Introduction
Hypoxia is a general characteristic of malignant
tumors (1,2) which occurs in numerous solid tumors
(3–5). It regulates a variety of
transcription factors, including hypoxia-inducible factor-1 (HIF-1)
which is a heterodimeric transcription factor consisting of 2
subunits; a constitutively stable β subunit and an oxygen sensitive
α subunit (6). The α subunit is
rapidly degraded through the ubiquitin-proteasome pathway in the
physiological microenvironment (7). However, the α subunit is stabilized
and accumulates under hypoxic conditions (8) due to the inactivation or absence of
the von Hippel-Lindau (VHL) tumor suppressor gene (9,10).
HIF-1α products regulate cell adaptation to hypoxic
microenvironments by modulating a number of downstream genes
involved in vascular growth and cellular metabolism. Among these
genes, vascular endothelial growth factor (VEGF) is vital as a
regulatory gene of angiogenesis in the adaptation to hypoxic
microenvironments (11). Studies
have demonstrated the correlation between HIF-1α and VEGF in tumor
cells (12) and solid tumors
(13) and high levels of HIF-1α
expression appear to predict a poor prognosis for various types of
cancer (4,14,15).
However, relevant studies on tongue squamous cell
carcinoma (TSCC) are rare. As a type of common malignant tumor in
the oral cavity, TSCC has a high mortality rate due to early
metastasis and recurrence. Although the level of healthcare has
greatly improved, the cure rate of TSCC remains unsatisfactory and
the 5-year survival rate is ∼50% (16).
The aim of the present study was to investigate the
association of HIF-1α expression with various clinical parameters
using reverse transcription-polymerase chain reaction (RT-PCR),
immunohistochemistry and western blot analysis to evaluate the
impact of HIF-1α expression on the prognosis of TSCC.
Patients and methods
Clinical cases
A cohort of 49 patients (28 males and 21 females;
mean age, 69.2 years; range, 45–84) was eligible for the present
study. All the patients were treated between January 2000 and
December 2005 at the Department of Oral and Maxillofacial Surgery,
Hospital of Stomatology, Tongji University (Shanghai, China). A
total of 49 paraffin-embedded tumor specimens of TSCC and 15
adjacent non-tumor tissue specimens were obtained for
immunohistochemistry. Additionally, 15 fresh frozen tumor tissue
specimens and their corresponding adjacent tissue specimens were
obtained for RT-PCR. All the patients included in the study had a
primary tumor in the oral cavity which was diagnosed as a squamous
cell carcinoma (SCC) by clinical, radiological and pathological
examination. Surgical treatment involved radical resection of the
whole tumor with a free histopathological margin of at least 15 mm
from the tumor borders. Bilateral selective neck dissection was
performed in cases of suspect results from pre-operative tumor
staging by computerized tomography and sonographic examination or
in cases of a tumor size of >2 cm. The tissues were confirmed
post-surgically as TSCC or adjacent non-tumor tissue using
hematoxylin and eosin (H&E) staining. All tumors were
classified according to the UICC TNM staging system which was
revised in 2002 (17).
Follow-up
All patients were followed up by telephone or
postoperative questionnaires monthly and underwent ultra-sound or
computed tomography examinations at least once every 3 months at
the outpatient clinic, particularly in the first 2 years. The
average overall survival time was 36.73 months (ranging from 3 to
72 months).
RT-PCR
Total RNA was isolated from the frozen tissues using
the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was
synthesized using a PrimeScript RT reagent kit (Takara, Beijing,
China). mRNA expression was evaluated quantitatively using
real-time RT-PCR with SYBR Premix Ex Taq™ (Takara) and an ABI
PRISM® 7900HT real-time PCR system. The thermocycler
conditions were pre-denaturing at 95°C for 30 sec, followed by 35
cycles of denaturing at 95°C for 30 sec, annealing at 60°C for 30
sec and extension at 68°C for 1 min. The relative amount of the PCR
product was defined as the threshold cycle (CT value) of the sample
divided by that of β-actin. All experiments were performed in
triplicate. The primers were synthesized (Sangon Biotech, Shanghai,
China) and the sequences were as follows: for HIF-1α, the forward
primer was 5′-GAA CCT GAT GCT TTA AAC T-3′ and the reverse primer
was 5′-CAA CTG ATC GAA GGA ACG-3′; for VEGF, the forward primer was
5′-TTT CTG CTG TCT TGG GTG CAT TGG-3′ and the reverse primer was
5′-TCT GCA TGG TGA TGT TGG ACT CCT-3′; for β-actin, the forward
primer was 5′- TGG, CAC, CCA, GCA, CAA, TGA, A- 3′ and the reverse
primer was 5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′.
Immunohistochemisty
Sections (thickness, 4 μm) of
paraffin-embedded TSCC and adjacent tissues were dewaxed in xylene
and rehydrated. Antigen retrieval was performed by heating the
sections with 10 mM citrate buffer (pH 6.0) for 15 min in a
microwave. The sections were washed in PBS buffer and blocked for
30 min in 10% goat serum containing 1% BSA and 0.02% Triton X-100.
