Introduction
Hepatocellular carcinoma (HCC) is the most common
malignant tumor of the liver and its incidence is particularly high
in China relative to other countries (1,2). The
development of standardized surveillance strategies and the
introduction of the Barcelona Clinic Liver Cancer (BCLC)
classification for the clinical management of HCC have
significantly improved the outcomes of patients with early or
intermediate-stage HCC (3). As
local ablative therapies, including transarterial chemoembolization
(TACE), have limited efficacy against large HCC and yield
incomplete necrosis, the tumors often progress following local
therapy (4) and the prognosis may
be extremely poor. Meanwhile, there is a lack of convincing
evidence showing that systemic chemotherapy lengthens overall
survival (OS) for advanced HCC (5).
A fuller understanding of the molecular pathogenesis
of HCC has led to the development of molecular-targeted therapies.
The oral multikinase inhibitor sorafenib (Nexavar®) has
been reported to block angiogenesis and cell proliferation in HCC
(6). Two international randomized
controlled trials of sorafenib conducted in Caucasian and Asian
patients with advanced HCC revealed beneficial effects of sorafenib
on the time to tumor progression (TTP) and OS (7,8).
Thus, sorafenib is now well-established as a standard of care for
HCC. Nevertheless, the efficacy of sorafenib alone for advanced HCC
remains moderate and certain patients have extremely short survival
(9). The mechanisms underlying
tumor resistance to sorafenib therapy are not well known and
prognostic factors have not been clearly defined. In a phase II
trial conducted by Abou-Alfa et al (10), it was found that pretreatment tumor
phosphorylated ERK levels were correlated with TTP. However, in
patients with advanced HCC who are amenable only to systemic
therapy, tumor tissue is generally not available as needle tract
metastases may arise from biopsy, hindering further attempts to
understand the molecular biology of tumor resistance to therapy. A
more recent phase II open-label study conducted by Yau et al
(11) revealed that the presence
of lung metastasis was a poor prognostic factor and implied that a
high tumor load may render the patients refractory to sorafenib
treatment. Meanwhile, Vincenzi et al (12) reported that early skin toxicity may
be a predictive factor for tumor control in HCC patients treated
with sorafenib. Despite these reports, it remains unclear whether
the established prognostic factors, including Child-Pugh
classification, α-fetoprotein (AFP), portal vein thrombosis (PVT),
hepatitis B virus (HBV) DNA and tumor differentiation and size, are
relevant to patients treated with sorafenib.
Therefore, the aim of this study was to
prospectively investigate the efficacy and determine the prognostic
factors for progression-free survival (PFS) and OS in patients with
advanced HBV-related HCC treated with sorafenib as first-line
therapy.
Materials and methods
Patients
Based on the BCLC staging classification, 326
consecutive patients with HBV-related advanced HCC were screened
between August 2008 and May 2010 at the Center of Therapeutic
Research for Hepatocellular Carcinoma, Beijing 302nd Hospital
(Beijing, China). A total of 67 patients were Child-Pugh C, 58
patients were Child-Pugh B8 or B9 with serum bilirubin level
>51.3 μmol/l. A total of 91 patients had a history of either
hepatectomy (14), preoperative
chemotherapy (11), prior TACE or
local ablation (47) or radiotherapy (19). As a result, 216 patients were
excluded from the analyses and 110 patients were included in the
present study (Table I). HCC was
diagnosed based on a serum AFP level >400 ng/ml and typical
imaging findings consistent with the criteria of the European
Association for the Study of the Liver (13). Liver biopsies were obtained in 58
patients with uncertain diagnosis and assessed histologically to
confirm diagnosis. The BCLC classification was used to identify
tumor stages (14). The presence
of PVT, representing macroscopic vascular invasion and extrahepatic
spread, was used to define advanced HCC. Performance status (PS)
was evaluated according to the Eastern Cooperative Oncology Group
criteria. Patients who met the following criteria were included in
the study: diagnosis of advanced HCC, first-line treatment with
sorafenib, ECOG PS ≤2, Child-Pugh class A or B and total serum
bilirubin level <51.3 μmol/l, alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) levels less than five times the
normal upper limit, adequate hematological function (platelet count
greater than 50×109/l and hemoglobin level more than 80
g/l) and adequate renal function (serum creatinine level less than
1.5 times the normal upper limit). Baseline demographic, clinical
and laboratory data were collected for all patients using a uniform
database template to ensure consistent data collection. Outcomes,
including PFS and OS, were collected from patient charts. All
treatments were approved by the Beijing 302nd Hospital Research
Ethics Committee, and written informed consent was obtained from
the patients who met the inclusive criteria prior to the collection
of data and blood and tumor specimens and analysis being
performed.
 | Table I.Patient characteristics (n=110). |
Table I.
Patient characteristics (n=110).
| Clinical
features | Values |
|---|
| Gender, n (%) | |
| Male | 100 (90.9) |
| Female | 10 (9.1) |
| Age (years), median
(range) | 54 (31–76) |
| ECOG PS, n (%) | |
| 0 | 22 (20.0) |
| 1 | 46 (41.8) |
| 2 | 42 (38.2) |
| Tumor
differentiation, n | |
| Medium | 29 |
| Low | 29 |
| Tumor diameter (cm),
median (range) | 8 (2.2–19.3) |
| No. of tumors, n
(%) | |
| 1 | 34 (30.9) |
| 2 | 16 (14.5) |
| 3 | 16 (14.5) |
| 4 | 44 (40.1) |
| Invasion of portal
vein, n (%) | |
| Branch | 84 (76.4) |
| Trunk | 26 (23.6) |
| Extrahepatic
metastasis, n (%) | |
| Lung | 36 (32.7) |
| Adrenal | 2 (1.8) |
| Bone | 2 (1.8) |
| HBV DNA (IU/ml), n
(%) | |
| 0–9,999 | 70 (63.6) |
|
10,000-99,999 | 14 (12.7) |
| ≥100,000 | 26 (23.7) |
| Combined treatment,
n (%) | |
| Sorafenib
alone | 32 (29.1) |
| TACE | 38 (34.5) |
| TACE and
cryoablation | 40 (36.4) |
| Child-Pugh class, n
(%) | |
| A | 87 (79.1) |
| B | 23 (20.9) |
| Platelet count
(×109/l), median (range) | 110 (27–351) |
| AFP (ng/ml), median
(range) | 1019 (7–20000) |
Sorafenib administration
All the patients received sorafenib. The dosage was
400 mg twice daily (the standard dose); treatment interruptions and
dose reductions (first 400 mg twice daily, then 200 mg twice daily)
were permitted for adverse drug reactions (ADRs) according to the
National Cancer Institute Common Toxicity Criteria (15). For ADRs of grade 3–4, sorafenib was
reduced to 200 mg twice daily until the ADRs improved to grade 2 or
below, then increased to 400 mg twice daily if well tolerated. The
criteria for the discontinuation of therapy were as follows: ADRs
that required termination of medication, deterioration of ECOG PS
score to 4 and withdrawal of consent. If disease progression was
observed, sorafenib was continued if the patient was considered to
have a good clinical status (e.g., PS, liver function and tolerable
side effects) and wished to continue the treatment. Following
sorafenib treatment, TACE or cryoablation were conducted in those
without absolute contraindications to TACE or cryoablation, based
on the potential clinical benefits expected from the treatment and
the patient's consent. Sorafenib therapy was continued without
interruption during local therapies.
