Introduction
Although surgery-based comprehensive therapy has
greatly improved the treatment of gliomas, the prognosis of
high-level gliomas remains poor (1–3).
There is little evidence addressing the reasons for glioma
recurrence following extensive tumor resection and post-surgical
sequential treatments, such as radiotherapy and chemotherapy
(4). The tumor stem cell theory
has provided novel hypotheses for the further understanding of
gliomas and related treatments.
According to the tumor stem cell theory, the
majority of tumor cells are not tumorigenic; rather it is the tumor
stem cells that determine tumor occurrence, development, metastasis
and recurrence. Existing conventional treatments fail to diminish
tumor stem cell numbers or functions and thus tumor recurrence is
common (5–8). Tumor stem cells may be present in
other locations far from the clinical lesions, and may form tumors
through migration and seeding. Thus, they are difficult to detect
and remove by conventional surgical resection. Tumor stem cells may
also possess the capacity for immune escape (9) which may be significant in the
establishment of tumor microcirculation (10). An understanding of the
characteristics of tumor stem cells and the mechanisms associated
with their ‘escape’ from clinical therapy is required, in
conjunction with the use of new treatments based on the clearance
and intervention of tumor stem cells, to advance the treatment of
glioma.
In 2003, Singh et al were the first to
identify a cell subpopulation with an unlimited proliferative
potential and specific differentiation potential in medulloblastoma
and glioblastoma multiforme. These cells possessed different
molecular genetics and cell biology characteristics compared with
common brain tumor cells since they expressed neural stem cell
markers, such as nestin, Musashi-1, Bmi-1 and CD133. Additionally,
these cells had an increased self-renewal and proliferation ability
compared with neural stem cells (11). These cells were able to
differentiate into tumor cells with the same phenotype as the
original tumor in vitro and form tumors in vivo
following transplantation. Tumor stem cells in the brain are
resistant to radiotherapy and chemotherapy and consequently an
increasing amount of glioma stem cell research has been carried
out. However, there is controversy with regard to the sorting and
identification criteria of brain tumor stem cells. For example,
nestin was considered to be a specific brain tumor stem cell marker
(12) but was later found to be
expressed in progenitor cells during differentiation (13). CD133 is a transmembrane protein
with a relative molecular weight of 120,000 Da and was initially
used as a hematopoietic stem cell marker. CD133 is a common marker
in neural stem cells, rather than a specific marker of brain tumor
stem cells (14). However, CD133
may be useful for identifying brain tumor stem cell-specific
markers and is considered to be important for the separation and
purification of brain tumor stem cells, as well as in tumor
development and prognosis.
In the present study, we aimed to conduct
CD133+/− cell selection in the glioblastoma tissues of 8
individuals from Northern China and to analyze the biological
characteristics of the two cell subtypes through in vivo and
in vitro observations. The results verified the high
tumorigenicity and invasiveness of CD133+ tumor cells
and demonstrated the limitations and deficiencies of using CD133
alone as a marker to distinguish tumor stem cells.
Materials and methods
Tissue specimens
Eight samples of glioblastoma tissue were obtained
from patients admitted to the Department of Neurosurgery at the
Fifth Central Hospital of Tianjin (Tianjin, China) between August
2009 and September 2011. All patients were Han Chinese individuals
from the Beijing, Tianjin, Hebei Province and Shandong Province
regions of Northern China. All patients and their family members
agreed and signed a consent to enroll in the study.
Tumor tissue digestion and detection of
the CD133+ cell percentage
Glioblastoma blocks were rinsed twice with D-Hank’s
solution on an ultraclean table and the tissue block was sheared
into paste after vessels and necrotic tissue were eliminated. The
paste was then digested with 0.25% trypsin (Invitrogen, Carlsbad,
CA, USA) and cells were pipetted into a single-cell suspension.
Digestion was terminated using 10% fetal bovine serum (Hangzhou
Sijiqing Biological Materials Co., Ltd., Hangzhou, China). Tissues
were filtered through a 30-μm mesh and centrifuged at 1,000
rpm for 5 min. Following the removal of the supernatant, cells were
collected and counted. Cells (1×106) were suspended in
100 μl phosphate-buffered saline (PBS), stained with
isothiocyanate-labeled anti-CD133 antibody (Chemicon, Temecula, CA,
USA) for 30 min in darkness and re-suspended with 1%
paraformaldehyde (600 μl). The percentage of
CD133+ cells was analyzed using flow cytometry.
Immunomagnetic separation of CD133
glioblastoma cells
Cells were suspended with PBE incubation solution
(0.5% bovine serum albumin, 0.08% EDTA in PBS, pH 7.2) to a final
concentration of 1×108 cells in 0.5 ml, then incubated
with anti-CD133 antibody (final antibody concentration 20
μg/ml) at 4°C for 30 min and incubated with antibody-coated
superfine magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany) at 10°C for 15 min and suspended in 20 times the total
volume of PBE solution. The separation column was installed into a
magnetic field and pretreated with 0.5 ml PBE which was naturally
eluted due to gravity. The incubated cell suspension was added to
the separation column and naturally eluted Then 0.5 ml PBE was
added to the separation column and naturally eluted. The column was
rinsed twice and then separated from the magnetic field. The column
was subsequently inserted into a new tube and 1–2 ml PBE was
administered along the needle core to remove the CD133-positive
cells. Simultaneously, negative cells were collected and the two
types of cells were rinsed with medium.
Immunocytofluorescence assay
CD133+ and CD133− cells were
prepared on cell slides and the coverslip was pre-coated with
polylysine. Immunocytofluorescence was performed 12 h later to
determine the expression levels of surface neural stem cell nestin
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), glial cell
glial fibrillary acidic protein (GFAP, Chemicon) and
neuron-specific enolase (NSE, Chemicon). Immunocytofluorescence
procedures were performed as follows. Tissues were fixed with 4%
paraformaldehyde at room temperature for 30 min, treated with 1%
Triton X-100, washed with PBS for 3 min 3 times and blocked with
goat serum for 30 min. After the serum was removed, cells were
incubated with the appropriate dilution of antibody in a wet box at
4°C overnight. Cells were then incubated with FITC- or
TRITC-labeled antibodies (1:100) in a wet box at 37°C for 45 min,
followed by rinsing with PBS and deionized water. Sections were
then mounted with Vectashield sealing agent and observed under a
fluorescence microscope. Antibody dilutions were as follows: nestin
(1:200), GFAP (1:500) and NSE (1:100).