Serial sections were incubated with rabbit anti-human HIF-1α 67
monoclonal antibody (1:100; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) and rabbit anti-human VEGF polyclonal antibody
(1:100; Santa Cruz Biotechnology Inc.) separately for 20 min (with
PBS instead of primary antibody as the negative control) and washed
in PBS 3 times for 15 min. Subsequently, a catalyzed signal
amplification system (Santa Cruz Biotechnology Inc.) was used for
HIF-1α staining according to the manufacturer’s instructions. The
antibodies were detected using a standard avidin-biotin complex
method [biotinylated rabbit anti-mouse antibody (Santa Cruz
Biotechnology Inc.) and an avidin-biotin complex (Santa Cruz
Biotechnology Inc.)] and developed with diaminobenzidine. All
sections were then counterstained for 45 sec with hematoxylin and
dehydrated in alcohol and xylene prior to mounting. Non-tumor
tissue samples adjacent to the paraffin-embedded tumor specimens
were examined in a similar way for H&E HIF-1α staining.
Classification of HIF-1α
expression
The HIF-1α protein was mainly present inside the
nucleus, shown as brown or brown-yellow granules located inside the
tumor cell. The VEGF protein was present inside the tumor cell or
the cell membrane. Each section was examined independently by 2
pathologists. Five fields of view were randomly selected under an
optical microscope at a x200 magnification. The positive cells were
examined for staining intensity and counted 3 times to calculate
the average number in each section. Based on the relative number of
positive cells and staining intensity, 4 levels were defined to
identify the staining activity of tumor cells: level I, no positive
cells; level II, <10% positive cells, weak staining; level III,
10–50% positive cells, moderate staining; level IV, >50%
positive cells, strong staining.
Statistical analysis
Correlations between clinicopathological features
and the expression of HIF-1α were evaluated using the Chi-square
test. Disease-free survival (DFS) and overall survival (OS) were