TACE
Patients were eligible for TACE if their tumor
burden was <50% of the total liver volume. Hepatic angiography
was routinely performed to determine tumor location, size or number
and blood vessels using the Seldinger method. Super-selective
catheterization was performed to the arteries supplying the tumor
where possible. Then 40 mg of cisplatin, 1,000 mg of 5-fluorouracil
and 20 mg of doxorubicin were infused via the arteries for
chemotherapy, after which the blood vessels supplying the tumor
were filled with a suspension of 10–20 ml of 40% ultra-fluid
lipiodol and 10 mg of ADM. TACE was carried out with an interval of
4–6 weeks between cycles. Total course of TACE was terminated if
more than 75% of the tumor volume was occupied by iodine oil on
computed tomography (CT) scans 1 month after 1, 2 or 3 cycles of
TACE. In our experience, if three cycles of TACE do not achieve
adequate iodine deposition, the likelihood of increasing iodine
accumulation is low with further TACE cycles. Therefore, at our
institution, we limit TACE to a maximum of three cycles.
Argon-helium cryoablation
Argon-helium cryoablation was performed as
previously described (16).
Briefly, an argon-helium gas-based CRYOcare system (EndoCare,
Irvine, CA, USA) and cryoprobes were used to freeze the tumor with
a dual freeze-thaw cycle under ultrasound (US) guidance. After
sonographically determining the most favorable percutaneous
approach, we inserted the cryoprobes into the tumor under US
guidance and advanced the tip to reach the distal margin of the
targeted lesion. The number of probes used depended on the location
and size of the lesions to be ablated. The dual freeze-thaw cycle
consisted of a 20-min freeze, followed by a 10-min thaw and a
15-min freeze. The dimensions of the frozen tissue were monitored
by US. The cryoprobe temperatures were reduced with 1 min to
−135±2°C. Upon removal of the probes, all tracts were packed with
Surgicel (Johnson & Johnson, Inc., Arlington, TX, USA), to
control bleeding. We aimed to ablate all the tumors with a curative
intent in a single or repeated cryoablation, especially for tumors
less than 5 cm in diameter. For large tumors, complete ablation
using percutaneous modality is not possible, so in these cases we
reduced tumor load larger than 50% as much as possible. We limited
cryoablation to a maximum of three procedures.
Disease assessment
Disease status was assessed using CT scans or
magnetic resonance imaging (MRI) performed approximately every 8
weeks. The response was classified as complete response (CR),
partial response (PR), stable disease (SD) or progressive disease
(PD) according to Modified RECIST (mRECIST) Assessment for
Hepatocellular Carcinoma (17).
Patients who achieved CR, PR or SD were defined as achieving
clinical benefits (CB). PFS was calculated from the date of
starting sorafenib treatment to the date of disease progression or
mortality. OS was calculated from the date of starting sorafenib to
