epithelial cancer cell migration: a role for
chemokine receptors? Cancer Res. 61:4961–4965. 2001.PubMed/NCBI
|
|
14
|
Schimanski CC, Schwald S, Simiantonaki N,
et al: Effect of chemokine receptors CXCR4 and CCR7 on the
metastatic behavior of human colorectal cancer. Clin Cancer Res.
11:1743–1750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: new insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan MC, Goedegebuure PS, Belt BA, et al:
Disruption of CCR5-dependent homing of regulatory T cells inhibits
tumor growth in a murine model of pancreatic cancer. J Immunol.
182:1746–1755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu JY, Deng XY, Bian XW, et al: The
expression of functional chemokine receptor CXCR4 is associated
with the metastatic potential of human nasopharyngeal carcinoma.
Clin Cancer Res. 11:4658–4665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ginestier C, Liu S, Diebel ME, et al:
CXCR1 blockade selectively targets human breast cancer stem cells
in vitro and in xenografts. J Clin Invest. 120:485–497. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S, Nannuru KC, Sadanandam A, Varney
ML and Singh RK: CXCR1 and CXCR2 enhances human melanoma
tumourigenesis, growth and invasion. Br J Cancer. 100:1638–1646.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eck M, Schmausser B, Scheller K, Brandlein
S and Muller-Hermelink HK: Pleiotropic effects of CXC chemokines in
gastric carcinoma: differences in CXCL8 and CXCL1 expression
between diffuse and intestinal types of gastric carcinoma. Clin Exp
Immunol. 134:508–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitadai Y, Takahashi Y, Haruma K, et al:
Transfection of interleukin-8 increases angiogenesis and
tumorigenesis of human gastric carcinoma cells in nude mice. Br J
Cancer. 81:647–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin BR, Chang CC, Chen LR, et al:
Cysteine-rich 61 (CCN1) enhances chemotactic migration,
transendothelial cell migration, and intravasation by concomitantly
up-regulating chemokine receptor 1 and 2. Mol Cancer Res.
5:1111–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Croker AK, Goodale D, Chu J, et al: High
aldehyde dehydrogenase and expression of cancer stem cell markers
selects for breast cancer cells with enhanced malignant and
metastatic ability. J Cell Mol Med. 13:2236–2252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitadai Y, Haruma K, Mukaida N, et al:
Regulation of disease-progression genes in human gastric carcinoma
cells by interleukin 8. Clin Cancer Res. 6:2735–2740.
2000.PubMed/NCBI
|
|
29
|
Ju DW, Wei PK, Lin HM, Sun DZ, Yu S and
Xiu LJ: Effects of Xiaotan Sanjie decoction on expressions of
interleukin-8 and its receptors in gastric tumor xenografts and
gastric tissue adjacent to the tumor in mice. Zhong Xi Yi Jie He
Xue Bao. 8:74–79. 2010.(In Chinese).
|
|
30
|
Wulfing P, Kersting C, Tio J, et al:
Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression
is correlated with vascular endothelial growth factor expression
and angiogenesis in breast cancer. Clin Cancer Res. 10:2393–2400.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyazaki H, Patel V, Wang H, Edmunds RK,
Gutkind JS and Yeudall WA: Down-regulation of CXCL5 inhibits
squamous carcinogenesis. Cancer Res. 66:4279–4284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Richards BL, Eisma RJ, Spiro JD, Lindquist
RL and Kreutzer DL: Coexpression of interleukin-8 receptors in head
and neck squamous cell carcinoma. Am J Surg. 174:507–512. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors, and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruffini PA, Morandi P, Cabioglu N,
Altundag K and Cristofanilli M: Manipulating the
chemokine-chemokine receptor network to treat cancer. Cancer.
109:2392–2404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balakin KV, Ivanenkov YA, Tkachenko SE,
Kiselyov AS and Ivachtchenko AV: Regulators of chemokine receptor
activity as promising anticancer therapeutics. Curr Cancer Drug
Targets. 8:299–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koizumi K, Hojo S, Akashi T, Yasumoto K
and Saiki I: Chemokine receptors in cancer metastasis and cancer
cell-derived chemokines in host immune response. Cancer Sci.