used as the 2 end-points of the survival analysis. OS was defined
as the number of months from the day the patient left the hospital
to patient mortality. Patients who succumbed to other causes or
remained alive at the final follow-up were considered to be review
events. DFS was defined as the tumor-free time between the initial
treatment and the first local recurrence or distant metastasis.
Survival curves of DFS and OS were analyzed using the Kaplan-Meier
method. The log-rank test was used to assess the differences
between the groups and multivariate survival analysis was performed
using Cox’s regression model. P<0.05 was considered to indicate
statistically significant differences.
Results
Immunohistochemical analysis of HIF-1α
and VEGF expression in TSCC
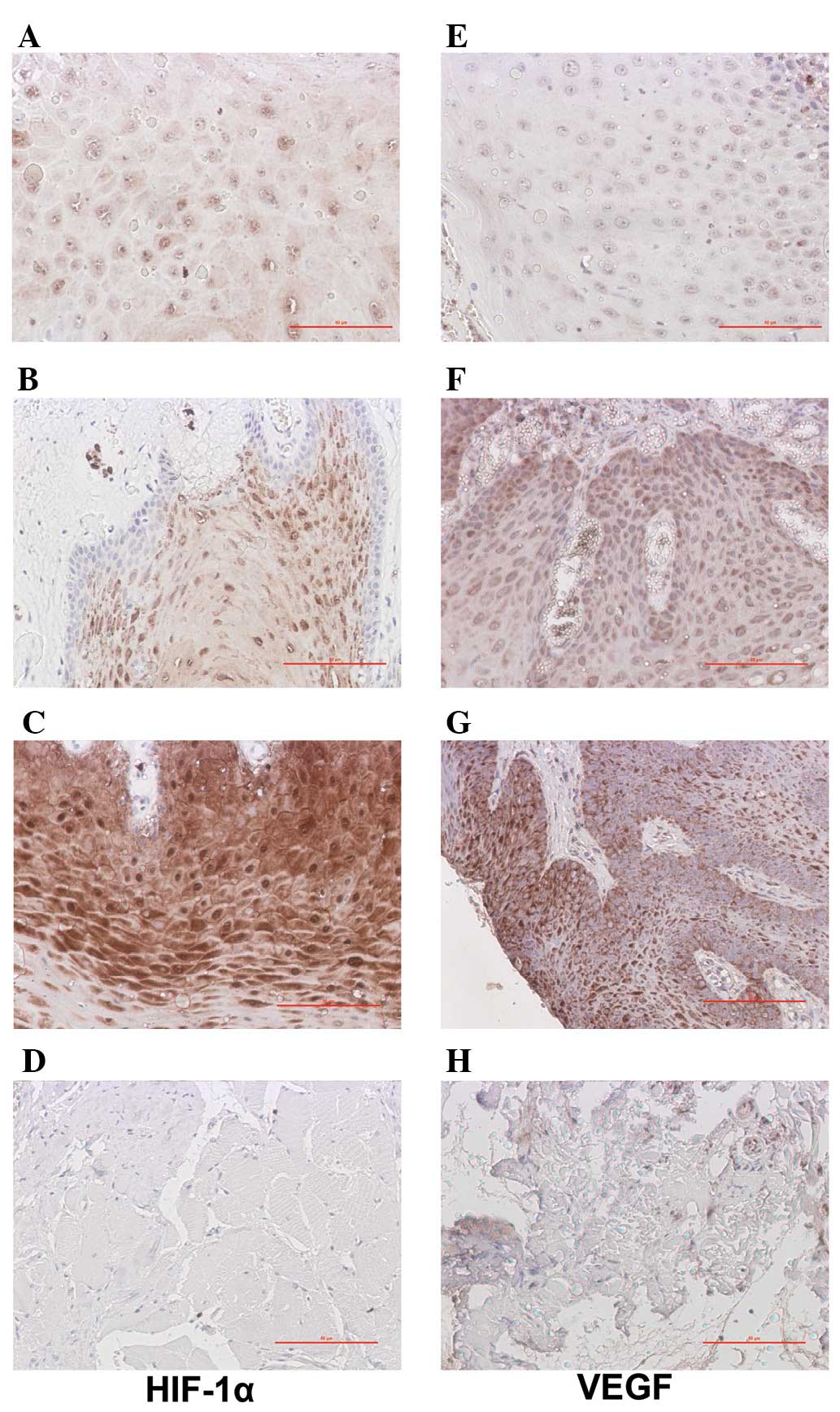
Fig. 1 shows the
results of the immunohistochemisty analysis. In the 49 cases of
TSCC, there were 6 (12.24%) negative cases, 21 (42.86%) weak
positive cases (Fig. 1A), 12
(24.49%) medium positive cases (Fig.
1B) and 10 (20.41%) strong positive cases (Fig. 1C); the total positive rate was
87.76%. By contrast, there were only 5 (33.33%) cases of weak
expression of HIF-1α in the 15 adjacent non-tumor tissue specimens
(Fig. 1D). For VEGF, there were 8
(16.33%) negative cases, 17 (34.69%) weak positive cases (Fig. 1E), 16 (32.65%) medium positive
cases (Fig. 1F) and 8 (16.33%)
strong positive cases in the present study (Fig. 1G) and the overall positive rate was
83.67%. There were only 3 (20%) cases of weak expression of VEGF in
the 15 adjacent non-tumor tissue specimens (Fig. 1H). The differences were observed to
be significant (P<0.001).
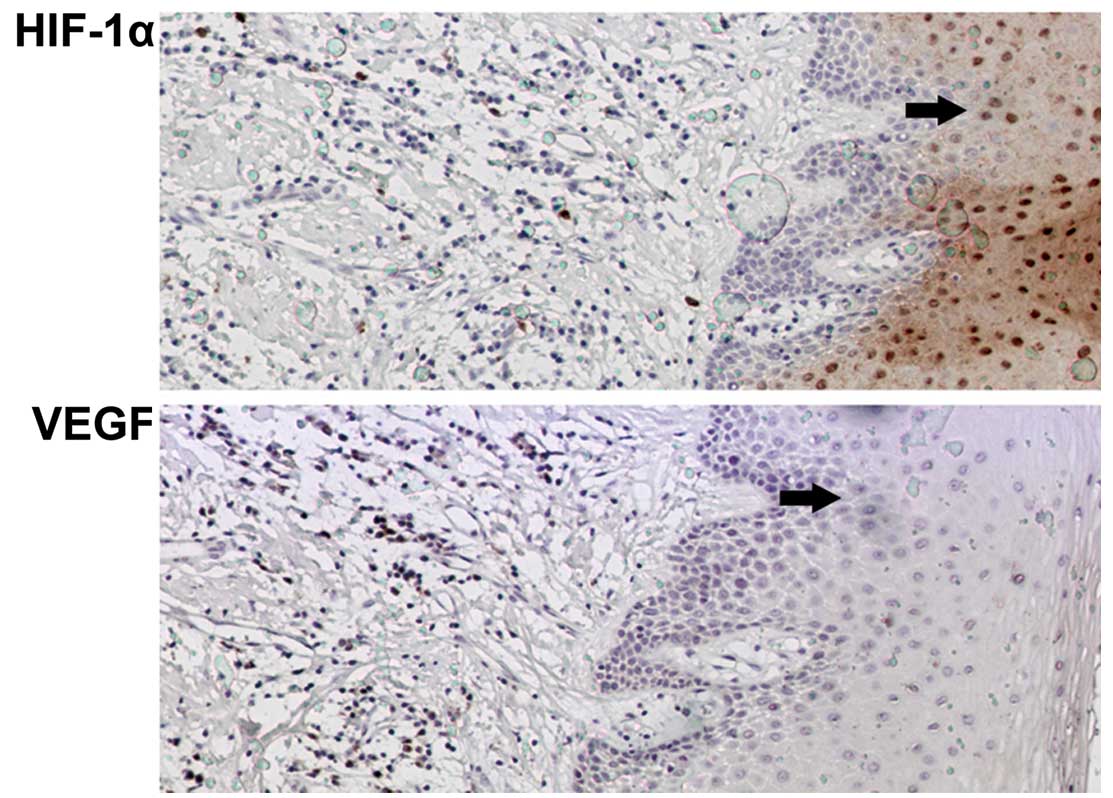
HIF-1α was observed to be markedly expressed in the
TSCC tissue, while no expression was observed in the adjacent
tissue of the representative section shown in Fig. 2. In the same regions, HIF-1α and
VEGF were expressed at relatively low levels. This was supported by
the results of the RT-PCR.