the date of mortality or last follow-up.
Statistical analysis
Continuous data are expressed as medians and range.
All continuous data were classified into subgroups according to the
median value. Univariate associations between OS, PFS and potential
prognostic factors were assessed using the Kaplan-Meier method with
the log-rank test. Cox's proportional hazards model was used for
multivariate analyses with a step-wise procedure and a significance
level of 0.10 to enter and remove variables. All statistical
analyses were performed using SPSS software version 16.0 for
Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant result.
Results
Patient characteristics and clinical
outcomes
The baseline characteristics of the patients are
presented in Table I. The median
follow-up was 9 months (range, 3–18) and the median duration of
sorafenib treatment was 6.5 months (range, 1.5–18). A total of 15
patients discontinued sorafenib at 6–24 weeks due to liver function
deterioration (10 cases) and esophagogastric variceal bleeding (5
cases). A total of 27 (24.5%) patients reduced sorafenib dosage to
200 mg twice daily due to grade 3–4 ADRs, but all these patients
were restored to 400 mg twice daily after 1–2 weeks. Overall, 14
(12.7%) patients achieved CR, 16 (14.5%) achieved PR and 40 (36.4%)
achieved SD lasting >8 weeks. Therefore, the overall clinical
benefit rate (CBR) was 63.6% (70/110). The median OS and PFS for
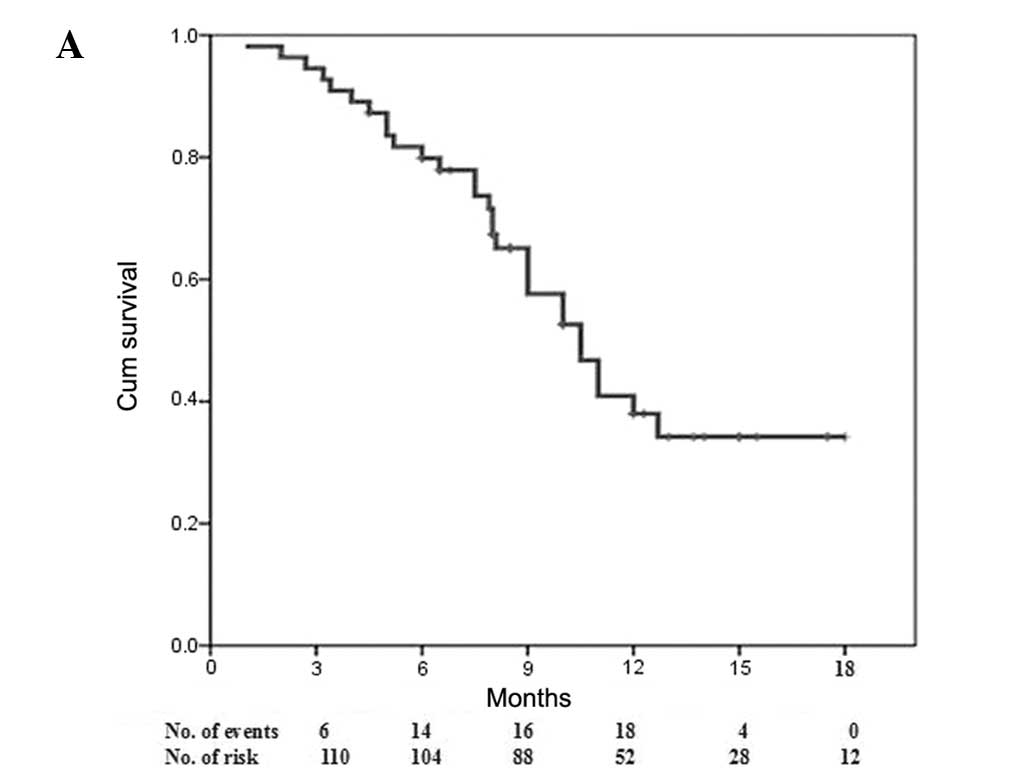
the whole cohort were 10.5 [95% confidence interval (CI), 8.7–12.3]
and 5.0 months (95% CI, 3.7–6.3), respectively (Fig. 1). Disease progression occurred in
100 (90.9%) patients. Furthermore, a total of 58 (52.7%) patients
died during the study; 25 (22.7%) succumbed to
recurrence/metastasis, 14 (12.7%) to liver failure, 10 (9.1%) to
esophagogastric variceal bleeding, 6 (5.5%) to refractory
ascites-induced renal failure and 3 (2.7%) to tumor
rupture/hemorrhage.
Treatment-related adverse effects
Hand-foot skin reaction (65.5%) was the most common
adverse event, followed by rash (63.6%), hypertension (55.5%),
alopecia (50.9%), fatigue (46.3%), weight loss (45.5%), diarrhea
(40.0%) and liver toxicity (elevated bilirubin levels, 39.1%).
Hematological toxicities occurred in 38 (34.5%) patients, including
leucopenia (14.5%), hemorrhage (12.7%), anemia (3.6%) and
thrombocytopenia (3.6%) and were the most frequently encountered
grade 3–4 toxicities (16.4%). The most common grade 3 toxicities
were hand-foot skin reaction (15.5%), liver toxicity (8.2%),
diarrhea (4.5%), hypertension (3.6%) and hemorrhage (2.7%). Liver
toxicity (6.4%), hemorrhage (4.5%), leucopenia (3.6%), anemia
(1.8%) and diarrhea (1.8%) were the most common grade 4 toxicities.
Liver toxicity occurred in 43 (39.1%) patients and grade 3–4
toxicity was observed in 16 (14.5%), 10 of whom succumbed to liver
failure.
Univariate analysis of factors associated
with PFS and OS
