Introduction
Survivin is a protein that inhibits apoptosis and
regulates cell division (1).
Vascular endothelial growth factor (VEGF) is a critical
pro-angiogenic factor that induces proliferation and migration of
endothelial cells within cancer vasculature (2). The expression levels of survivin and
VEGF have been implicated to be associated with a wide range of
clinical disorders, including cancer and cardiovascular diseases
(3). For instance, survivin
promotes DNA synthesis through the activation of CDK2/cyclin E and
Rb gene phosphorylation and induces cancer cell proliferation and
migration (4), while VEGF has been
demonstrated to be a significant promoter of tumor neovascularity;
positive associations between tumor VEGF expression and
aggressiveness have been demonstrated in various types of cancer
(5,6).
More recently, interactions of survivin with caspase
and Fas were revealed to be associated with the development of
leukemia (7). Based on these
observations, we hypothesized that survivin and VEGF may be
significant in acute lymphoblastic leukemia. In the current study,
we carried out a systematic analysis of the expression of survivin
and VEGF in patients with acute lymphoblastic leukemia (ALL) and
performed a quantitative analysis of survivin and VEGF to discern
whether they play a significant role in ALL.
Materials and methods
Study design and patients
Between 2011 and 2012, a total of 40 patients with
ALL and 40 healthy controls, matched for age and body mass index
(BMI), were recruited in this study. The selection for these
patients was in accordance with the following criteria: i) a
clinical and bone marrow diagnosis of ALL; ii) not receiving any
chemotherapy, radiotherapy or anticancer drugs; iii) not previously
treated for this disease. Patients who had a family history of
autoimmune diseases or cardiovascular diseases were excluded.
The patients then underwent the following
chemotherapy: idarubicin, 8–10 mg/(m2 per day) for three
days; cytosine arabinoside, 100 mg/(m2 per day) for five
days; and etoposide, 75–100 mg/(m2 per day) for five
days. For comparison purposes, we considered that there were three
groups: the 40 patients prior to treatment were defined as Group A;
these patients following chemotherapy were defined as Group B; and
the healthy controls were defined as Group C. The study was
approved by the ethics committe of The First Affiliated Hospital of
Xinxiang Medical University and informed consent was obtained from
all patients.
Reverse transcription (RT)-PCR
Total RNA was prepared using the Qiagen RNeasy Kit
(Qiagen Inc., Valencia, CA, USA) and RT-PCR was performed using the
SuperScript III One-Step RT-PCR kit (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The specific primer pairs are as follows: for survivin, 5′-GGA CCA
CCG CAT CTC TAC ATT-3′ (forward) and 5′-AGA AGA AAC ACT GGG CCA AGT
C-3′ (reverse); for VEGF, 5′-TTG CTG CTC TAC CTC CAC-3′ (forward)
and 5′-AAT GCT TTC TCC GCT CTG-3′ (reverse); and for β-actin,
5′-GCT CAC CAT GGA TGA TGA TAT C-3′ (forward) and 5′-GCC AGA TTT
TCT CCA TGT CGT C-3′ (reverse).
Western blot analysis
We further obtained 5-ml blood samples from the
patients and healthy controls, mixed the blood (1 ml) with 5X
loading buffer and then incubated the samples at 100°C for 80 min.
All the samples were analyzed using the standard western blot
method according to the manufacturer’s instructions (Biyuntian,
Inc., Haimen, Jiangsu, China). For survivin, VEGF and β-actin
measurement, we used the monoclonal antibody specific for the
respective protein (BioVision, Inc., San Francisco, CA, USA), at a
dilution ratio of 1:1,000.
Determination of survivin and VEGF
concentrations in plasma
After collecting the blood samples, we immediately
placed them into sterile EDTA test tubes and centrifuged at 1,500 ×
g for 20 min at 4°C to collect plasma. The plasma was stored at
−70°C until assayed. The concentrations of survivin and VEGF were
analyzed by enzyme-linked immunosorbent assay (ELISA; Merck
Millipore, Darmstadt, Germany) according to the manufacturer’s
instructions.