Measurement of cell invasiveness
Transwell (Chemicon, Temecula, CA, USA) upper
chambers were pre-coated with a matrix adhesive (Sigma, St. Louis,
MO, USA) and CD133+ and CD133− glioblastoma
cells were incubated in the upper chamber at a density of
1×104 cells/well with each type of cell repeated in
eight wells. Each well in the lower chamber was cultured with 600
μl DMEM containing 10% fetal bovine serum. After 48 h, the
upper chamber was removed and the non-adherent cells on the
membrane surface were wiped away with a wet cotton swab. Following
hematoxylin staining, tissue was mounted at 80°C and dried. The
adherent cells on the membrane were observed under an inverted
microscope.
Measurement of tumor cell serum
dependence
CD133+ or CD133− glioblastoma
cells were cultured in conditioned DMEM containing 10% fetal bovine
serum and eight wells out of a 96-well culture dish were selected
for trypan blue staining. The average number of living cells and
the percentage of the total number of cells was calculated using
light microscopy.
Cell cloning
A 6-well plate was pre-coated with 0.7% agar
(Zhongshan Biocorp.) and allowed to coagulate. Low-melting agarose
(Zhongshan Biocorp.) working fluid at a final concentration of
0.35% was mixed with CD133+ and CD133−
glioblastoma cell suspensions and then added into a 6-well plate.
Cells were coagulated in an incubator overnight and the following
day 200 μl full medium was added to each well. Three weeks
later, 10 fields of vision were randomly selected under an inverted
microscope and if there were >40 clones in these fields, the
mean number of cells was calculated.
Subcutaneous cell transplantation
experiments in nude mice
A total of 60 C57BL/6 male thymectomized mice,
weighing 80 g, were purchased from the Animal Breeding Center of
the Academy of Military Medical Sciences of Chinese PLA, and
maintained at the Animal Experiment Center of Tianjin Medical
University, at 20–25°C and in 50±5% humidity (lot no. Beijing
0195). All experimental procedures were carried out according to
the regulations and internal biosafety and bioethics guidelines of
Tianjin Medical University and the Tianjin Municipal Science and
Technology Commission. CD133+ and CD133−
glioblastoma cells were adjusted to a final concentration of
4×1010 cells/l. Mice were divided into two groups of 30
mice. The area between the right leg and abdominal cavity in the
nude mice was disinfected with iodine and a single cell suspension
was injected into the mice subcutaneously using a 50-μl
micro syringe. The needle was held in place for 1 min and then
gradually withdrawn to prevent liquid return. After inoculation,
mice were housed in a sterile barrier system at constant
temperature (25±2°C) and humidity (45–50%). Tumor formation and
growth were observed daily.
Tumor tissue sampling and
preparation
CD133+ and CD133− glioblastoma
cell-induced subcutaneous tumors were observed in 26 and 2 nude
mice, respectively, and extracted on a super-clean table. Tumor
tissue was divided into four sections of varying volumes according
to the requirements of subsequent experiments, and used for frozen
sections, protein extraction, digestion for preparation of a single
cell suspension and tumor transplantation.
In situ apoptosis analysis
Frozen sections were washed with PBS for 5 min
twice, soaked in a semipermeable membrane for 5 min, and incubated
with TUNEL (Beijing Zhongshan, China) labeling reaction mixture (25
μl) in a wet box at 37°C for 60 min and then rinsed with PBS
for 5 min 3 times. After the PBS was removed, each section was
incubated with Hoechst 33258 (1:1,000; Santa Cruz) to counterstain
cell nuclei. Cells were incubated in darkness for 10 min and rinsed
with PBS and deionized water, then tissue was mounted with a
fluorescence agent and observed under fluorescence microscopy. The
cell apoptotic rate was calculated according to the following
formula: (number of apoptotic cells/total cell number) × 100%.
Western blot assay
A total of 40 μg lysates were subjected to
SDS-PAGE on 8% SDS-acrylamide gel. Separated proteins were
transferred to PVDF membranes (Millipore, Bedford, MA, USA) and
incubated with primary antibodies against Oct4 (1:1,000; Zhongshan
Bio Corp.), Sox2 (1:1,000; Zhongshan Bio Corp.), PCNA (1:200;
Zhongshan Bio Corp.), EGFR (1:1,000; Santa Cruz Biotechnology,
Inc.), Ang2 (1:500; Chemicon), MMP2 (1:500; Zhongshan Bio Corp.)
and MMP9 (1:200; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
The following day, cells were incubated with horseradish
enzyme-labeled antibody (1:500) at 7°C for 2 h. A chemiluminescence
reagent kit was used to develop the reaction and a Bio-Rad gel
imaging system was used to determine the absorbance value which was
analyzed with Quantity One software.
Flow cytometry
After blood vessels and necrotic tissue in tumors
were eliminated, tumor tissue was sheared into a paste, digested
with 0.25% trypsin and pipetted repeatedly into a single-cell
suspension. The digestion was terminated with 10% fetal bovine
serum, cells were filtered with a 30-μm mesh and centrifuged
at 1,000 rpm for 5 min. Cells were counted following the removal of
the supernatant, and 1×106 cells were suspended in 100
μl PBS, stained with isothiocyanate-labeled anti-CD133
antibody for 30 min in darkness and then resuspended in 600
μl 1% paraformaldehyde. The percentage of CD133+
cells was detected by flow cytometric analysis.
Tumor formation rate following in vivo
transplantation
Tumor tissue was carefully dissected on a superclean
table and fish-shaped tissues were harvested and cut with scissors
into 1-mm3 sections which were stored in PBS for further
use. The neck dorsal skin of nude mice was fixed with the left
thumb and forefinger, the other three fingers fixed the dorsal skin
individually and the little finger was used to fix the left hind
leg of nude mice. A 3-mm incision was cut in the left groin and a
1-mm3 section of tumor tissue was inserted into the
subcutaneous inguinal region to a depth of ∼5 mm. The inoculated
mice were housed in a decontamination sterile barrier system at a
constant temperature (25±2°C) and constant humidity (45–50%) and
tumor formation and growth were recorded daily.
Statistical analysis
Data are expressed as the mean ± SE. Statistics were
determined using analysis of variance, the χ2 test or
the Student’s t-test using SPSS 11.0 software (Windows). P<0.05
or P<0.01 were considered to indicate statistically significant
differences.
Results
Percentage of CD133+ tumor
cells for cell sorting
Flow cytometric analysis showed that the percentage
of CD133+ glioblastoma cells in the total cell number
was 2.31±0.57%, which complied with the immunomagnetic bead sorting
system requirements for cell separation.