Univariate analysis (Table II) revealed that ECOG PS ≥1,
extrahepatic metastasis, high HBV DNA level, Child-Pugh class B and
AFP >1019 ng/ml were significantly associated with reduced PFS.
Meanwhile, ECOG PS ≥1, tumor diameter, extrahepatic metastasis,
high HBV DNA level and AFP >1019 ng/ml were associated with
reduced OS. Use of local therapy was associated with longer PFS and
OS.
 | Table II.Univariate analysis of factors
associated with PFS and OS. |
Table II.
Univariate analysis of factors
associated with PFS and OS.
| | PFS (months)
| OS (months)
|
|---|
| Parameter | No. of
mortalities | Median | P-value | Median | P-value |
|---|
| Gender | | | 0.214 | | 0.898 |
| Male | 53 | 6.0 | | 10.5 | |
| Female | 5 | 3.0 | | 9.0 | |
| Age (years) | | | 0.668 | | 0.228 |
| ≤54 | 33 | 5.0 | | 9.0 | |
| >54 | 25 | 6.0 | | 10.5 | |
| ECOG PS | | | <0.001 | | <0.001 |
| 0 | 2 | 8.0 | | 17.2 | |
| 1 | 20 | 6.0 | | 11.0 | |
| 2 | 36 | 3.0 | | 7.5 | |
| Tumor
differentiation | | | 0.255 | | 0.401 |
| Medium | 21 | 3.0 | | 8.0 | |
| Low | 21 | 4.5 | | 9.0 | |
| Tumor diameter
(cm) | | | 0.125 | | 0.007 |
| ≤8 | 22 | 6.0 | | 12.0 | |
| >8 | 36 | 5.0 | | 8.1 | |
| Tumor number | | | 0.165 | | 0.995 |
| 1 | 15 | 6.0 | | 12.7 | |
| 2 | 11 | 6.0 | | 11.0 | |
| 3 | 11 | 5.0 | | 10.0 | |
| 4 | 21 | 4.0 | | 10.0 | |
| Invasion of portal
vein | | | 0.856 | | 0.399 |
| Branch | 43 | 5.0 | | 11.0 | |
| Trunk | 15 | 6.0 | | 10.0 | |
| Extrahepatic
metastasis | | | 0.019 | | 0.040 |
| No | 34 | 6.0 | | 11.0 | |
| Yes | 24 | 4.0 | | 9.0 | |
| HBV DNA
(IU/ml) | | | <0.001 | | <0.001 |
| 0–9,999 | 26 | 6.0 | | 12.7 | |
|
10,000–99,999 | 10 | 4.0 | | 10.0 | |
| ≥100,000 | 22 | 3.0 | | 8.0 | |
| Combined
treatment | | | <0.001 | | <0.001 |
| Sorafenib
alone | 24 | 3.0 | | 8.0 | |
| TACE | 18 | 6.0 | | 12.0 | |
| TACE and
cryoablation | 16 | 6.0 | | 12.7 | |
| Child-Pugh
class | | | <0.001 | | 0.246 |
| A | 47 | 6.0 | | 11.0 | |
| B | 11 | 3.0 | | 9.0 | |
| Platelet count
(×109/l) | | | 0.555 | | 0.427 |
| ≤110 | 26 | 6.0 | | 10.5 | |
| >110 | 32 | 5.0 | | 10.0 | |
| AFP (ng/ml) | | | 0.005 | | 0.001 |
| ≤1019 | 24 | 6.0 | | 12.7 | |
| >1019 | 34 | 4.0 | | 9.0 | |
Multivariate analysis of factors
associated with PFS and OS
Cox proportional hazards model analyses revealed
that local therapy was independently associated with improved PFS
[odds ratio (OR), 0.576; 95% CI, 0.399–0.831; P= 0.003] whereas
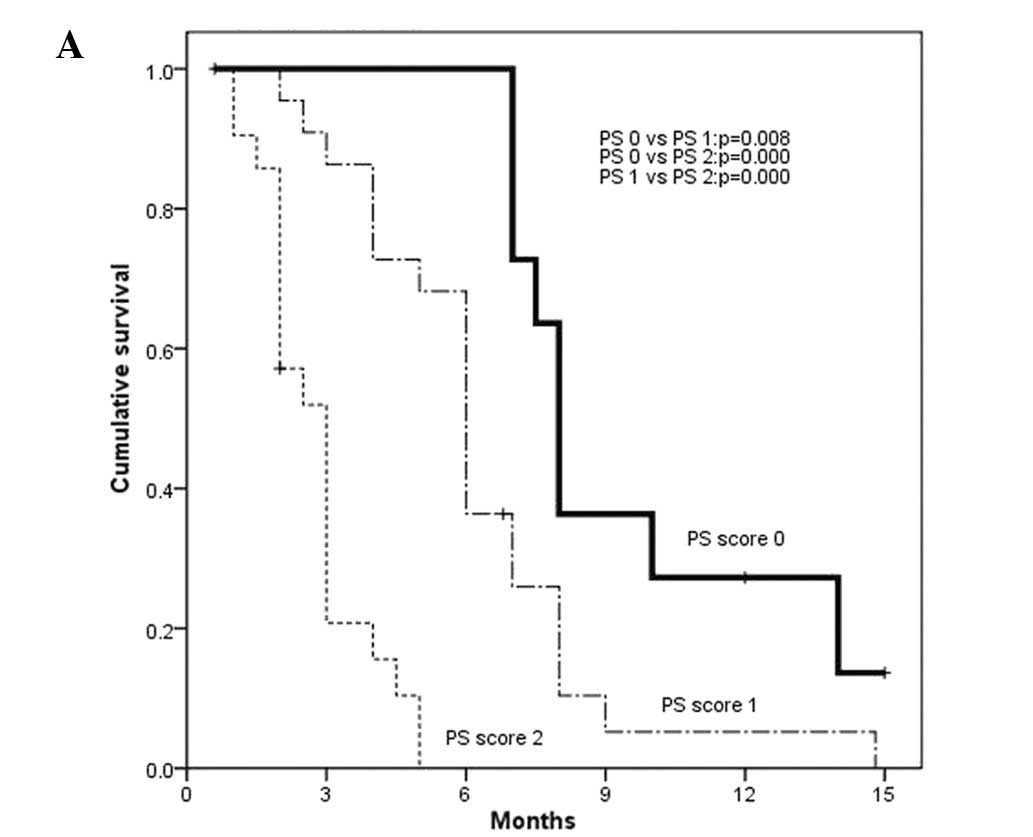
ECOG PS (OR, 5.705; 95% CI, 3.352–9.709; P= 0.000) and Child-Pugh
class (OR, 2.628; 95% CI, 1.416–4.878; P=0.002) were independently
associated with reduced PFS (Fig.
2). Moreover, local therapy (OR, 0.245; 95% CI, 0.071–0.846; P=
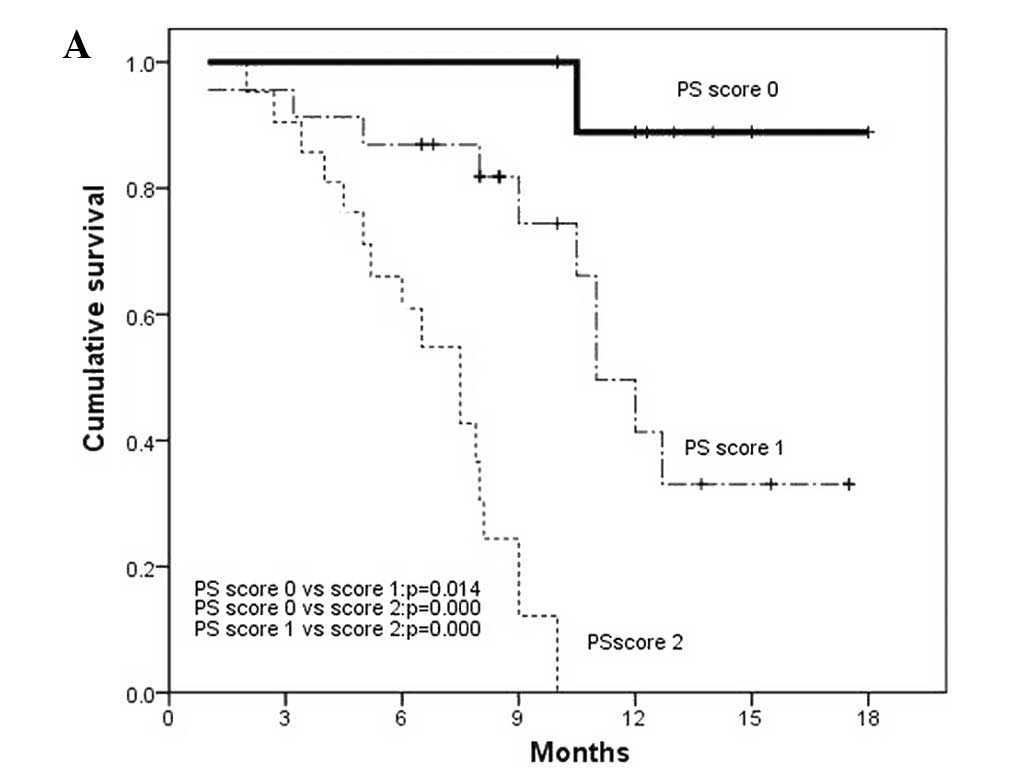
0.026) was independently associated with improved OS while ECOG PS
(OR, 8.998; 95% CI, 4.275–18.938; P=0.000) and AFP (OR, 2.260; 95%
CI, 1.174–4.352; P= 0.015) were independently associated with
reduced OS (Fig. 3).