RT-PCR analysis of HIF-1α and VEGF
expression in TSCC
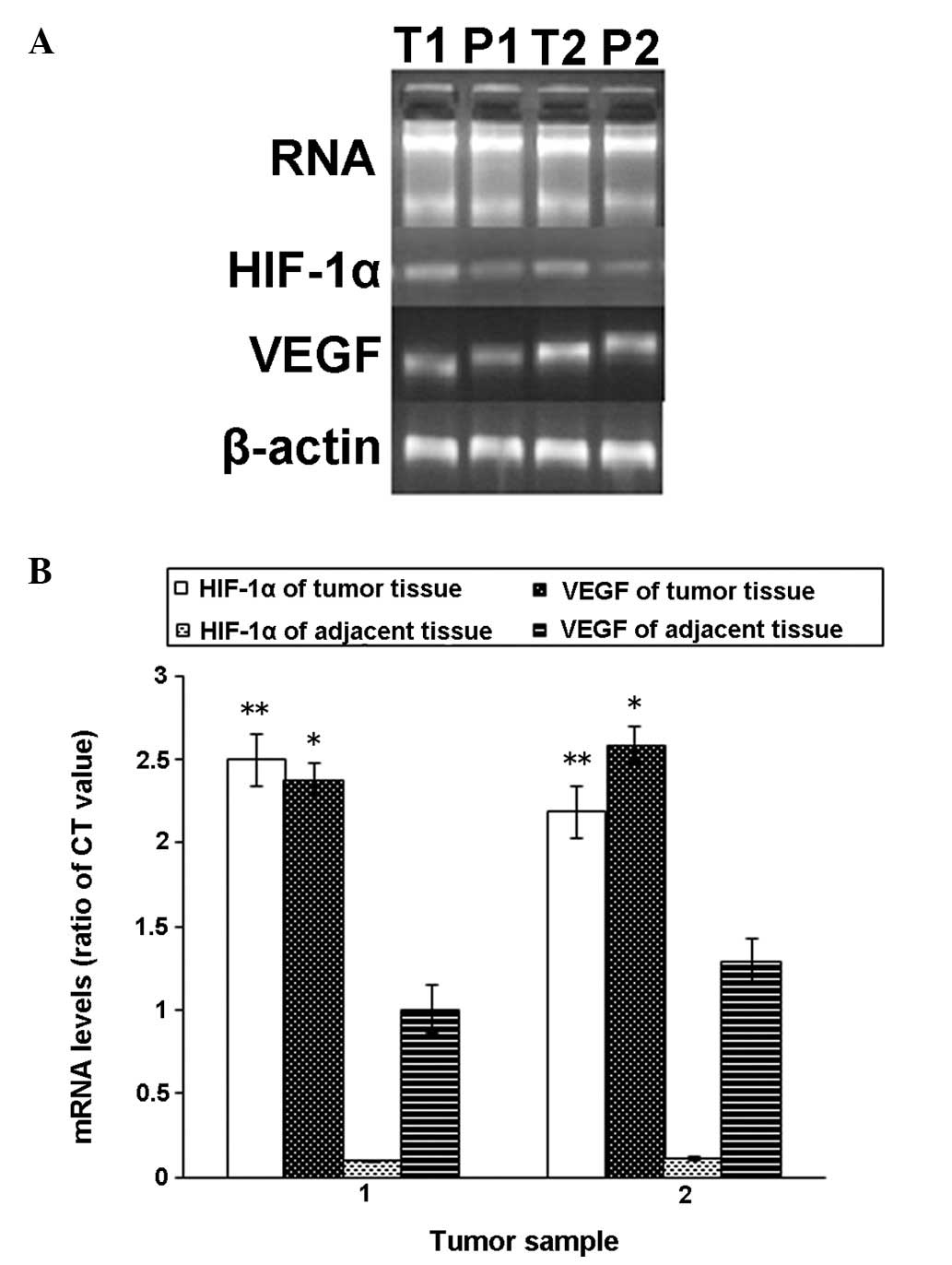
As shown in Fig.