Statistical analysis
Statistical analysis was performed using SPSS 12.0
(SPSS Inc., Chicago, IL, USA). All values were expressed as the
mean ± SD. Comparisons were made using the χ2
test or Fisher’s exact test. We also analyzed the correction
between the concentrations of survivin and VEGF using Pearson’s
two-tailed model. P<0.05 was considered to indicate a
statistically significant result.
Results
Survivin and VEGF mRNA expression in
patients with ALL
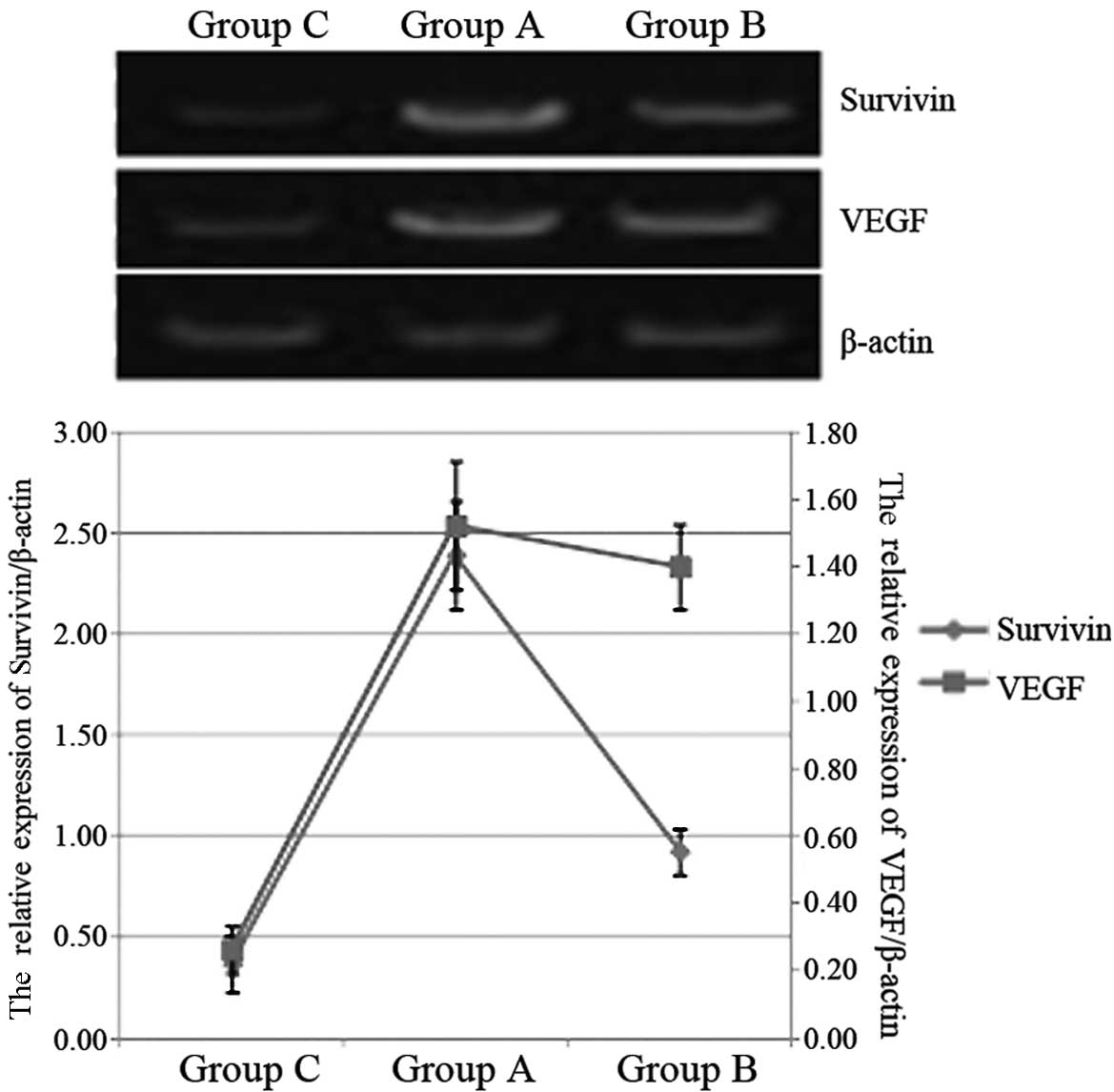
Using an RT-PCR approach, we detected the mRNA
levels of survivin and VEGF in patients with ALL. As shown in
Fig. 1, in Group A, the survivin
and VEGF mRNA levels were significantly higher than those in Group
C (P<0.05), while the level of survivin mRNA in Group A was
significantly lower than that in Group B (P<0.05). Compared with
the VEGF mRNA levels of Groups B and C, that of Group A was not
statistically significantly different (P>0.05).
