Introduction
Epithelial ovarian cancer (OC) is the most common
cause of mortality from gynecological malignancy and the majority
of ovarian carcinomas are of serous type. Serous carcinomas are
further subclassified as high- or low-grade, based on histological
features (1). The group designated
‘atypical proliferative serous tumor (APST)’ performed in a benign
fashion, and a second, smaller group designated ‘micropapillary
serous carcinoma (MPSC)’ or ‘noninvasive low-grade serous
carcinoma’ performed as a low-grade malignant tumor (2). The latter subset was closely
associated with invasive low-grade serous carcinoma (LGSC) and the
investigators proposed that MPSC was the immediate precursor of
LGSC. LGSC is a distinct entity that differs from high-grade serous
carcinoma (HGSC) in several ways. For example, LGSC has specific
mutations in genes such as BRAF and KRAS.
Compelling evidence is emerging that numerous
high-grade ovarian serous carcinomas originate from the epithelium
of the distal fimbrial portion of the Fallopian tube (FT) (3). The FT mucosa was suggested as a
strong candidate for the primary source of pelvic (ovarian, tubal
or peritoneal) serous carcinoma. Serous tubal intraepithelial
carcinoma (STIC) has been implicated in the origins of not only
HGSC but also serous carcinomas and primary peritoneal carcinomas.
It has been proposed that the earliest neoplastic change begins in
secretory-type cells (3). Further
evidence supporting the proposal that STICs are precursors was the
identification of STICs in females without OC as well as the
presence of identical p53 mutations in STICs and concomitant
ovarian HGSCs, indicating a clonal relationship between them
(4).
HMGA2, a high-mobility-group AT-hook (HMGA) protein,
is a non-histone DNA-binding factor that binds to AT-rich sequences
in the minor groove of the DNA helix. HMGA2 is expressed in
embryonic tissue, but not in the majority of adult tissues and is
an important regulator for cell growth, differentiation, apoptosis
and malignant transformation (5).
HMGA2 overexpression has been observed to be an early genetic event
in tumorigenesis. The expression of HMGA2 is regulated by microRNA.
Previous studies show that let-7s specifically repress HMGA2
expression both in vivo and in vitro, revealing the
regulatory role of let-7 in HMGA2 expression (5–7).
Downregulation of let-7s is common in OC. OC patients with high
HMGA2 and low let-7 expression in cancerous cells had a lower
survival than patients with a low HMGA2/high let-7 ratio (6,7).
Levels of ovarian and peritoneal FSH and LH appear
to be elevated in OC patients (8).
Although the mutagenic effect of FSH remains controversial, the
proliferative effect has been demonstrated in ovarian surface
epithelium (OSE), in OC in vitro and in several OC cell
lines in a dose- and time-dependent manner in vitro(9,10).
However, little is known about the exact mechanism of FSH
stimulation in the tumorigenesis of OC. In addition, the
correlation of HMGA2 expression, p53 mutation and FSH, is poorly
understood.
The purpose of the present study was to investigate
the effect and mechanism of FSH on let-7, HMGA2 and p53 expression
in the normal fimbrial epithelial cell of HGSCs and reveal the
different susceptibilities to FSH of fimbria in HGSCs and
LGSCs.
Materials and methods
Tissue samples
Fresh FT fimbria specimens were obtained from the
Obstetrics and Gynecology Hospital of Fudan University (Shanghai,
China) with the approval of the review board of the Obstetrics and
Gynecology Hospital, Fudan University. The fimbrial tissues used in
this study were collected from surgical procedures for 18 cases of
HGSCs and 16 cases of LGSCs between January 2011 and December 2011
in the Obstetrics and Gynecology Hospital of Fudan University. None
of the patients had a history of other neoplasms or had undergone
radiotherapy, chemotherapy, hormone replacement therapy,
immunotherapy or any other therapy prior to surgery. Following
surgery, 2 pathologists reviewed the hematoxylin and eosin-stained
sections from each specimen to exclude STIC and fimbria involvement
for each case. p53 immunohistochemistry was performed to exclude
positive cases. The histological diagnosis and tumor grade of HGSCs
and LGSCs were based on the conventional criteria (1). In addition, clinical characteristics,
including age and disease stage are listed in Table I.
