Introduction
Secreted frizzled-related proteins (sFRPs) are
palmitoylated secreted glycoproteins that are involved in cell
proliferation and neoplastic growth (1,2).
sFRPs consist of ∼300 amino acids and are composed of a
cysteine-rich domain (CRD) at their amino terminal ends with 30–50%
homology to the active site of the Frizzled receptor (3,4). The
CRD of Frizzled serves as the active site for Wnt binding and
subsequent signal transduction. This class of CRD is conserved in
diverse proteins, including the seven-transmembrane class of
tyrosine receptor kinases of the receptor tyrosine kinase-like
orphan receptor (ROR) family (5).
sFRPs act as extracellular signaling ligands and are able to
downregulate Wnt signaling by forming an inhibiting complex with
the Frizzled receptors (6). Since
Wnt causes cancer cells to grow, it was originally hypothesized
that sFRPs are inhibitors of cancer cell growth (7) but a subsequent study revealed that
sFRP-3 (also known as FrzB) is present at high levels in metastatic
renal cancer tissues (8). This
study also demonstrated that sFRP-3 promotes invasion by renal
cancer cells (8). sFRPs have been
linked to tumor-promoting activities in other types of cancer
(9). The elevated levels of sFRP-3
in various types of cancer suggest that it may be a valuable
therapeutic target (7).
Four endogenous cardiac hormones [vessel dilator,
kaliuretic peptide (KP), atrial natriuretic peptide (ANP) and
long-acting natriuretic peptide (LANP)] have anticancer effects
in vivo(10–12), and in vitro have been
reported to decrease the numbers of human renal carcinoma cells by
up to 81% (13), human colorectal
cancer cells by 89–97% (14) and
pancreatic cancer cells by up to 65% (15). The present investigation was
designed to determine whether the four cardiac hormones inhibit
sFRP-3 in human renal carcinoma, human pancreatic cancer and human
colorectal cancer cells as part of their anti-cancer mechanism(s)
of action. The results showed that each of the four cardiac
hormones potently inhibited sFRP-3 in the three different types of
cancer.
Materials and methods
Cardiac hormones
The four cardiac hormones were obtained from Phoenix
Pharmaceuticals, Inc. (Belmont, CA, USA).
Human colorectal, pancreatic and renal
cancer cells
Human colorectal cancer (ATCC number CCL-225),
pancreatic carcinoma (ATCC number CRL-1469, panc-1) and renal
adenocarcinoma (CRL-1611) cells were obtained from American Type
Culture Collection (ATCC; Manassas, VA, USA). The ATCC
authenticated these cell lines and performed the genotype and
phenotype evaluations, including DNA profiles (STR) and cytogenetic
analyses.
Culturing of human colorectal
adenocarcinoma cells
The propagation of the human colorectal
adenocarcinoma cells was performed in Roswell Park Memorial
Institute (RPMI)-1640 medium with 2 mM glutamine adjusted with the
addition of 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM
HEPES, 1 mM 90% sodium pyruvate and 10% fetal bovine serum (FBS;
Sigma Chemical Co., St. Louis, MO, USA) at a temperature of 37°C
with 5% CO2 as recommended by the ATCC. Cells were
dispensed into new flasks with sub-culturing every 6–8 days. The
growth medium was changed every three days.
Culturing of human pancreatic carcinoma
cells
The propagation of the human pancreatic carcinoma
cells was carried out in Dulbecco’s modified Eagle’s plus Ham’s
F12A 1:1 mixture containing 1.2 g/l sodium bicarbonate (Sigma
Chemical Co.) supplemented with 15 mM HEPES and FBS 10% with 5%
CO2 at a temperature of 37°C, as recommended by the
ATCC. Cells were dispensed into new flasks with subculturing every
6–8 days. The growth medium was changed every 3 days.
Culturing of human renal adenocarcinoma
cells
The propagation of the human renal cell
adenocarcinoma cells was carried out in Eagle’s Minimum Essential
Medium supplemented with 2 mM glutamine adjusted by the addition of
1.5 g/l sodium bicarbonate, 1 mM 90% sodium pyruvate and 10% FBS
(Sigma Chemical Co.) with 5% CO2 at a temperature of
37°C, as recommended by the ATCC. Cells were dispensed into new
flasks with subculturing every 6–8 days. The growth medium was
changed every 3 days.
sFRP-3 ELISA
Analysis of sFRP-3 was carried out using the DuoSet
sFRP-3 immunoassay (R&D Systems, Inc., Minneapolis, MN, USA), a
6-hour solid phase ELISA designed to measure sFRP-3 levels in cell
culture. In this assay, an immobilized capture antibody specific
for sFRP-3 binds to sFRP-3 using a standard streptavidin conjugated
to horseradish peroxidase. This ELISA specifically recognizes
sFRP-3 without cross-reactivity or interference with FRP-1, FRP-4
and sFRP-2. The sFRP-3 ELISA was calibrated against a highly
purified NSO-expressed recombinant human sFRP-3 (R&D Systems,
Inc). The standard curve for this assay was calculated using a
four-parameter logistic (4-PL) curve fit.
sFRP-3 research protocol
The human colorectal cancer, pancreatic carcinoma
and renal adenocarcinoma cells were subcultured for 24 h, then
∼5,000 cells of each line in 50 μl of their respective media were
seeded in 96-well plates with 50 μl media containing 10 μM, 1 μM,
100 nM, 10 nM, 1 nM and 100 pM concentrations of each of the four
cardiac hormones separately (i.e. six concentrations of four
cardiac hormones measured six times at each concentration; n=6 for
each concentration). Standards from R&D Systems were diluted
using Reagent Diluent and added to blank wells to serve as
reference points of known sFRP-3 concentrations. In this assay,
absorbance was examined at a 540 nm wavelength using a 96-well
Gen5, Synergy Mx microplate reader (BioTek, Winooski, VT, USA) set
according to the parameters recommended by the manufacturer. There
were 32 controls for each cell line (n=32) and six experimental
determinations for each of the six concentrations of the four
cardiac hormones in the three cancer cell lines (n=6).