Effects of local therapy on PFS, OS and
safety
In terms of clinical efficacy, local treatment in
combination with sorafenib was superior to sorafenib alone. Indeed,
comparing sorafenib plus TACE plus cryoablation versus sorafenib
plus TACE versus sorafenib alone, we found significant differences
in CBR (80.0 vs. 73.7 vs. 31.3%; P=0.000), PFS (6.0 vs. 6.0 vs. 3.0
months; P=0.000) and OS (12.7 vs. 12.0 vs. 8.0 months; P=0.000)
among the three groups. However, the use of cryoablation yielded
only slight numerical increases in CBR and OS compared with
sorafenib plus TACE.
Continuation of sorafenib in a subset of
patients with radiological PD improves OS
At the end of follow-up, disease progression
occurred in 100 patients. In 42 patients, therapy of sorafenib was
discontinued due to new lesions or concomitant clinical
deterioration, but 58 patients with continuing clinically stable
presentation continued to receive sorafenib despite disease
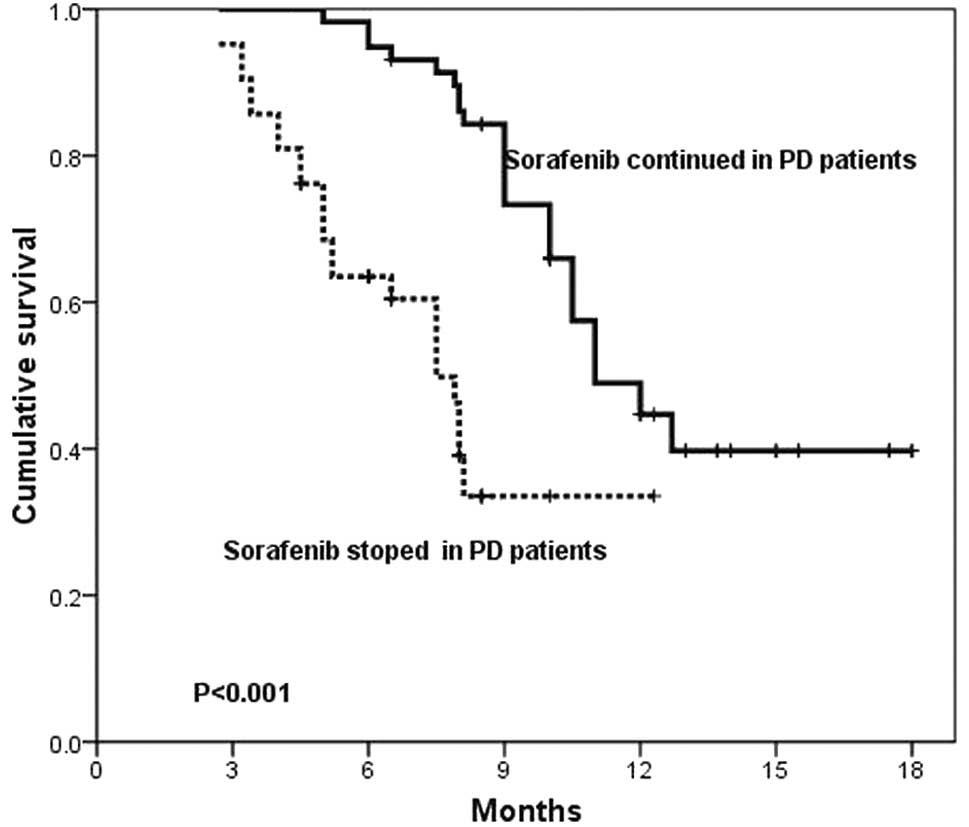
progression. There was a marked difference in OS between the
patients who continued to take sorafenib and those who discontinued
therapy (11 vs. 7.5 months, P<0.001; Fig. 4).
Discussion
Sorafenib has created a new era for advanced HCC
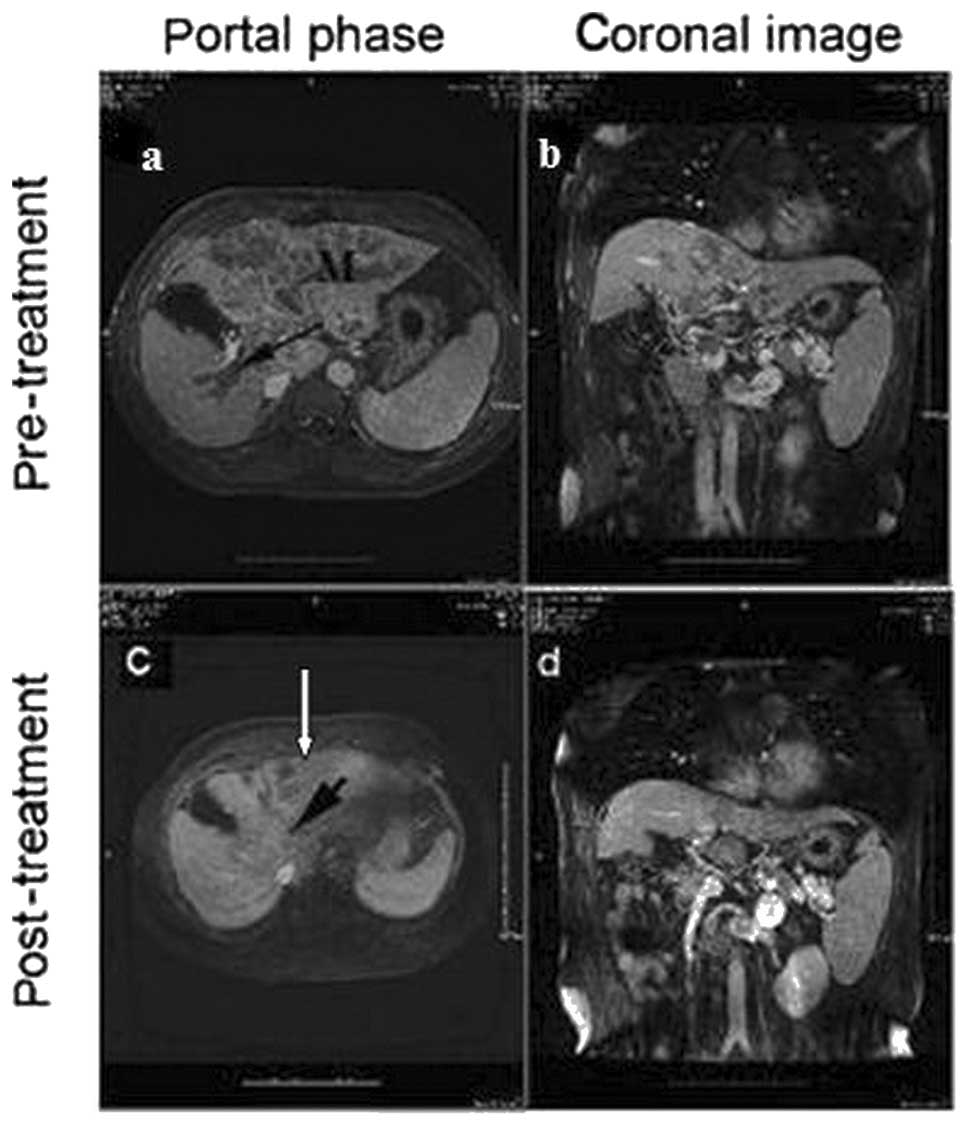
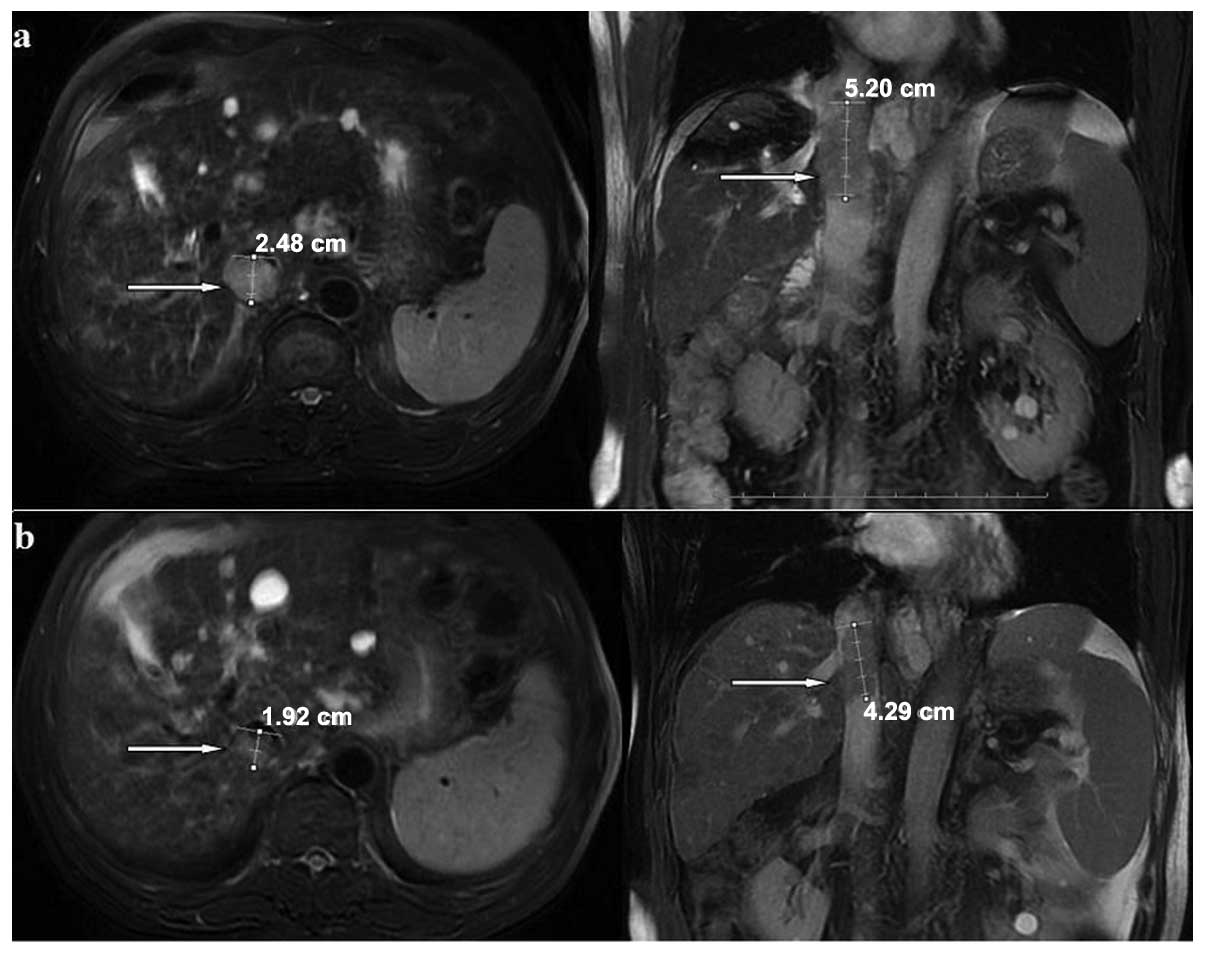
therapy. In the present study, 14 (12.7%) patients achieved CR
(Fig. 5), 16 (14.5%) achieved PR