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Characteristic | HGSC (n) | LGSC (n) |
|---|
| Total | 18 | 16 |
| Patient age
(years) | | |
| ≤50 | 6 | 7 |
| >50 | 12 | 9 |
| CA125 (U/ml) | | |
| ≤500 | 3 | 6 |
| >500 | 15 | 10 |
| Disease stage | | |
| I–II | 3 | 5 |
| III–IV | 15 | 11 |
| Residual disease | | |
| <1 cm | 14 | 15 |
| ≥1 cm | 4 | 1 |
The 15 cases of fimbrial tissues used for the
control group in this study were collected from surgical procedures
for benign gynecological indications. Cases of inflammatory
disease, infection and extensive adhesions were excluded. The
primary human FT epithelium ex vivo culture system was
completed according to a previous study (11,12).
Western blot analysis
Cell lysates from the culture were collected and
quantified using the BCA method. Following 8, 12 and 15%
denaturating sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, 30 μg of protein lysates was separated from the
gel and transferred to a nitrocellulose filter. The membranes were
sealed with PBS containing 5% non-fat milk for 1 h at room
temperature and then sealed with a primary antibody (anti-HMGA2
antibody, 1:50, Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA; anti-FSHR antibody, 1:400, Lab Vision Co., Fremont, CA, USA;
anti-p53 antibody, 1:1000, Abcam, Cambridge, MA, USA) overnight at
4°C. The following day, the membranes were mixed with
HRP-conjugated secondary antibodies for 1 h at 37°C. GAPDH was used
as a loading control. The signal was detected with an enhanced
chemiluminescence assay (PerkinElmer, Waltham, MA, USA) and the
protein was analyzed semiquantitatively using the software Quantity
One (Bio-Rad, Hercules, CA, USA).
RNA extraction and reverse transcription
(RT)-PCR
The levels of let-7b microRNA were determined by
RT-PCR. Total cellular RNA was extracted from cells using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. cDNA was synthesized from 2 μg RNA
using a reverse transcription kit (Promega, Madison, WI, USA) and
PCR primers by Yingjun Biotechnology Corporation (Shanghai, China).
The mature let-7b (Applied Biosystems, Carlsbad, CA, USA) sequence
was 5′-UGAGGUAGUAGGUUGUGUGGUU-3′. The conditions for amplification
were as follows: one cycle at 94°C for 5 min, followed by 50 cycles
at 94°C for 30 sec, 57°C for 30 sec and 70°C for 30 sec. In total,
20 μl PCR product was used for agarose electrophoresis.
FSH stimulation
FSH was purchased from Sigma Chemical Co. (St.
Louis, MO, USA). GAPDH monoclonal antibody was purchased from
Kangchen Bioengineering Corporation (Shanghai, China). The
Fallopian tube epithelium (FTE) cells were plated at
4×104 or 4×105 and 1×104 or
1×105 cells per well onto 96-well or 6-well plates,
respectively. Twenty-four hours after plating, RPMI-1640 medium
without serum was replaced and the cells were serum-starved for 18
h. The cells were then stimulated with FSH at 40 mIU/ml for
different time periods (up to 120 min for signaling or up to 24 h
for protein expression), PBS was used as a control. Transfected
cells were also starved for 18 h and then stimulated with FSH at 40
mIU/ml for an additional 24 h. The cells were then harvested and
the proteins were extracted for western blot analysis.
Anti-let-7b transfection
FTE cells of HGSCs were transfected in 12-well
plates with 60 pmol of anti-miR let-7b or equivalent amounts of
negative control #1 miRNA inhibitor (Ambion, Austin, TX, USA) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions and cells were incubated for 48 h after
transfection.
Statistical analysis
The results of the experiments were analyzed using
the χ2 test for positive rate comparison and one-way
analysis of variance for the other comparisons. P<0.05 was
considered to indicate a statistically significant result. The SPSS
software program (version 12.0; SPSS, Inc., Chicago, IL, USA) was
used for all statistical analysis.