Expression of survivin and VEGF proteins
in patients with ALL
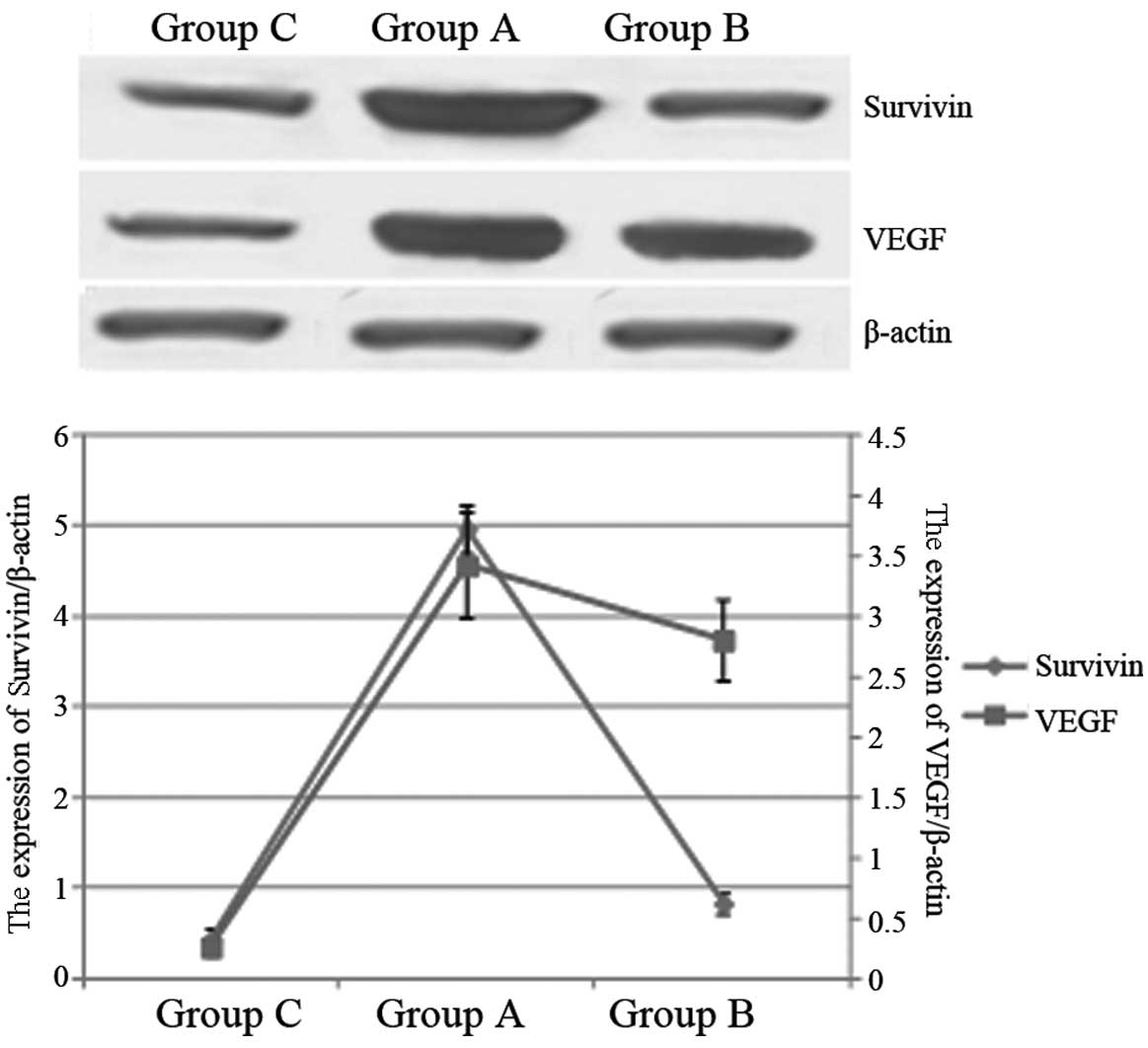
We further used western blotting to analyze the
survivin and VEGF protein expression in these groups. As shown in
Fig. 2, the quantities of survivin
and VEGF protein expressed in Group A were markedly higher than in
Group C (P<0.05). Moreover, the survivin protein expression
level in Group A was significantly lower than that in Group B
(P<0.05), while there was no statistically significant
difference between the VEGF protein expression levels in Groups A
and B (P>0.05).