3A, the mRNA expression levels in fresh tissues obtained from 2
cases were observed, demonstrating that HIF-1α mRNA was highly
expressed in the tumor tissue but expressed at a low level in the
adjacent tissue. However, VEGF mRNA expression was observed in the
tumor tissue and the adjacent tissue, although the expression in
tumor was higher. These data indicated that HIF-1α expression was
markedly higher in tumor tissue when compared with that of the
corresponding adjacent tissue specimens and that for VEGF, the
difference in mRNA expression levels was less significant (Fig. 3B).
Association between HIF-1α and
clinicopathological parameters
Table I shows the
correlation between the parameters, including gender, age,
histological differentiation, surgical approach, lymphatic
metastasis, T stage and HIF-1α expression. In the present study,
the 4 classes of expression of HIF-1α were combined into 2 classes:
class I/II and class III/IV. No significant correlation was
observed between gender, age, surgical approach and expression of
HIF-1α (class I/II and class III/IV) using the Chi-square test.
However, the T stage (Tis/T1 vs. T2/T3) and histological
differentiation (G1 vs. G2 + G3) were observed to correlate with
HIF-1α overexpression and the results were statistically
significant (P<0.05).
 | Table ICorrelation between HIF-1α expression
and clinical parameters in TSCC. |
Table I
Correlation between HIF-1α expression
and clinical parameters in TSCC.
| | HIF-1α
| |
|---|
| Parameter | n (%) | I/II | III/IV | P-value |
|---|
| Total | 49 (100) | 27 | 22 | - |
| Gender | | | | 0.158 |
| Male | 28 (57.14) | 18 | 10 | |
| Female | 21 (42.86) | 9 | 12 | |
| Age (years) | | | | 0.246 |
| <70 | 21 (42.86) | 14 | 7 | |
| ≥70 | 28 (57.14) | 13 | 15 | |
| Neck dissection | | | | 0.395 |
| Yes | 30 (61.22) | 15 | 15 | |
| No | 19 (38.78) | 12 | 7 | |
| T stage | | | | 0.010a |
| T1 + Tis | 24 (48.98) | 18 | 6 | |
| T2 + T3 | 25 (51.02) | 9 | 16 | |
| Lymphatic
metastasis | | | | 0.005a |
| Negative | 43 (87.76) | 27 | 16 | |
| Positive | 6 (12.24) | 0 | 6 | |
| Histological
differentiation | | | | 0.000a |
| G1 | 23 (46.94) | 21 | 2 | |
| G2 + G3 | 26 (53.06) | 6 | 20 | |
Association between HIF-1α and
prognosis
Univariate analysis of clinical
parameters with DFS and OS
Table II shows the
results of the Kaplan-Meier analysis. The results revealed
significantly poorer DFS (P<0.05) and OS (P<0.05) with
histological differentiation (P= 0.020, P=0.008), lymphatic
metastasis (P<0.001, P<0.001) and HIF-1α expression (P=
0.001, P<0.001). No significant associations between survival
rates and gender, age or neck dissection were observed.
 | Table IICorrelation of clinical parameters
with DFS and OS in TSCC. |
Table II
Correlation of clinical parameters
with DFS and OS in TSCC.
| Parameter | DFS (months) | P-value | OS (months) | P-value |
|---|
| Gender | | 0.107 | | 0.115 |
| Male | 33.00±21.96 | | 39.93±25.04 | |
| Female | 26.00±18.99 | | 32.48±21.14 | |
| Age (years) | | 0.292 | | 0.218 |
| <70 | 31.81±20.53 | | 39.38±23.60 | |
| ≥70 | 28.64±21.32 | | 34.75±23.675 | |
| Neck
dissection | | 0.094 | | 0.088 |
| Yes | 25.53±20.06 | | 31.33±22.561 | |
| No | 37.05±20.57 | | 45.26±22.99 | |
| T stage | | 0.132 | | 0.108 |
| T1 + Tis | 31.33±20.31 | | 39.42±22.58 | |
| T2 + T3 | 28.72±21.66 | | 34.16±24.55 | |
| Lymphatic
metastasis | | 0.000b | | 0.000b |
| Negative | 32.98±20.46 | | 40.33±22.78 | |
| Positive | 8.67±5.20 | | 11.00±6.29 | |
| Histological
differentiation | | 0.020a | | 0.008a |
| G1 | 36.35±20.00 | | 46.83±21.96 | |
| G2 + G3 | 24.38±20.28 | | 27.81±21.45 | |
| HIF-1α
expression | | 0.001a | | 0.000b |
|
Negative/weak | 37.63±20.61 | | 46.96±21.85 | |
|
Moderate/strong | 20.64±17.30 | | 24.18±19.30 | |
Multivariate analysis of clinical
parameters with DFS and OS
In multivariate Cox analysis the OS and DFS were
compared according to clinical parameters (lymphatic metastasis,
histological differentiation) with the HIF-1α expression. Lymphatic
metastasis and HIF-1α expression were identified by Cox regression
as independent predictors of DFS and OS. Lymphatic metastasis
(P=0.010, P=0.011) and HIF-1α expression (P=0.050, P=0.030) were
also predictors of tumor-free survival in multivariate regression
(Table III).
 | Table IIIMultivariate analysis of DFS and OS
in TSCC. |
Table III
Multivariate analysis of DFS and OS
in TSCC.
| DFS
| OS
|
|---|
| Parameter | P-value | RR | P-value | RR |
|---|
| Lymphatic
metastasis | | | | |
| Negative vs.