Results
The expression of HMGA2, let-7, p53 and
FSHR in FTEs
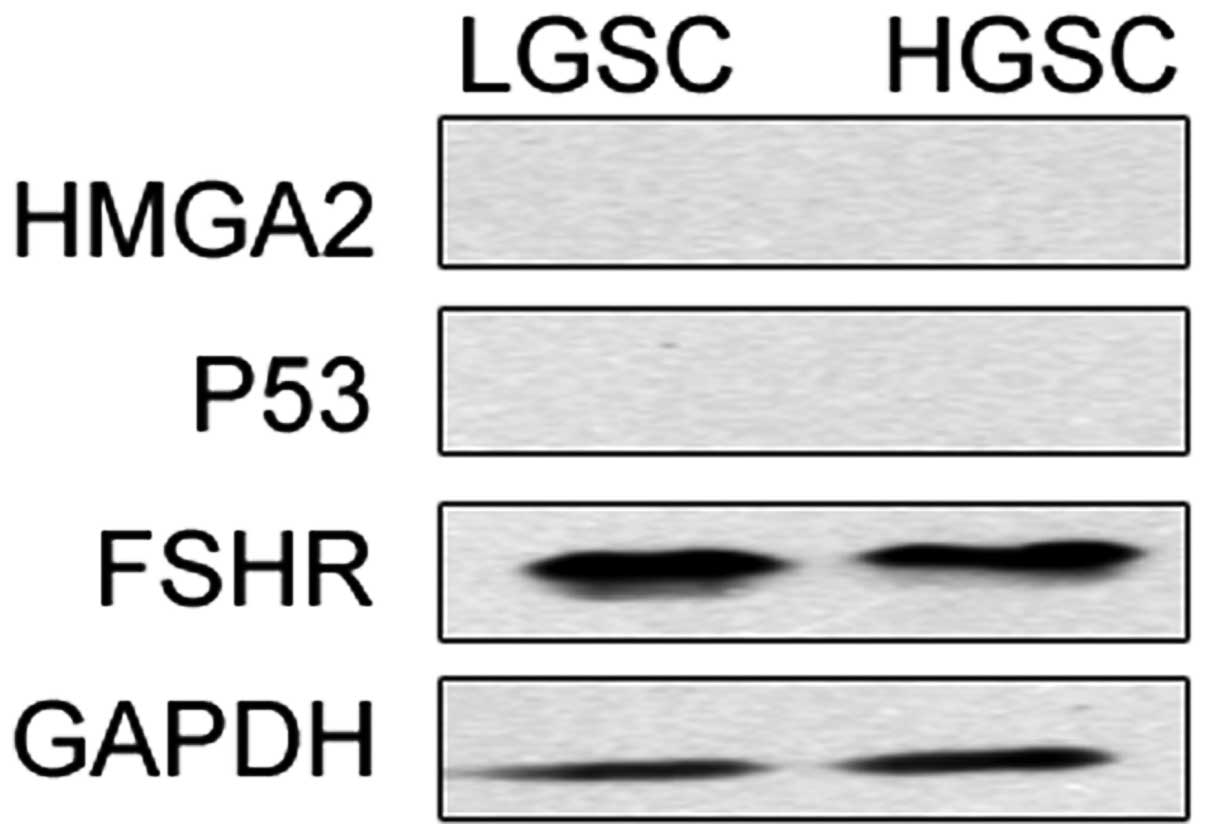
HMGA2, let-7, p53 and FSHR had similar expression
levels in FTE cells of LGSCs and HGSCs. This result was confirmed
by RT-PCR and western blot analysis. All 34 samples expressed
let-7b. HMGA2 and p53 expression were not detected in any samples.
FSHR mRNA expression revealed by western blot analysis was observed
in 100% of the FTE cells of HGSCs and LGSCs (Fig. 1).
FSH increases expression of HMGA2 and
decreases expression of let-7 in normal fimbria of HGSCs
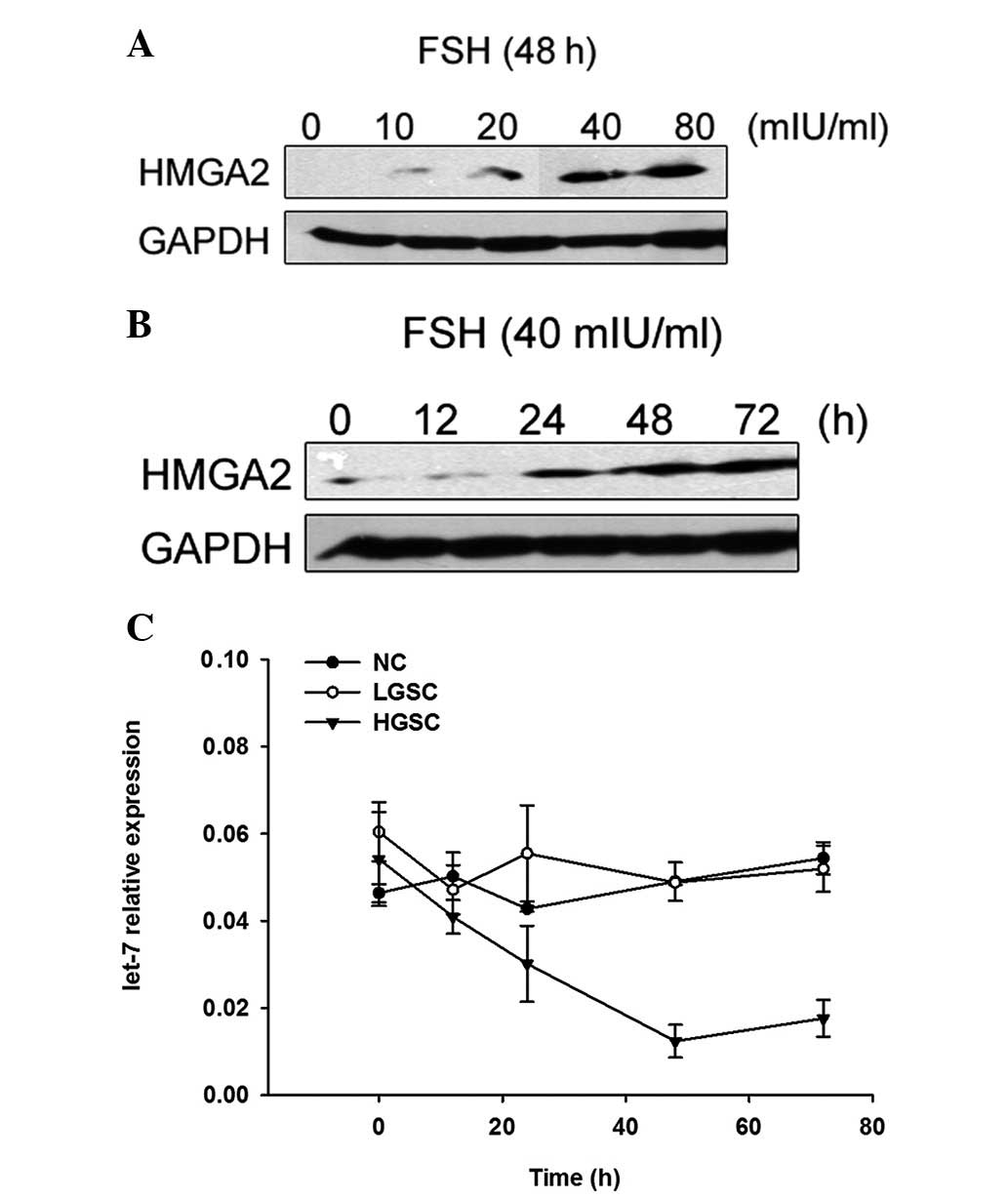
We stimulated the FTE cells with FSH at various
concentrations and for various time courses. Western blot analysis
showed that HMGA2 expression levels increased gradually in addition
to the FSH concentration in FTE cells of the HGSCs, peaked at an
FSH concentration of 40 mIU/ml, and then began to decline slightly,
indicating a dose-dependent correlation (Fig. 2). When FTE cells of the HGSCs were
stimulated with 40 mIU/ml of FSH for 0, 12, 24, 48 or 72 h, HMGA2
expression in the 24, 48 and 72 h groups of cells increased in
comparison to the control group, indicating a time-dependent
correlation. HMGA2 expression peaked from 48 h after stimulation
with 40 mIU/ml FSH (Fig. 2).
Notably, we observed that the let-7 expression levels decreased
gradually with time and an inverse correlation between the
expression of let-7b and HMGA2 in FTE cells of the HGSCs was
observed following FSH stimulation (r=−0.55, P=0.006). p53 was not
detected in the normal fimbrial epithelial cells before or after
FSH administration. However, we did not observe changes of HMGA2,
let-7b or p53 in FTE cells of the LGSCs due to FSH stimulation. The
results demonstrated that the susceptibility of fimbria of LGSCs
and HGSCs was different.
FSH stimulates HMGA2 expression by
downregulating let-7
We observed that FSH stimulation of the FTE cells of
HGSCs significantly increased the expression of HMGA2 and decreased
the expression of let-7b, but had no effect on let-7b and HMGA-2 of
LGSCs. To confirm that let-7 regulated HMGA2 expression, we
pretreated the FTE cells of HGSCs with anti-miR let-7b transfection
for 48 h. HMGA2 expression was detected and p53 was absent, as
demonstrated by western blot analysis. These data indicate that
let-7 regulated HMGA2 expression and FSH stimulates HMGA2
expression by down-regulating let-7.