(Fig. 6) and 54 (49.1%) had SD,
according to Modified RECIST (mRECIST) Assessment for
Hepatocellular Carcinoma. The median OS and PFS were 10.5 and 5.0
months, respectively. These findings are encouraging and similar to
those of other studies (7,10,18),
although our patients had a poorer prognosis than those of the
aforementioned studies due to the presence of advanced tumors
(including PVT). For comparison, in two previous studies the median
OS of patients with similar advanced tumors was 4 months (19,20).
Notably, compared with studies with similar populations of
patients, our results are superior to those of other studies
(8,11). For example, in the Asia-Pacific
study, the median OS and PFS were 6.5 and 2.8 months, respectively,
although this is unsurprising as patients in that study had poorer
PS, with 74% having ECOG PS ≥1, and more advanced cancer, with 96%
at BCLC stage C (8). In the study
by Yau et al (11), the
median OS and PFS were 5 and 3 months, respectively; in their
cohort, 47% of patients had major vessel invasion, 39% had lung
metastasis and 29% were Child-Pugh class B or C. Nevertheless, the
10.5-month OS and 5-month PFS achieved in patients with PVT in the
current study are impressive. These encouraging results are at
least partly due to the use of local therapy, as 70.9% (78/110) of
patients received sorafenib in combination with local therapy (TACE
or cryoablation).