Positive | 0.010a | 0.080–0.705 | 0.011a | 0.086–0.732 |
| Histological
differentiation | | | | |
| G1 vs. G2 +
G3 | 0.749 | 0.329–2.224 | 0.619 | 0.296–2.066 |
| HIF-1α
expression | | | | |
| Negative/weak vs.
moderate/strong | 0.050a | 0.146–1.001 | 0.030a | 0.122–0.898 |
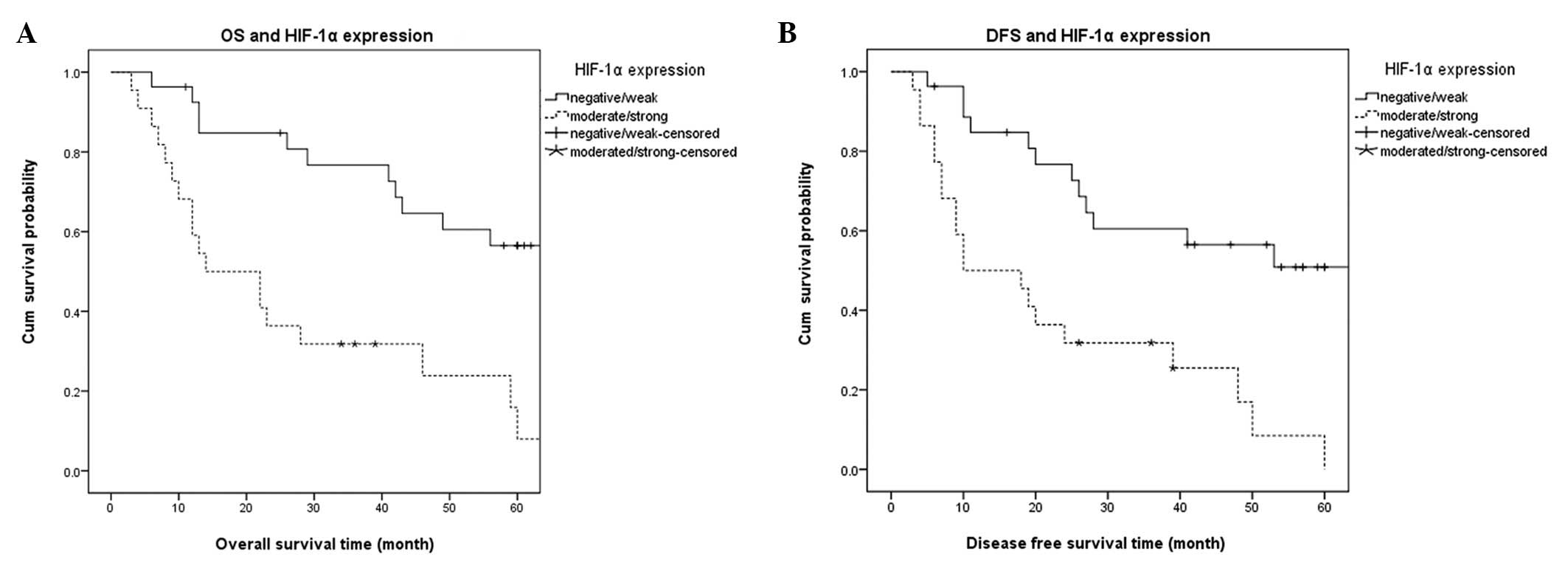
Fig. 4 shows the
Kaplan-Meier survival curve analysis of DFS and OS. The expression
of HIF-1α (class I/II and class III/IV) affected the OS (Fig. 4A) and DFS (Fig. 4B) of patients with TSCC. The
patients with high HIF-1α expression levels had poorer DFS and OS,
suggesting that the overexpression of HIF-1α was associated with a
poor prognosis.