Specific marker protein expression
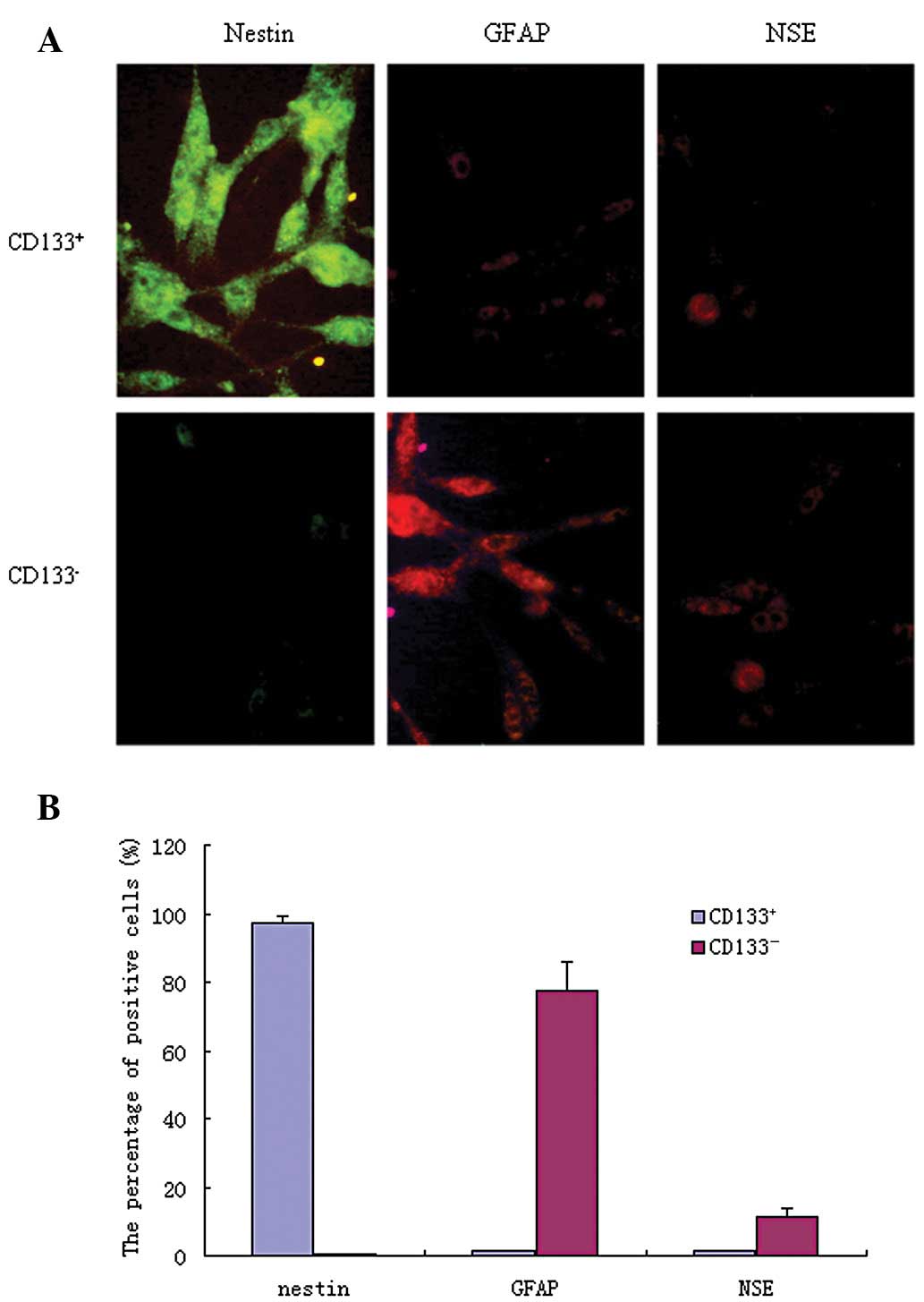
As shown in Fig. 1,
FITC-labeled antibodies appeared as green fluorescence, while
TRITC-labeled antibodies appeared as red fluorescence. In
CD133+ glioblastoma cells, the nestin-positive
expression rate was 97.34±2.14%, GFAP was 1.44±0.27% and NSE was
1.35±0.24%. In CD133− cells, the nestin-positive
expression rate was 0.47±0.06%, GFAP was 77.41±8.49% and NSE was
11.38±2.21%. The expression levels of these molecules were
significantly different between the CD133+ and
CD133− cells (P<0.05).
Cell invasion assay
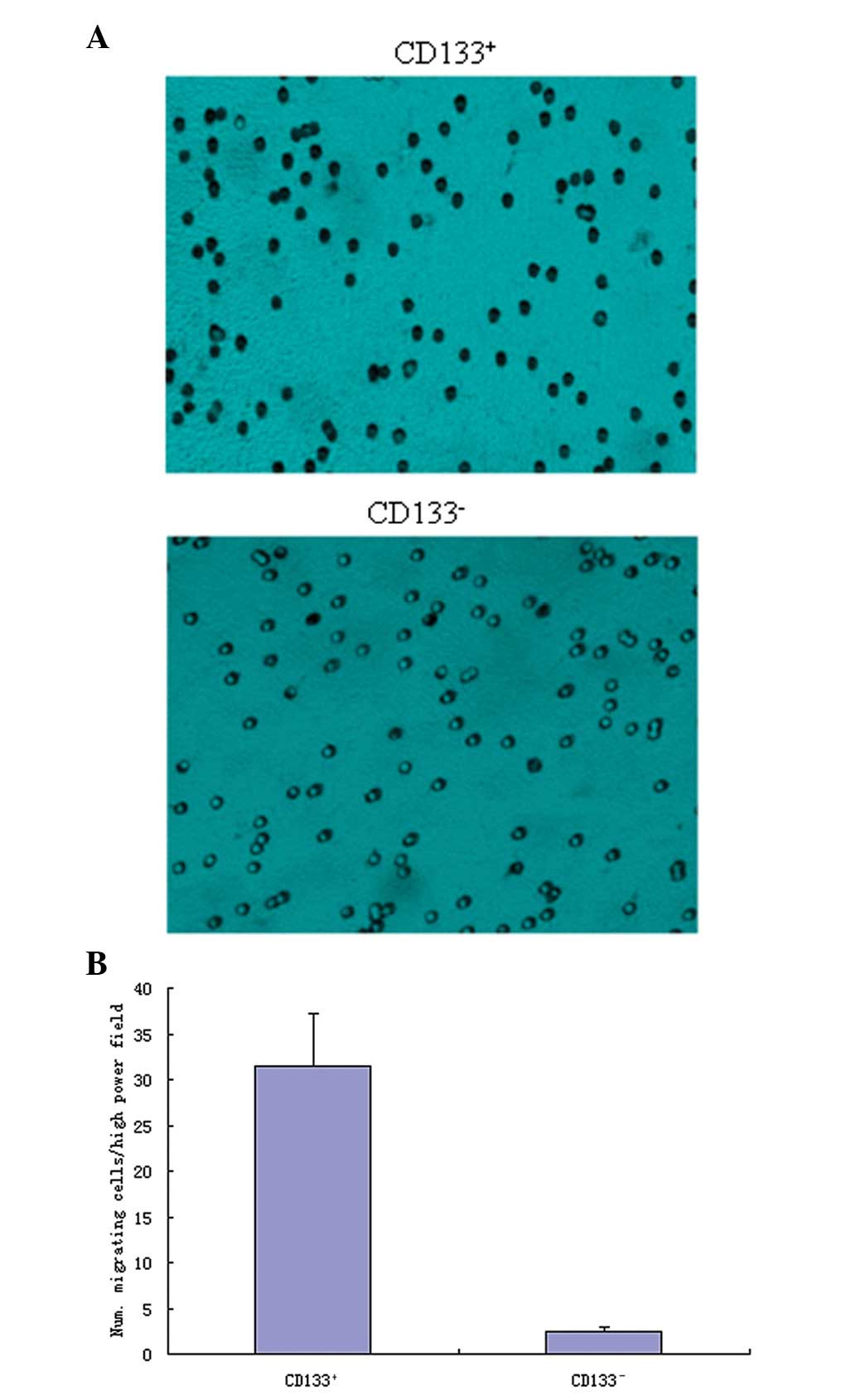
As shown in Fig. 2,
the CD133+ glioblastoma cells showed a high invasive
capacity, and had a significantly higher mean number of invasive
cells in each high-power field (31.46±5.73), compared with the
CD133− cells (2.47±0.53; P<0.05).