98:1652–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D, Sai J, Carter G, Sachpatzidis A,
Lolis E and Richmond A: PAK1 kinase is required for CXCL1-induced
chemotaxis. Biochemistry. 41:7100–7107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schraufstatter IU, Chung J and Burger M:
IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac
signaling pathways. Am J Physiol Lung Cell Mol Physiol.
280:L1094–L1103. 2001.PubMed/NCBI
|
|
39
|
Venkatakrishnan G, Salgia R and Groopman
JE: Chemokine receptors CXCR-1/2 activate mitogen-activated protein
kinase via the epidermal growth factor receptor in ovarian cancer
cells. J Biol Chem. 275:6868–6875. 2000. View Article : Google Scholar
|
|
40
|
Bonacchi A, Romagnani P, Romanelli RG, et
al: Signal transduction by the chemokine receptor CXCR3: activation
of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls
cell migration and proliferation in human vascular pericytes. J
Biol Chem. 276:9945–9954. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chandrasekar B, Melby PC, Sarau HM, et al:
Chemokinecytokine cross-talk. The ELR+ CXC chemokine LIX
(CXCL5) amplifies a proinflammatory cytokine response via a
phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem.
278:4675–4686. 2003.PubMed/NCBI
|
|
42
|
Chandrasekar B, Bysani S and Mummidi S:
CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa
B kinase, and nuclear factor-kappa B and induces cell-cell adhesion
and aortic smooth muscle cell proliferation. J Biol Chem.
279:3188–3196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Caruso RA, Bellocco R, Pagano M, Bertoli
G, Rigoli L and Inferrera C: Prognostic value of intratumoral
neutrophils in advanced gastric carcinoma in a high-risk area in
northern Italy. Mod Pathol. 15:831–837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Carlo E, Forni G, Lollini P, Colombo
MP, Modesti A and Musiani P: The intriguing role of
polymorphonuclear neutrophils in antitumor reactions. Blood.
97:339–345. 2001.PubMed/NCBI
|
|
45
|
Welch DR, Schissel DJ, Howrey RP and Aeed
PA: Tumor-elicited polymorphonuclear cells, in contrast to ‘normal’
circulating polymorphonuclear cells, stimulate invasive and
metastatic potentials of rat mammary adenocarcinoma cells. Proc
Natl Acad Sci USA. 86:5859–5863. 1989.PubMed/NCBI
|
|
46
|
De Larco JE, Wuertz BR and Furcht LT: The
potential role of neutrophils in promoting the metastatic phenotype
of tumors releasing interleukin-8. Clin Cancer Res. 10:4895–4900.
2004.PubMed/NCBI
|
|
47
|
Schmausser B, Josenhans C, Endrich S, et
al: Downregulation of CXCR1 and CXCR2 expression on human
neutrophils by Helicobacter pylori: a new pathomechanism in
H. pylori infection? Infect Immun. 72:6773–6779. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Backhed F, Torstensson E, Seguin D,
Richter-Dahlfors A and Rokbi B: Helicobacter pylori
infection induces interleukin-8 receptor expression in the human
gastric epithelium. Infect Immun. 71:3357–3360. 2003. View Article : Google Scholar
|
|
49
|
Gabellini C, Trisciuoglio D, Desideri M,
et al: Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on
human malignant melanoma progression. Eur J Cancer. 45:2618–2627.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Charafe-Jauffret E, Ginestier C, Iovino F,
et al: Breast cancer cell lines contain functional cancer stem
cells with metastatic capacity and a distinct molecular signature.
Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Folkman J: Angiogenesis and apoptosis.
Semin Cancer Biol. 13:159–167. 2003. View Article : Google Scholar
|
|
52
|
White ES, Flaherty KR, Carskadon S, et al:
Macrophage migration inhibitory factor and CXC chemokine expression
in non-small cell lung cancer: role in angiogenesis and prognosis.
Clin Cancer Res. 9:853–860. 2003.PubMed/NCBI
|