Discussion
The observations that FSH increases the risk of
ovarian malignancy and that pregnancies or oral contraceptives
protect the ovaries by suppressing FSH secretion led to numerous
studies (13). The majority of
studies addressing the role of FSH observed that it has a growth
stimulation effect in normal or immortalized ovarian surface
epithelial cells (10). SV-40
transformed benign ovarian epithelial tumor cells and certain OC
cells in a dose- and time-dependent manner in vitro, as
observed in our previous study that examined the role of FSH in
ovarian carcinogenesis (14).
Since a comparable amount of FSHR is also present in
the FT, including the fimbriated end (15), certain high grade ovarian
epithelial cancers with likely tubal origin do not negate the role
of FSH in ovarian carcinogenesis. Our results showed that FSHR mRNA
expression revealed by western blot analysis was observed in 100%
of the FTE cells of HGSCs and LGSCs and was consistent with the
literature. The FTE cells show a limited ability to resolve the
damage over time, potentially leaving the cells more susceptible to
the accumulation of additional mutagenic injury (11). However, susceptibility to hormones,
such as FSH, is not clear.
In this study, we observed that FSH stimulation of
the FTE cells of HGSCs significantly increased the expression of
HMGA2 and decreased the expression of let-7b, but had no effect on
that of LGSCs. This suggested significant biological differences in
the behavior of the fimbria in high-grade and low-grade ovarian
serous cancers (OSCs) and we presumed that the susceptibility to
FSH of fimbria of high- and low-grade OSCs are different. In
addition, we detected FSHR expression in all FTE cells of HGSCs and
LGSCs. It is likely that FSH regulated let-7b via FSHR. However,
thus far little is known about the mechanism of FSH stimulation of
let-7 and HMGA2 expression and it was lack of investigation in
vitro and in vivo to confirm the mechanism.
HMGA2 overexpression has been associated with tumor
growth, differentiation, metastasis, unfavorable outcome and
resistance to treatment (5).
Silencing of HMGA2 expression in OC cells has been reported to have
a therapeutic effect on OC growth (6). HMGA2 upregulation occurs early during
OC progression before the tumors begin to metastasize both in human
patients and in an OC mouse model (7).
Serous intraepithelial carcinoma in the FT has a
high rate and level of HMGA2 overexpression in addition to
p53-dominant mutations (16). The
immunohistochemistry results indicate that HMGA2 overexpression is
an early event in the tumorigenesis of high-grade papillary serous
carcinoma. In addition, it suggests that the later event of HMGA2
over-expression is after p53 mutations. However, we observed the
expression of HMGA2 but no occurrence of p53 mutation with the
stimulation of FSH. The mutation of p53 may have caused the
expression change of HMGA2. The detailed mechanism requires further
research. Levanon et al(17) proposed a sequential model of HGSC,
progressing from precursor with p53 signature, to STIC and then to
invasive carcinoma (3,17). However. the cause of the p53
signature remains unknown.
We identified HMGA2 as a significant molecule for
future studies of HGSCs due to its potential use in the early
detection of disease and its functional role in tumorigenesis. FSH
likely plays a key role in the early carcinogenesis and has no
effect in the advanced process.
Members of the let-7/miR-98 family are induced late
in mammalian embryonic development to suppress the expression of
embryonic genes that are not expressed in the adult organism.
Reported let-7 targets include RAS, c-myc and HMGA2. let-7 is
frequently downregulated in human neoplasms, suggesting that
embryonic target genes of let-7 are upregulated in cancer.
let-7 expression causes degradation of HMGA2 mRNA.
The efficient degradation of HMGA2 mRNA may be due to the high
degree of complementarity of let-7 to certain let-7 seed matches
present in the HMGA2 untranslated region (3′-UTR) (18–23).
There was significant downregulation of let-7b in high-grade
papillary serous carcinomas in comparison to matched FTE. Therefore
loss of let-7 expression plays a key role in the regulation of FTE
cells (24).
In the present study, we observed that the let-7
expression levels decreased gradually over time and an inverse
correlation between the expression of let-7b and HMGA2 in FTE cells
of the HGSCs stimulated by FSH was present. A previous study showed
that HMGA2 was a direct target for let-7 in human cancer cell lines
and let-7 regulated HMGA2 expression in OC and predicts disease
progression (20).