Discussion
HIF-1α is the most significant nuclear transcription
factor identified as mediating the hypoxic response. As a global
regulatory factor, it is able to activate a wide range of genes
mediating physiological responses to hypoxia and consequently
regulates oxygen concentration in cell metabolism (18). The transcriptional activity of
HIF-1α is activated by hypoxia, thus triggering a series of
adaptive responses leading to glucose metabolism, tumor
angiogenesis and erythropoietin generation (19). Hypoxia is a general characteristic
of malignant tumors which occurs in numerous solid tumors (20). Due to the continuous growth and
expansion of the solid tumor, the intratumoral oxygen concentration
is continuously reduced until hypoxia occurs. This causes HIF-1α
accumulation inside the cell nucleus which activates various types
of downstream genes for hypoxia adaptation. As a regulatory gene of
angiogenesis in the adaptation to hypoxic microenvironments, VEGF
is vital in tumor recurrence and metastasis (21). It is a highly specific vascular
endothelial cell mitogen which directly stimulates endothelial
cells, thus promoting endothelial cell proliferation, migration and
increases in vascular permeability. The normal functions of VEGF
include creating new blood vessels during embryonic development and
following injury. However, under certain pathological conditions,
VEGF may contribute to diseases such as tumors. Studies have
demonstrated the function of VEGF in tumorigenesis and revealed it
to be an independent indicator for predicting malignant tumors with
poor prognoses (22–24). Sugiura et al examined 160
oral SCC specimens from the oral cavity using immunohistochemistry
and revealed that VEGF-C may be used to predict the lymphatic
metastasis of oral SCC (25).
In the present study, the expression of VEGF was
observed to be significantly associated with that of HIF-1α. The
results of RT-PCR suggested that the HIF-1α was present,
overexpressed in TSCC and closely associated with VEGF. These
results were consistent with those reported by Yasuda et
al(13). The authors used
in situ hybridization and immunohistochemistry to observe
the expression of the HIF-1α gene and its association with the VEGF
protein and microvessel density (MVD) and revealed that the
expression of HIF-1α mRNA positively correlated with the VEGF
protein expression and MVD in colorectal adenoma. Additionally, in
the present study, HIF-1α and VEGF were mainly expressed in the
TSCC tissue and were barely detected in the adjacent non-tumor
tissue suggesting that HIF-1α was present and overexpressed in
TSCC.
Since a number of studies have revealed HIF-1α
overexpression to be significantly associated with poor prognoses
in certain solid tumors, including pancreatic cancer, breast
cancer, rectal adenocarcinoma and cervical cancer (26–29),
it was investigated whether HIF-1α may function as a prognostic
factor of TSCC. Using the the immunohistochemical staining
performed on the 49 TSCC specimens of HIF-1α, the association
between HIF-1α expression and the prognoses of patients with TSCC
was studied. The data demonstrated that patients with no or weak
expression of HIF-1α had higher survival rates (approximately 60%)
than those with moderate or high expression of HIF-1α
(approximately 30%). HIF-1α overexpression was closely associated
with clinicopathological parameters, including histological
differentiation, T stage and lymph node metastasis. The data also
revealed unfavorable effects on survival rate. In multivariate Cox
regression analysis, lymphatic metastasis and HIF-1α expression
were significantly associated with DFS and OS, suggesting that the
subgroup of patients with HIF-1α overexpression may have a high
risk of TSCC and a poor prognosis. This hypothesis was supported by
a number of studies in various research fields as mentioned
previously. Bos et al(30)
investigated the expression levels of HIF-1α, HER-2/neu, estrogen
receptor and progesterone receptor in 150 patients with early-stage
breast carcinoma using immunohistochemistry and HER-2/neu gene
amplification with automated fluorescent in situ
hybridization. The authors observed that increased levels of HIF-1α
were associated independently with lower survival rates in patients
with lymph node negative breast carcinoma.
However, the results of certain studies are
inconsistent with those of the present study. Fillies et
al(31) investigated 85
patients with histologically demonstrated surgically treated T1/2
SCC of the oral floor and the results suggested that HIF-1α
overexpression was an indicator of favorable prognosis in T1 and T2
SCC of the oral floor. This contradiction may be due to a uniform
cut-off point of HIF-1α expression or various types of tendentious
treatment for the tumor. Considering this, HIF-1α may function as
an independent prognostic marker of TSCC.
In conclusion, the present study is the first to
clinically study patients with TSCC in the East China region. The
findings suggested that the overexpression of HIF-1α predicts a
poor prognosis in TSCC. Further studies should be performed to
explore the potential functional role of HIF-1α in malignant tumors
and to determine whether HIF-1α may be regarded as an indicator or
target in the diagnosis and treatment of TSCC.
Acknowledgements
This study was supported by a grant
from the Shanghai Science and Technology Committee
(09QA1406400).
References
|
1
|
Hochachka PW: Mechanism and evolution of
hypoxia-tolerance in humans. J Exp Biol. 201:1243–1254.
1998.PubMed/NCBI
|
|
2
|
Vaupel P, Thews O and Hoeckel M: Treatment
resistance of solid tumors: role of hypoxia and anemia. Med Oncol.