Correlation between survivin and VEGF
concentrations
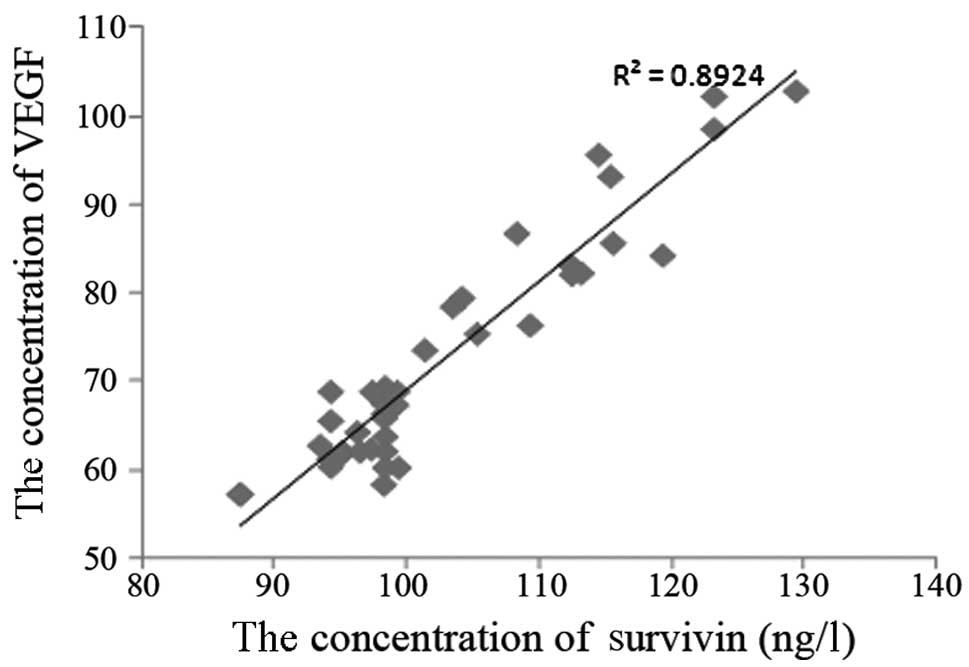
With the purpose of understanding the correlation
between the concentrations of survivin and VEGF in plasma, we
carried out an ELISA to analyze the concentrations. Fig. 3 shows the correlation between
survivin and VEGF concentrations; it is clear that there is a
significant correlation between survivin and VEGF
(R2=0.8924).
Discussion
ALL is the most common pediatric malignancy,
affecting one in four children and adolescents younger than 20
years old. It accounts for 80% of all leukemia cases in children
(8). Of the numerous variables
that influence prognosis, genetic subsets, initial white blood cell
count (WBC), age at diagnosis and early treatment response are the
most significant (9). The clinical
manifestations of ALL always include the symptoms of anemia,
bleeding and organ infiltration. The standard treatment of ALL
includes various phases (induction, consolidation/intensification
and maintenance phases) in which combination chemotherapy, central
nervous system (CNS) sanctuary therapy with intrathecal
chemotherapy and high dose chemotherapy are administered.