Serum dependence of tumor cells
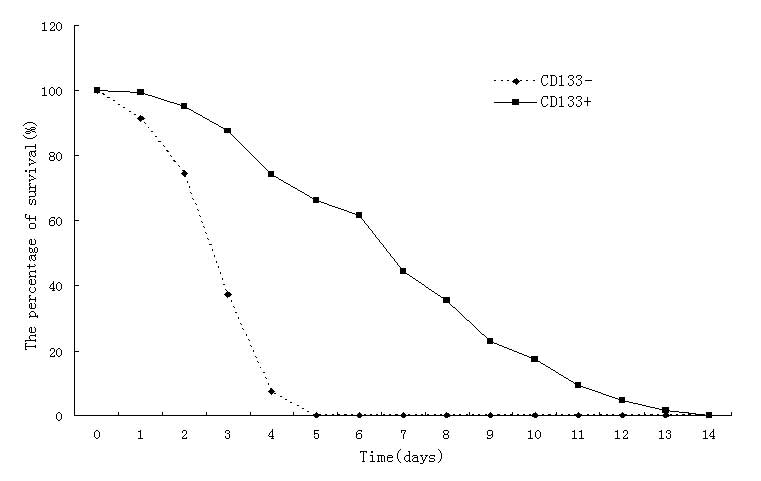
As shown in Fig. 3,
the CD133− glioblastoma cells did not survive beyond 5
days when grown in low serum conditions, while the
CD133+ glioblastoma cells survived for up to 13 days
under the same low serum culture conditions, which indicated a
greater tolerance to a low nutrient environment.
Soft agar colony formation
experiment
As shown in Fig. 4,
10 high-power fields of vision were randomly analyzed and the
results revealed that the mean number of colonies forming >40
cells per high-power field in the CD133+ glioblastoma
cell group was 7.18±1.17 compared with no colony formation in the
CD133− glioblastoma cell group.
Comparison of the in vivo tumor formation
rate
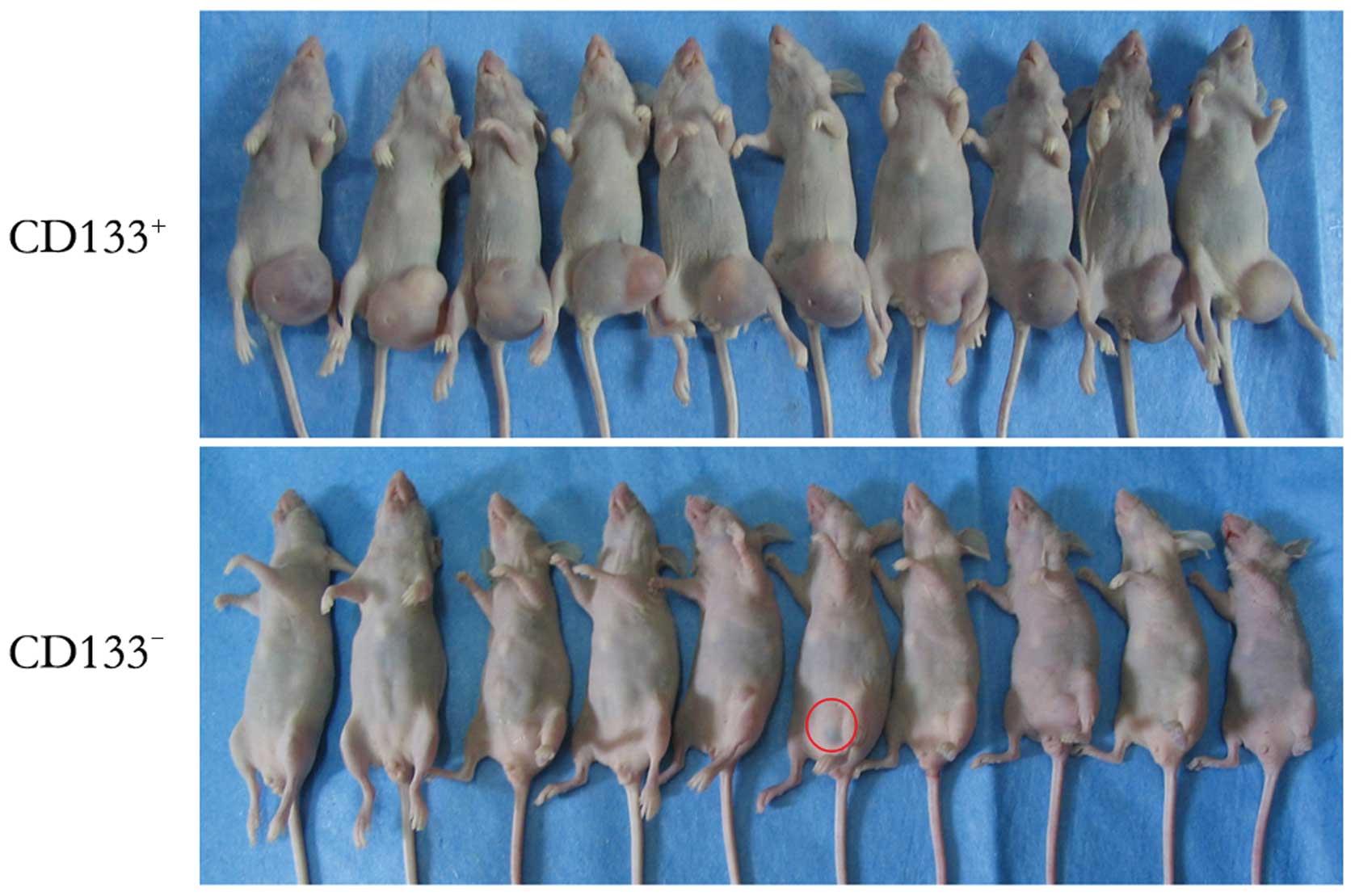
As shown in Fig. 5,
the incidence of subcutaneous tumor formation in the thymectomized
mice receiving CD133+ glioblastoma cells was 26/30 at 28
days post-transfer, compared with 2/30 in the CD133−
glioblastoma cell group. The CD133− induced tumor
volumes were also smaller.