In conclusion, our results suggest that FSH
stimulation of HMGA2 expression is mediated by let-7. Further
studies to understand the role of FSH in tumorigenesis of FTE cells
at cellular and molecular levels are required, as this may
elucidate the etiology of OC development.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Nos. 81072131 and
81072130), and the Shanghai Health Bureau Youth Research Project
(No. 2008Y008).
References
|
1
|
Malpica A, Deavers MT, Lu K, et al:
Grading ovarian serous carcinoma using a two-tier system. Am J Surg
Pathol. 28:496–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burks RT, Sherman ME and Kurman RJ:
Micropapillary serous carcinoma of the ovary. A distinctive
low–grade carcinoma related to serous borderline tumors. Am J Surg
Pathol. 20:1319–1330. 1996.
|
|
3
|
Lee Y, Miron A, Drapkin R, et al: A
candidate precursor to serous carcinoma that originates in the
distal fallopian tube. J Pathol. 211:26–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kindelberger DW, Lee Y, Miron A, et al:
Intraepithelial carcinoma of the fimbria and pelvic serous
carcinoma: Evidence for a causal relationship. Am J Surg Pathol.
31:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar
|
|
6
|
Malek A, Bakhidze E, Noske A, et al: HMGA2
gene is a promising target for ovarian cancer silencing therapy.
Int J Cancer. 123:348–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SM, Shell S, Radjabi AR, et al: Let-7
Prevents Early Cancer Progression by Suppressing Expression of the
Embryonic Gene HMGA2. Cell Cycle. 6:2585–2590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rzepka-Górska I, Chudecka-Glaz A and
Kosmowska B: FSH and LH serum/tumor fluid ratios and malignant
tumors of the ovary. Endocr Relat Cancer. 11:315–321.
2004.PubMed/NCBI
|
|
9
|
Ohtani K, Sakamoto H, Kikuchi A, et al:
Follicle-stimulating hormone promotes the growth of human
epithelial ovarian cancer cells through the protein kinase
C-mediated system. Cancer Lett. 166:207–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji Q, Liu PI, Chen PK, et al: Follicle
stimulating hormone-induced growth promotion and gene expression
profiles on ovarian surface epithelial cells. Int J Cancer.
112:803–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levanon K, Ng V, Piao HY, et al: Primary
ex vivo cultures of human fallopian tube epithelium as a model for
serous ovarian carcinogenesis. Oncogene. 29:1103–1113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fotheringham S, Levanon K and Drapkin R:
Ex Vivo Culture of Primary Human Fallopian Tube Epithelial Cells. J
Vis Exp. pii. 27282011.PubMed/NCBI
|
|
13
|
Brekelmans CT: Risk factors and risk
reduction of breast and ovarian cancer. Curr Opin Obstet Gynecol.
15:63–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Hua K, Zhou X, et al: Activation
of the PI3K/AKT pathway mediates FSH-stimulated VEGF expression in
ovarian serous cystadenocarcinoma. Cell Res. 18:780–791. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng W, Magid MS, Kramer EE and Chen YT:
Follicle-stimulating hormone receptor is expressed in human ovarian
surface epithelium and fallopian tube. Am J Pathol. 148:47–53.
1996.PubMed/NCBI
|
|
16
|
Wei JJ, Wu J, Luan C, et al: HMGA2: a
potential biomarker complement to P53 for detection of early-stage
high-grade papillary serous carcinoma in fallopian tubes. Am J Surg
Pathol. 34:18–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levanon K, Crum C and Drapkin R: New
insights into the pathogenesis of serous ovarian cancer and its
clinical impact. J Clin Oncol. 26:5284–5293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar MS, Lu J, Mercer KL, et al: Impaired
microRNA processing enhances cellular transformation and
tumorigenesis. Nat Genet. 39:673–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shell S, Park SM, Radjabi AR, et al: Let-7
expression defines two differentiation stages of cancer. Proc Natl
Acad Sci USA. 104:11400–11405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mayr C, Hemann MT and Bartel DP:
Disrupting the pairing between let-7 and Hmga2 enhances oncogenic
transformation. Science. 315:1576–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hebert C, Norris K, Scheper MA, et al:
High mobility group A2 is a target for miRNA-98 in head and neck
squamous cell carcinoma. Mol Cancer. 6:52007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahajan A, Liu Z, Gellert L, et al: HMGA2:
A biomarker significantly overexpressed in high-grade ovarian
serous carcinoma. Modern Pathology. 23:673–681. 2010. View Article : Google Scholar : PubMed/NCBI
|