18:243–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakayama K, Kanzaki A, Hata K, et al:
Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in
human ovarian carcinoma. Cancer Lett. 176:215–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuwai T, Kitadai Y, Tanaka S, et al:
Expression of hypoxia-inducible factor-1alpha is associated with
tumor vascularization in human colorectal carcinoma. Int J Cancer.
105:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ioannou M, Mylonis I, Kouvaras E, et al:
Validated analysis of HIF-1α expression in cancer cells using a
controlled and comparative immunoassay. Oncol Rep. 24:161–169.
2010.
|
|
6
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar
|
|
8
|
Wang GL and Semenza GL: General
involvement of hypoxia-inducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krieg M, Haas R, Brauch H, Acker T, Flamme
I and Plate KH: Up-regulation of hypoxia-inducible factors
HIF-1alpha and HIF-2alpha under normoxic conditions in renal
carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of
function. Oncogene. 19:5435–5443. 2000. View Article : Google Scholar
|
|
10
|
Iliopoulos O, Levy AP, Jiang C, Kaelin WG
Jr and Goldberg MA: Negative regulation of hypoxia-inducible genes
by the von Hippel-Lindau protein. Proc Natl Acad Sci USA.
93:10595–10599. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianco F, Basini G, Santini S and
Grasselli F: Angiogenic activity of swine granulosa cells: effects
of hypoxia and the role of VEGF. Vet Res Commun. 29(Suppl 2):
157–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SB, Park JB, Song TJ and Choi SY:
Molecular mechanism of HIF-1-independent VEGF expression in a
hepatocellular carcinoma cell line. Int J Mol Med. 28:449–454.
2011.PubMed/NCBI
|
|
13
|
Yasuda S, Arii S, Mori A, et al:
Hexokinase II and VEGF expression in liver tumors: correlation with
hypoxia-inducible factor 1 alpha and its significance. J Hepatol.
40:117–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi R, Tanaka S, Hiyama T, et al:
Hypoxia-inducible factor-1α expression and angiogenesis in
gastrointestinal stromal tumor of the stomach. Oncol Rep.
10:797–802. 2003.
|
|
15
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: Relation of hypoxia inducible factor 1 alpha and 2 alpha
in operable non-small cell lung cancer to angiogenic/molecular
profile of tumours and survival. Br J Cancer. 85:881–890. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casiglia J and Woo SB: A comprehensive
review of oral cancer. Gen Dent. 49:72–82. 2001.
|
|
17
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classification revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gordan JD and Simon MC: Hypoxia-inducible
factors: central regulators of the tumor phenotype. Curr Opin Genet
Dev. 17:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: Hypoxia, clonal selection, and
the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol.
35:71–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
21
|
Richard DE, Berra E and Pouysségur J:
Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res
Commun. 266:718–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyzas PA, Stefanou D, Batistatou A and
Agnantis NJ: Prognostic significance of VEGF immunohistochemical
expression and tumor angiogenesis in head and neck squamous cell
carcinoma. J Cancer Res Clin Oncol. 131:624–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vidal O, Soriano-Izquierdo A, Pera M, et
al: Positive VEGF immunostaining independently predicts poor
prognosis in curatively resected gastric cancer patients: results
of a study assessing a panel of angiogenic markers. J Gastrointest
Surg. 12:1005–1014. 2008. View Article : Google Scholar
|
|
24
|
Zhao ZQ, Yang S and Lu HS: Expression of
midkine and vascular endothelial growth factor in gastric cancer
and the association of high levels with poor prognosis and
survival. Mol Med Report. 5:415–419. 2012.PubMed/NCBI
|
|
25
|
Sugiura T, Inoue Y, Matsuki R, et al:
VEGF-C and VEGF-D expression is correlated with lymphatic vessel
density and lymph node metastasis in oral squamous cell carcinoma:
Implications for use as a prognostic marker. Int J Oncol.
34:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibaji T, Nagao M, Ikeda N, et al:
Prognostic significance of HIF-1 alpha overexpression in human
pancreatic cancer. Anticancer Res. 23:4721–4727. 2003.PubMed/NCBI
|
|
27
|
Gruber G, Greiner RH, Hlushchuk R, et al:
Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an
independent prognostic parameter? Breast Cancer Res. 6:R191–R198.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu XG, Xing CG, Feng YZ, Chen J and Deng
C: Clinical significance of immunohistochemical expression of
hypoxia-inducible factor-1alpha as a prognostic marker in rectal
adenocarcinoma. Clin Colorectal Cancer. 5:350–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
30
|
Bos R, van der Groep P, Greijer AE, et al:
Levels of hypoxia-inducible factor-1alpha independently predict
prognosis in patients with lymph node negative breast carcinoma.
Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fillies T, Werkmeister R, van Diest PJ,
Brandt B, Joos U and Buerger H: HIF1-alpha overexpression indicates
a good prognosis in early stage squamous cell carcinomas of the
oral floor. BMC Cancer. 5:842005. View Article : Google Scholar : PubMed/NCBI
|