There is a strong theoretical rationale for
combining sorafenib with local therapy. Sorafenib is able to
prolong survival in advanced HCC patients, but sorafenib
monotherapy rarely elicits HCC shrinkage (18). Furthermore, a high tumor load may
render the patients refractory to sorafenib (11). TACE has been widely used for
non-surgical HCC patients (21),
but residual tissue at the margin of treatment and tumor
progression or metastasis following TACE remain limiting factors
(22,23). The upregulation of angiogenic
factors in surviving tumor cells following TACE is associated with
tumor growth and invasiveness (24,25).
Percutaneous cryoablation offers a promising treatment modality for
HCC due to the ability to ablate larger zones than other ablation
procedures (16,26,27).
In this context, we believe that the combination of sorafenib with
local therapies offers several advantages. First, local therapy is
able to reduce the tumor load to increase the efficacy of
sorafenib. Second, sorafenib-mediated blockade of the Raf/MAPK and
VEGFR pathways may enhance the efficacy of local therapy if
sorafenib is continued during and following TACE or cryoablation.
Third, TACE plus cryoablation promotes necrosis of the treated
tumor. In mice with implanted renal tumors, the efficacy of
radiofrequency ablation in combination with sorafenib on tumor
ablation increased in a sorafenib dose-dependent manner (28). In our study, the combination of
sorafenib plus local therapy was an independent predictor of PFS
and OS, resulting in marked survival benefits compared with
sorafenib alone. Meanwhile, although there were no significant
differences between sorafenib plus TACE plus cryoablation versus
sorafenib plus TACE in terms of CBR (P= 0.639), PFS (P=0.198) or OS
(P=0.588), the use of cryoablation did provide slight improvements
in these parameters. The clinical benefits may be due to a greater
reduction of tumor burden by cryoablation, based on prior studies
of local ablation combined with TACE (29). As this study had a relatively small
number of subjects, further prospective studies with a larger
number of patients are needed to confirm that cryoablation improves
the prognosis of patients with HCC when used in combination with
sorafenib and TACE.
Other than the benefits of combined therapy, one
aspect of this study may also contribute to prolonged survival. In
the majority of previous studies, sorafenib was discontinued upon
tumor progression. In the SHARP trial (7), the median survival time after disease
progression was 5.2 months. By contrast, in a Japanese phase I
study (18) of sorafenib, the
median TTP was 4.9 months, while median OS was 15.6 months. In the
study by Yau et al (11),
OS was substantially longer compared with their historical cohort,
even in patients who did not demonstrate any clinical benefits with
sorafenib. Wörns et al (30) reported that radiological disease
stabilization (PR and SD) was achieved in 50% of patients after a
median of 3.2 months, or stable clinical presentation was obtained
in a subset of patients with radiological PD leading to the
continuation of therapy. These results suggest that even patients
lacking demonstrable clinical benefits of sorafenib may gain some
survival benefit from the treatment. This is a phenomenon that has
also been observed using molecular-targeted agents for the
treatment of other types of solid tumors (31). If radiological progression criteria
are applied, sorafenib would be discontinued after 3–4 months in a
number of patients, possibly denying these patients the opportunity
for further clinical benefits and prolonging OS. We believe that
continuing sorafenib therapy following radiological progression is
justified in patients with a stable clinical presentation.
Therefore, sorafenib was continued in 58 patients despite disease
progression. Our results show that continuing sorafenib therapy in
patients with PD improved OS. Therefore, we argue that a sudden
stop of sorafenib treatment in advanced HCC patients may promote
tumor progression to a certain extent. It will be of interest to
further investigate the issue in future studies.
Multivariate analysis revealed that poor ECOG PS
predicted poor PFS and OS, which is consistent with the results of
previous studies (32,33). In the current study, none of the
patients had an ECOG PS greater than 2 or a Child-Pugh class worse
than B. Ideally, the tumor control rate increases with sorafenib
dose and the completion of local treatment. Patients with a better
PS had the opportunity for sorafenib maintenance therapy and
successful local treatment due to the acceptable adverse
effects.
Traditional prognostic factors, including tumor
number, tumor differentiation and PVT within the branch or trunk,
were not found to be associated with survival. Although tumor size
was significantly associated with OS based on the log-rank test,
which suggests that tumor load might be a mechanism involved in
refraction to sorafenib, it was excluded from multivariate
analysis. Similar to the results of previous studies, Child-Pugh
class and AFP were independently associated with PFS and OS,
respectively. The precise mechanism by which AFP influences
prognosis remains unclear. However, several studies have reported
that AFP is a novel protein-binding partner for caspase-3, blocks
the apoptotic signaling pathway and promotes the growth of human
hepatoma cells as a co-repressor in RA-RAR signaling (34,35).
The major limitation of the current study is its
nonrandomized design and that patients with a prior history of
treatment were excluded. Considering that patients with complete
PVT always have poor liver function (Child-Pugh class C) and an
expected survival time of less than 3 months, these patients were
also excluded.
In conclusion, taking into account the limitations
of the study, our results provide further evidence to show that
poor ECOG PS is associated with poor prognosis of sorafenib therapy
with/without local therapy for HCC. On the other hand, the use of
local therapy (TACE with/without cryoablation) improved the
prognosis of sorafenib therapy for HCC. The safety and efficacy of
sorafenib in combination with local therapies, including TACE or
local ablation, should be confirmed in well-designed, prospective
clinical studies.
Acknowledgements
This study was supported by grants
from the Key Scientific and Technological Research Foundation of
the National Special-Purpose Program (2008ZX10002-018) and the
Capital Medical Research and Development Fund (2007-1021,
2009-2041).
References
|
1.
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
2.
|
Yang HI, Lu SN, Liaw YF, You SL, Sun CA,
Wang LY, Hsiao CK, Chen PJ, Chen DS and Chen CJ; Taiwan
Community-Based Cancer Screening Project Group: Hepatitis B e
antigen and the risk of hepatocellular carcinoma. N Engl J Med.
347:168–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
4.