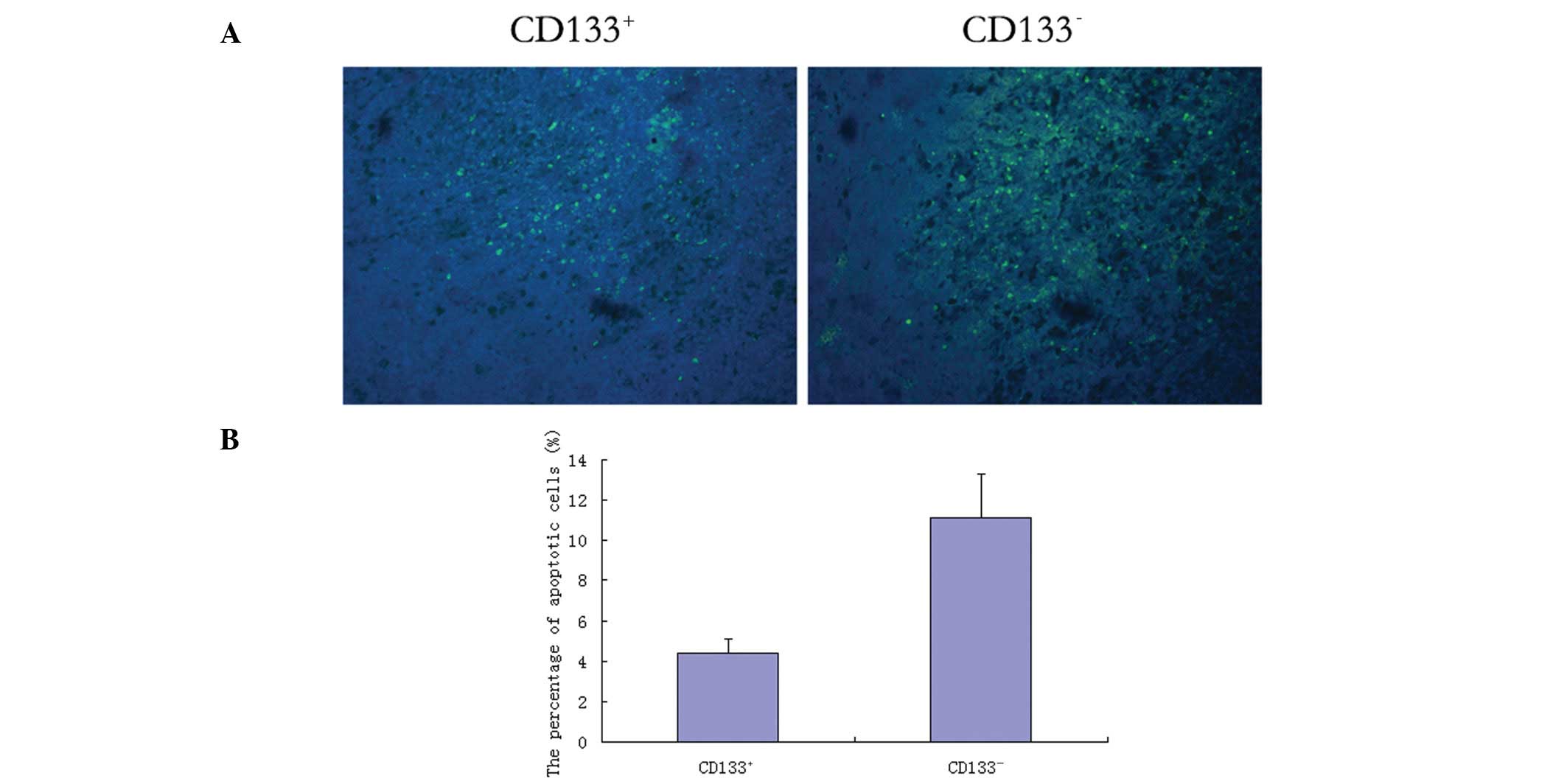
In situ apoptosis
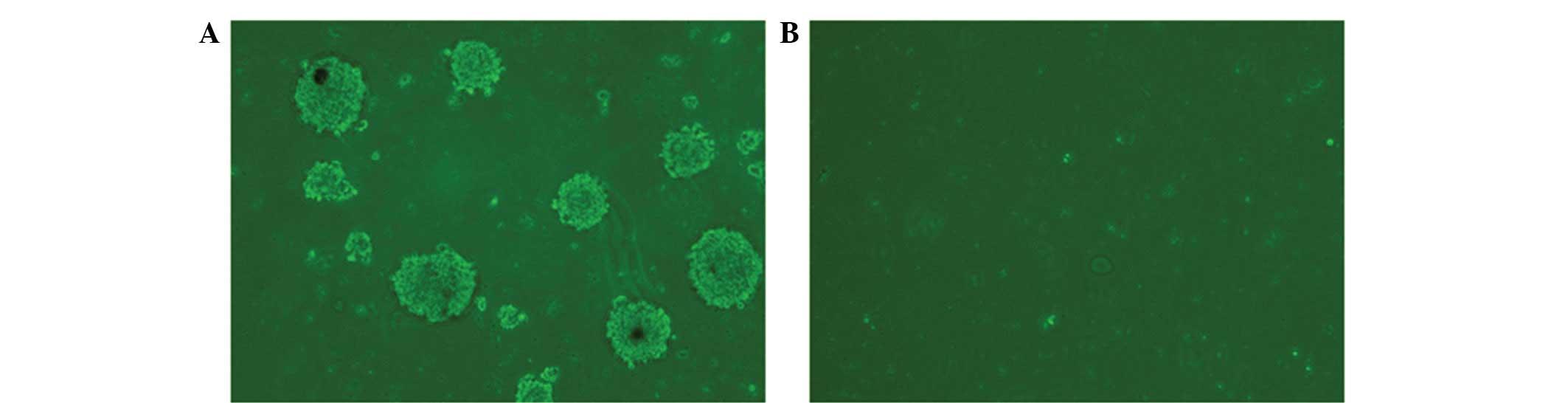
As shown in Fig. 6,
the CD133+ glioblastoma cell-induced tumor tissue
demonstrated scattered apoptotic cells (green stain). In 10
randomly selected high-power fields of vision, the apoptosis rate
of CD133+ cells was significantly lower (4.37±0.74%)
compared with that of CD133− cells (11.14±2.15%;
P<0.05).