Consequently, multimodal therapy and enhanced supportive care have
resulted in 5-year survival rates that approach 90% for those
diagnosed at 14 years of age and younger (9)
Genetic studies have indicated that cytokines or
intracellular transcription factors are involved in the the
pathogenesis of ALL. Of these, survivin, a unique member of the
inhibitor of apoptosis (IAP) protein family, is a cell-cycle
regulator and its expression in cancer has been associated with
cancer progression, drug resistance and shortened patient survival
(10,11). Moreover, there are a growing number
of studies concerning the association between survivin and ALL; Esh
et al(12) reported that
overexpression of survivin is a candidate parameter for determining
poor prognosis in ALL patients, while knockdown of survivin
improved the chemotherapeutic response in ALL models (13). A recent study suggested that
survivin/NF-κB signal transduction pathways may influence leukemia
treatment, while after treatment with drugs, survivin expression
decreased significantly in the HL60/adr cell line (14). This is in accordance with the
results revealed in the present paper. VEGF is a 45-kDa secretary
glycoprotein responsible for endothelial cell differentiation,
migration, proliferation, tubular formation and vessel assembly.
Demacq et al indicated that polymorphisms in VEGF are
associated with high relapse risk in ALL (15). In the present study, we
demonstrated that the ALL patients’ VEGF mRNA expression levels
were much higher than those of the control group. Moreover, we also
identified that survivin and VEGF have a positive correlation by
ELISA, but the molecular mechanism involving survivin and VEGF in
the pathogenesis of ALL remains to be further experimentally
investigated.
References
|
1
|
Altieri DC and Marchisio PC: Survivin
apoptosis: an interloper between cell death and cell proliferation
in cancer. Lab Invest. 79:1327–1333. 1999.PubMed/NCBI
|
|
2
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi J and Chang H: The expression of MAGE
and SSX, and correlation of COX2, VEGF, and survivin in colorectal
cancer. Anticancer Res. 32:559–564. 2012.PubMed/NCBI
|
|
4
|
Suzuki A, Hayashida M, Ito T, Kawano H,
Nakano T, Miura M, Akahane K and Shiraki K: Survivin initiates cell
cycle entry by the competitive interaction with Cdk4/p16(INK4a) and
Cdk2/cyclin E complex activation. Oncogene. 19:3225–3234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
6
|
Toi M, Inada K, Suzuki H and Tominaga T:
Tumour angiogenesis in breast cancer: its importance as a
prognostic indicator and the association with vascular endothelial
growth factor expression. Breast Cancer Res Treat. 36:193–204.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guenova ML, Balatzenko GN, Nikolova VR,
Spassov BV and Konstantinov SM: An anti-apoptotic pattern
correlates with multidrug resistance in acute myeloid leukemia
patients: a comparative study of active caspase-3, cleaved PARPs,
Bcl-2, Survivin and MDR1 gene. Hematology. 15:135–143. 2010.
View Article : Google Scholar
|
|
8
|
Redaelli A, Laskin BL, Stephens JM,
Botteman MF and Pashos CL: A systematic literature review of the
clinical and epidemiological burden of acute lymphoblastic
leukaemia (ALL). Eur J Cancer Care (Engl). 14:53–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pui CH, Campana D, Pei D, et al: Treating
childhood acute lymphoblastic leukemia without cranial irradiation.
N Engl J Med. 360:2730–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altieri DC: Survivin in apoptosis control
and cell cycle regulation in cancer. Prog Cell Cycle Res.
5:447–452. 2003.PubMed/NCBI
|
|
12
|
Esh AM, Atfy M, Azizi NA, El Naggar MM,
Khalil EE and Sherief L: Prognostic significance of survivin in
pediatric acute lymphoblastic leukemia. Indian J Hematol Blood
Transfus. 27:18–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrison DJ, Hogan LE, Condos G, et al:
Endogenous knockdown of survivin improves chemotherapeutic response
in ALL models. Leukemia. 26:271–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma W, Yao X, Tang F, et al: Regulation of
pathway of survivin-NF-κB of leukemia HL60/adr cells by Jiedu Huayu
Fang. J Beijing Univ Tradit Chin Med. 35:25–28. 2012.(In
Chinese).
|
|
15
|
Demacq C, Vasconcellos VB, Izidoro-Toledo
TC, et al: Vascular endothelial growth factor (VEGF) and
endothelial nitric oxide synthase (NOS3) polymorphisms are
associated with high relapse risk in childhood acute lymphoblastic
leukemia (ALL). Clin Chim Acta. 411:1335–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|