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, et al
Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: a randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar
|
|
5.
|
Palmer DH, Hussain SA and Johnson PJ:
Systemic therapies for hepatocellular carcinoma. Expert Opin Invest
Drugs. 13:1555–1568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: a phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar
|
|
9.
|
Furuse J: Sorafenib for the treatment of
unresectable hepato-cellular carcinoma. Biologics. 2:779–788.
2008.PubMed/NCBI
|
|
10.
|
Abou-Alfa GK, Letourneau R, Harker G,
Modiano M, Hurwitz H, Tchekmedyian NS, Feit K, Ackerman J, De Jager
RL, Eckhardt SG and O'Reilly EM: Randomized phase III study of
exatecan and gemcitabine compared with gemcitabine alone in
untreated advanced pancreatic cancer. J Clin Oncol. 24:4441–4447.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yau T, Chan P, Ng KK, Chok SH, Cheung TT,
Fan ST and Poon RT: Phase 2 open-label study of single-agent
sorafenib in treating advanced hepatocellular carcinoma in a
hepatitis B-endemic Asian population: presence of lung metastasis
predicts poor response. Cancer. 115:428–436. 2009. View Article : Google Scholar
|
|
12.
|
Vincenzi B, Santini D, Russo A, Addeo R,
Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M,
et al: Early skin toxicity as a predictive factor for tumor control
in hepatocellular carcinoma patients treated with sorafenib.
Oncologist. 15:85–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L and Colombo
M: Clinical management of hepatocellular carcinoma. Conclusions of
the Barcelona-2000 EASL conference. European Association for the
Study of the Liver. J Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: the BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar
|
|
16.
|
Wang C, Lu Y, Chen Y, Feng Y, An L, Wang
X, Su S, Bai W, Zhou L, Yang Y and Xu D: Prognostic factors and
recurrence of hepatitis B-related hepatocellular carcinoma after
argon-helium cryoablation: a prospective study. Clin Exp
Metastasis. 26:839–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Furuse J, Ishii H, Nakachi K, Suzuki E,
Shimizu S and Nakajima K: Phase I study of sorafenib in Japanese
patients with hepatocellular carcinoma. Cancer Sci. 99:159–165.
2008.PubMed/NCBI
|
|
19.
|
Rabe C, Pilz T, Klostermann C, Berna M,
Schild HH, Sauerbruch T and Caselmann WH: Clinical characteristics
and outcome of a cohort of 101 patients with hepatocellular
carcinoma. World J Gastroenterol. 7:208–215. 2001.PubMed/NCBI
|
|
20.
|
Han KH, Seong J, Kim JK, Ahn SH, Lee DY
and Chon CY: Pilot clinical trial of localized concurrent
chemoradiation therapy for locally advanced hepatocellular
carcinoma with portal vein thrombosis. Cancer. 113:995–1003. 2008.
View Article : Google Scholar
|
|
21.
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
22.
|
Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY and
Mack P: Experience with liver resection after hepatic arterial
chemoembolization for hepatocellular carcinoma. Cancer. 71:62–65.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kim YB, Park YN and Park C: Increased
proliferation activities of vascular endothelial cells and tumour
cells in residual hepato-cellular carcinoma following transcatheter
arterial embolization. Histopathology. 38:160–166. 2001. View Article : Google Scholar
|
|
24.
|
Poon RT, Ng IO, Lau C, Yu WC, Fan ST and
Wong J: Correlation of serum basic fibroblast growth factor levels
with clinicopathologic features and postoperative recurrence in
hepatocellular carcinoma. Am J Surg. 182:298–304. 2001. View Article : Google Scholar
|
|
25.
|
Ng IO, Poon RT, Lee JM, Fan ST, Ng M and
Tso WK: Microvessel density, vascular endothelial growth factor and
its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J
Clin Pathol. 116:838–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hinshaw JL and Lee FT Jr: Cryoablation for
liver cancer. Tech Vasc Interv Radiol. 10:47–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yang Y, Wang C, Lu Y, Bai W, An L, Qu J,
Gao X, Chen Y, Zhou L, Wu Y, et al: Outcomes of ultrasound-guided
percutaneous argon-helium cryoablation of hepatocellular carcinoma.
J Hepatobiliary Pancreat Sci. Dec 21–2011.(E-pub ahead of
print).
|
|
28.
|
Hakimé A, Hines-Peralta A, Peddi H, Atkins
MB, Sukhatme VP, Signoretti S, Regan M and Goldberg SN: Combination
of radiofrequency ablation with antiangiogenic therapy for tumor
ablation efficacy: study in mice. Radiology. 244:464–470.
2007.PubMed/NCBI
|
|
29.
|
Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL,
Zhang ZL and Yi CH: Chemoembolization combined with radiofrequency
ablation for patients with hepatocellular carcinoma larger than 3
cm: a randomized controlled trial. JAMA. 299:1669–1677. 2008.
View Article : Google Scholar
|
|
30.
|
Wörns MA, Weinmann A, Pfingst K,
Schulte-Sasse C, Messow CM, Schulze-Bergkamen H, Teufel A,
Schuchmann M, Kanzler S, Düber C, et al: Safety and efficacy of
sorafenib in patients with advanced hepatocellular carcinoma in
consideration of concomitant stage of liver cirrhosis. J Clin
Gastroenterol. 43:489–495. 2009.PubMed/NCBI
|
|
31.
|
Grothey A, Hedrick EE, Mass RD, Sarkar S,
Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM and Sargent DJ:
Response-independent survival benefit in metastatic colorectal
cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol.
26:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Si MS, Amersi F, Golish SR, Ortiz JA, Zaky
J, Finklestein D, Busuttil RW and Imagawa DK: Prevalence of
metastases in hepatocellular carcinoma: risk factors and impact on
survival. Am Surg. 69:879–885. 2003.PubMed/NCBI
|
|
34.
|
Li M, Li H, Li C, Guo L, Liu H, Zhou S,
Liu X, Chen Z, Shi S, Wei J, et al: Cytoplasmic alpha-fetoprotein
functions as a co-repressor in RA-RAR signaling to promote the
growth of human hepatoma Bel 7402 cells. Cancer Lett. 285:190–199.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Li M, Li H, Li C, Zhou S, Guo L, Liu H,
Jiang W, Liu X, Li P, McNutt MA and Li G: Alpha fetoprotein is a
novel protein-binding partner for caspase-3 and blocks the
apoptotic signaling pathway in human hepatoma cells. Int J Cancer.
124:2845–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|