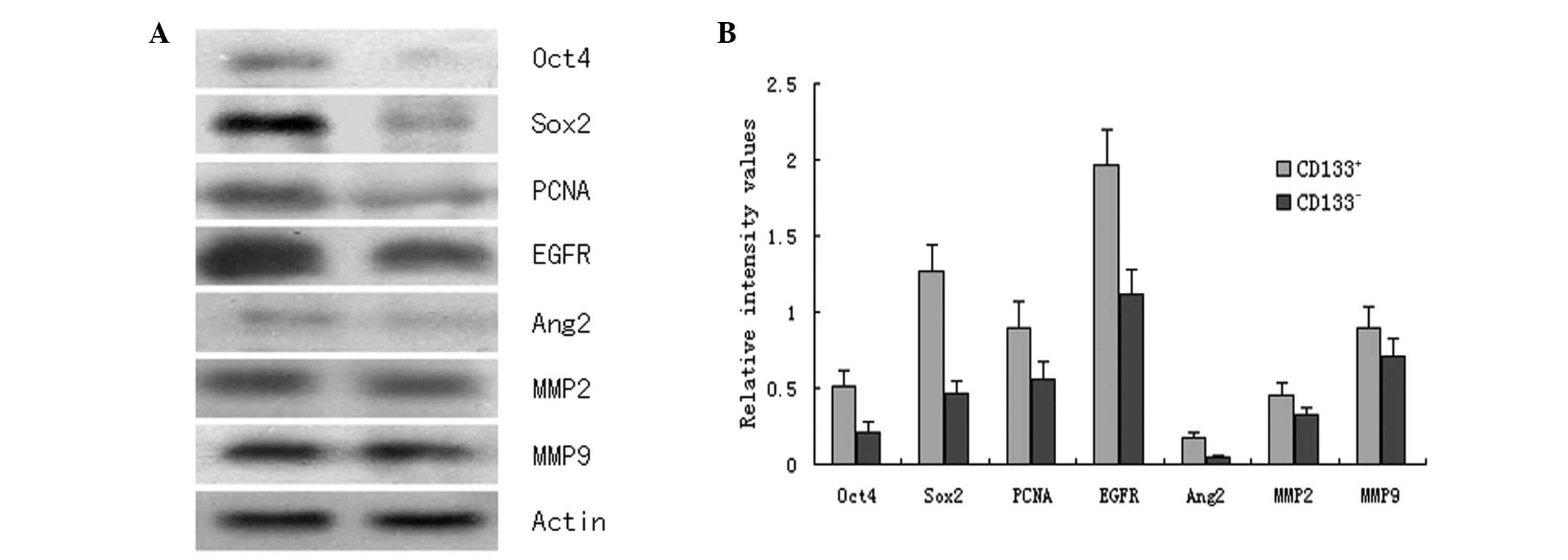
Western blot assay
As shown in Fig. 7,
the CD133+ glioblastoma cell induced-tumor tissue
expressed significantly higher levels of Oct4, Sox2, PCNA, EGFR,
Ang2, MMP2 and MMP9 proteins compared with the CD133−
glioblastoma cell induced-tumor tissue (P<0.05).
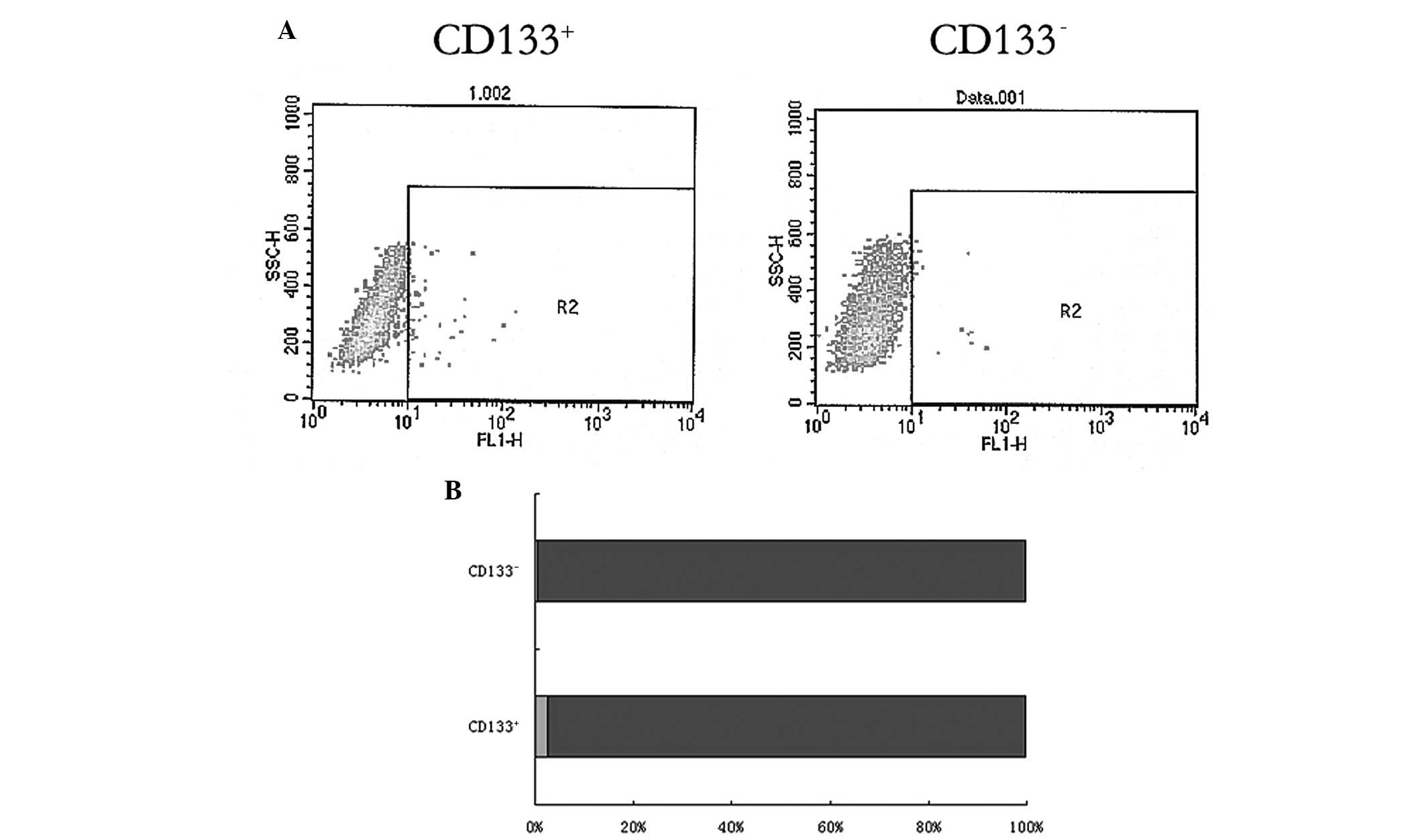
Flow cytometric detection
As shown in Fig. 8,
the R2 gate indicated the CD133+ glioblastoma cells.
Flow cytometry detection showed that in the CD133+
glioblastoma cell-induced tumors, there was a significantly higher
percentage of CD133+ cells (2.47±0.67% of the total
cells) compared with the CD133− glioblastoma
cell-induced tumors (0.44±0.14% of total cells; P<0.05).
In vivo transplantation tumor formation
rate
The tumor formation rate was 10/10 in
CD133+ glioblastoma cell-induced tumors following
transplantation, but only 4/10 in CD133− glioblastoma
cell-induced tumor tissue. CD133− tumor cell-induced
recurrent tumor volumes were also smaller compared with the
CD133+ cell-induced tumors.
Discussion
In the present study, CD133+/− tumor
cells from the glioblastoma cells of 8 Han Chinese patients living
in Northern China were obtained, and the biological characteristics
of the cells were analyzed. Magnetic bead sorting is a commonly
used technique for isolating tumor stem cells. Since
CD133+ cells accounted for 0.3–2% of the total glioma
cells (15–17), a low proportion does not favor
immunomagnetic sorting. In this study, tumor specimens
pathologically identified as type IV glioblastomas were used to
increase the chance of a higher proportion of tumor cells for
sorting. Flow cytometry analysis confirmed that CD133+
cells accounted for 2.31±0.57% of total cells in 8 cases of
glioblastoma, which was consistent with the immunomagnetic bead
sorting system requirements for cell separation. Not all tumor stem
cells expressed CD133+, as the CD133− cell
population also contained some tumor stem cells. However, most
CD133+ cells had high tumorigenicity and invasion
characteristics (18) and
therefore, CD133+ expressing glioblastoma cells were
considered as the observation subject with the aim of further
developing the scope and depth of understanding of glioma stem cell
research.
Nestin is a type VI intermediate filament protein
and its expression is restricted to central nervous system
precursor cells, such as neural and glioma stem cells. Therefore,
nestin may be used as a neural precursor cell marker (19). NSE occurs in mature neurons and may
be used as a surface marker of mature neuronal cells (20). GFAP, a type III intermediate
filament protein family member, is specifically expressed in
astrocytes and thus is often considered to be an astroglial marker
in neurobiological research (21).
This study revealed that CD133+ glioblastoma cells
expressed high levels of nestin but expressed low levels of GFAP
and NSE. By contrast, CD133− glioblastoma cells
expressed low levels of nestin (0.47±0.06%), but had high
expression levels of GFAP (77.41±8.49%) and NSE. This suggested
that the majority of CD133+ glioblastoma cells are
neural precursor cells, which rarely differentiate. Following 24 h
of in vitro culture, CD133− glioblastoma cells
began to exhibit increases in cell body size, cytoplasmic light
stain and a gradual extension of synapses. Immunofluorescence
detected high levels of glial cells and neuron-specific marker
protein expression which indicated differentiation to nerve
cells.
A transwell dual-chamber culture system was used to
detect the invasive capacity of the two types of tumor cells and
revealed that CD133+ glioblastoma cells had higher cell
invasion capacity than CD133− cells. The serum
dependence experiments compares the tolerance of less malignant or
normal cell lines and highly malignant cells, such as tumor cell
lines, to low nutrient conditions and the results are used as an
indicator of survivability and to evaluate the degree of
malignancy. The soft agar cell clone formation experiment was
designed to mimic the semi-solid growth environment of the in
vivo extracellular matrix and to determine in vitro cell
colony growth potential. Tumor cells are capable of proliferating
and show strong cloning ability while mature differentiated cells
do not form colonies (22). The
previous two experiments revealed that CD133+
glioblastoma cells had a greater tolerance to low nutrition and had
a higher colony forming ability compared with CD133−
cells.
In vivo experiments demonstrated a
significantly higher subcutaneous tumor incidence rate in the
CD133+ group (26/30) compared with the CD133−
group (2/30) following inguinal subcutaneous inoculation of
CD133+ or CD133− glioblastoma cells into
thymectomized mice. The in vivo experiments were repeated 3
times, but CD133+ cells did not induce tumors in all
mice. Additionally, subcutaneous tumors formed in two mice
inoculated with CD133− cells. Therefore, the data
suggest that CD133+ and CD133− cells may be
used as markers to distinguish tumor stem cells.
The present study also conducted histopathological
analysis on 26 cases of CD133+ and 2 cases of
CD133− glioblastoma cell-induced subcutaneous tumors.
Firstly, the tumor tissue was analyzed to detect in situ
apoptosis which indicated that CD133+ cell-induced
tumors had a low incidence of apoptosis compared with
CD133− glioblastoma cell-induced tumor cells. Secondly,
quantitative expression of proteins in the two types of tumor
tissues was determined. Oct4 and Sox2 are transcription factors
expressed by stem cells and are involved in self-renewal (23,24).
Oct4 belongs to the POU family, and maintains the undifferentiated
state of embryonic stem cells and promotes their proliferation.
Activation of Oct4 drives the reprogramming of somatic cells to
pluripotent stem cells (25). Sox2
is a specific transcription factor expressed by embryonic stem
cells and, similar to Oct4, is an essential gene for somatic cell
reprogramming. Sox2 acts synergistically with Oct4 to regulate and
maintain cellular pluripotent potential (26). In the present study, the expression
of Oct4, Sox2 and PCNA in CD133+ glioblastoma
cell-induced tumors was significantly higher than in
CD133− cell-induced tumors, indicating that
CD133+ glioblastoma cell-induced tumors expressed higher
levels of transcription factors to maintain pluripotent features
and induce proliferation. EGFR drives the evolution of glioma
malignancy through downstream signaling (27). Ang2 acts as an activator of
multiple tumor migration factors and is important in glioma
angiogenesis, matrix degradation and invasion (28). MMP2 and MMP9 protein expression
levels are key indicators of glioma cell invasion (29,30).
Notably, CD133+ glioblastoma cell-induced tumors
expressed higher levels of EGFR, Ang2, MMP2 and MMP9 proteins
compared with CD133− cell-induced tumors, suggesting
that CD133+ cell-induced tumors have a higher degree of
malignancy. This evidence may explain the low recurrence of tumor
formation following CD133− cell inoculation into mice
and the small tumor volume observed. However, the tumor recurrence
rates following subcutaneous CD133+ and
CD133− cell transplantation were 10/10 and 4/10,
respectively, which were higher than the tumor formation rates
following the initial transfer of tumor cells (26/30 and 2/30
respectively), particularly for the CD133−-induced tumor
cells. This suggests that recurrent tumors may have an increased
degree of malignancy due to genetic change.
In the present study, flow cytometry was used to
identify CD133 antigen-positive expression in tumor cells derived
from two subtypes of cells, and demonstrated that in
CD133+ cell-induced tumors, the percentage of
CD133+ cells was 2.47±0.67%, similar to the
CD133+ percentage obtained from human brain tumors
during the initial surgery (2.31±0.57%). CD133+ tumor
cells were also observed (0.44±0.14%) in the CD133−
cell-induced tumors. Therefore, CD133− cells may
differentiate into CD133+ cells and cells that do not
express CD133 at the initial stages may express CD133 under certain
environmental conditions. Thus, this study suggests that CD133 is
not the only marker protein for the identification of glioma stem
cells.
In summary, we successfully tested the biological
characteristics of CD133+ and CD133−
glioblastoma cells of 8 Han Chinese individuals, verified the high
tumorigenicity and invasiveness of CD133+ tumor cells
and validated the limitations and deficiencies of the CD133 antigen
as a marker to distinguish tumor stem cells. At present, research
addressing human glioma stem cells is in the initial stages and
consequently, it is vital to verify and further elucidate the
methods of glioma stem cell isolation and identification.
Acknowledgements
This study was supported by the China
National Natural Scientific Fund (81000901), the Tianjin Science
and Technology Committee (09JCYBJC09500), Key Laboratory Project of
Tianjin Programs for Science and Technology (10SYSYJC28800), Key
Project of Chinese National Programs for Fundamental Research and
Development (973 Program, 2010CB529405) and Tianjin Health Bureau
Science and Technology Projects (2011KZ24).
References
|
1
|
Gilbert MR: Recurrent glioblastoma: a
fresh look at current therapies and emerging novel approaches.
Semin Oncol. 38(Suppl 4): S21–S33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kesari S: Understanding glioblastoma tumor
biology: the potential to improve current diagnosis and treatments.
Semin Oncol. 38(Suppl 4): S2–S10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borodovsky A, Seltzer MJ and Riggins GJ:
Altered cancer cell metabolism in gliomas with mutant IDH1 or IDH2.
Curr Opin Oncol. 24:83–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navajas A and Giralt J: Evidence in
medulloblastomas. Clin Transl Oncol. 12:271–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pollina EA and Brunet A: Epigenetic
regulation of aging stem cells. Oncogene. 30:3105–3126. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Endersby R and Baker SJ: PTEN signaling in
brain: neuropathology and tumorigenesis. Oncogene. 27:5416–5430.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanchez-Martin M: Brain tumour stem cells:
implications for cancer therapy and regenerative medicine. Curr
Stem Cell Res Ther. 3:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taylor RA and Risbridger GP: Prostatic
tumor stroma: a key player in cancer progression. Curr Cancer Drug
Targets. 8:490–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao XG, Zhang X and Zhen HN: Progress on
potential strategies to target brain tumor stem cells. Cell Mol
Neurobiol. 29:141–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
12
|
Toda M: Therapeutic strategies targeting
brain tumor stem cells. Brain Nerve. 61:799–803. 2009.(In
Japanese).
|
|
13
|
Hide T and Kuratsu J: Progress in the
study of brain tumor stem cells as treatment targets. Brain Nerve.
61:781–789. 2009.(In Japanese).
|
|
14
|
Binello E and Germano IM: Targeting glioma
stem cells: a novel framework for brain tumors. Cancer Sci.
102:1958–1966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicolis SK: Cancer stem cells and
‘stemness’ genes in neuro-oncology. Neurobiol Dis. 25:217–229.
2007.
|
|
16
|
Nakano I and Saya H: Cancer stem cells in
malignant glioma - the mechanism of cancer initiation and the
therapeutic development. No Shinkei Geka. 38:879–889. 2010.(In
Japanese).
|
|
17
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu Q and Shi Y: Neural stem cells in the
developing and adult brains. J Cell Physiol. 221:5–9. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dell’Albani P: Stem cell markers in
gliomas. Neurochem Res. 33:2407–2415. 2008.
|
|
21
|
Pilkington GJ: Cancer stem cells in the
mammalian central nervous system. Cell Prolif. 38:423–433. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Z: Brain tumor stem cells. Neurochem
Res. 34:2055–2066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto N, Tsuchiya H and Hoffman RM:
Tumor imaging with multicolor fluorescent protein expression. Int J
Clin Oncol. 16:84–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun H and Zhang S: Arsenic trioxide
regulates the apoptosis of glioma cell and glioma stem cell via
down-regulation of stem cell marker Sox2. Biochem Biophys Res
Commun. 410:692–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du Z, Jia D, Liu S, et al: Oct4 is
expressed in human gliomas and promotes colony formation in glioma
cells. Glia. 57:724–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferletta M, Caglayan D, Mokvist L, et al:
Forced expression of Sox21 inhibits Sox2 and induces apoptosis in
human glioma cells. Int J Cancer. 129:45–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ischenko I, Seeliger H, Schaffer M, et al:
Cancer stem cells: how can we target them? Curr Med Chem.
15:3171–3184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu XS, Chopp M, Zhang RL, et al:
Angiopoietin 2 mediates the differentiation and migration of neural
progenitor cells in the subventricular zone after stroke. J Biol
Chem. 284:22680–22689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.
|
|
30
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrix metalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofia). 1:44–49. 2010.(In
Bulgarian).
|