Statistical analysis
Data are expressed as the means ± SEM. The
statistical analyses of the data were performed using a Student’s
t-test for unpaired values. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of sFRP-3 in human colorectal
cancer cells
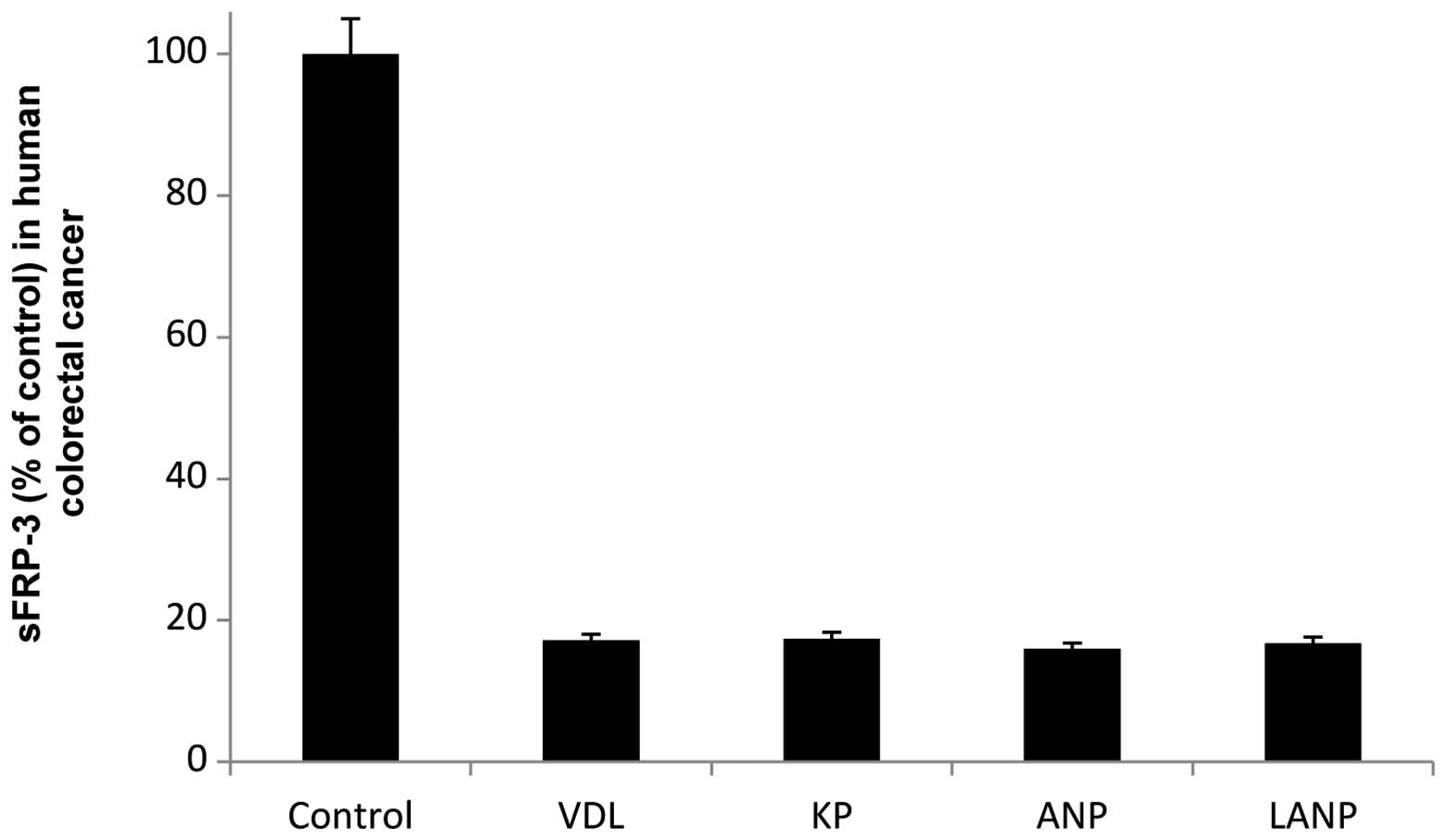
An 83% (P<0.0001) reduction of the sFRP-3 level
was observed in the human colorectal cancer cells following
treatment with 100 nM vessel dilator (Fig. 1). KP and ANP caused maximal
reductions of the sFRP-3 level in the human colorectal cancer cells
of 83% and 84% respectively, both at a concentration of 100 nM
(P<0.0001), while LANP caused a maximal decrease of 83%
(P<0.0001) at a concentration of 10 μM (Fig. 1). In the human colorectal cancer
cells, each of the cardiac hormones caused a similar significant
(P<0.0001) decrease in the sFRP-3 level.
Inhibition of sFRP-3 in human pancreatic
carcinoma cells
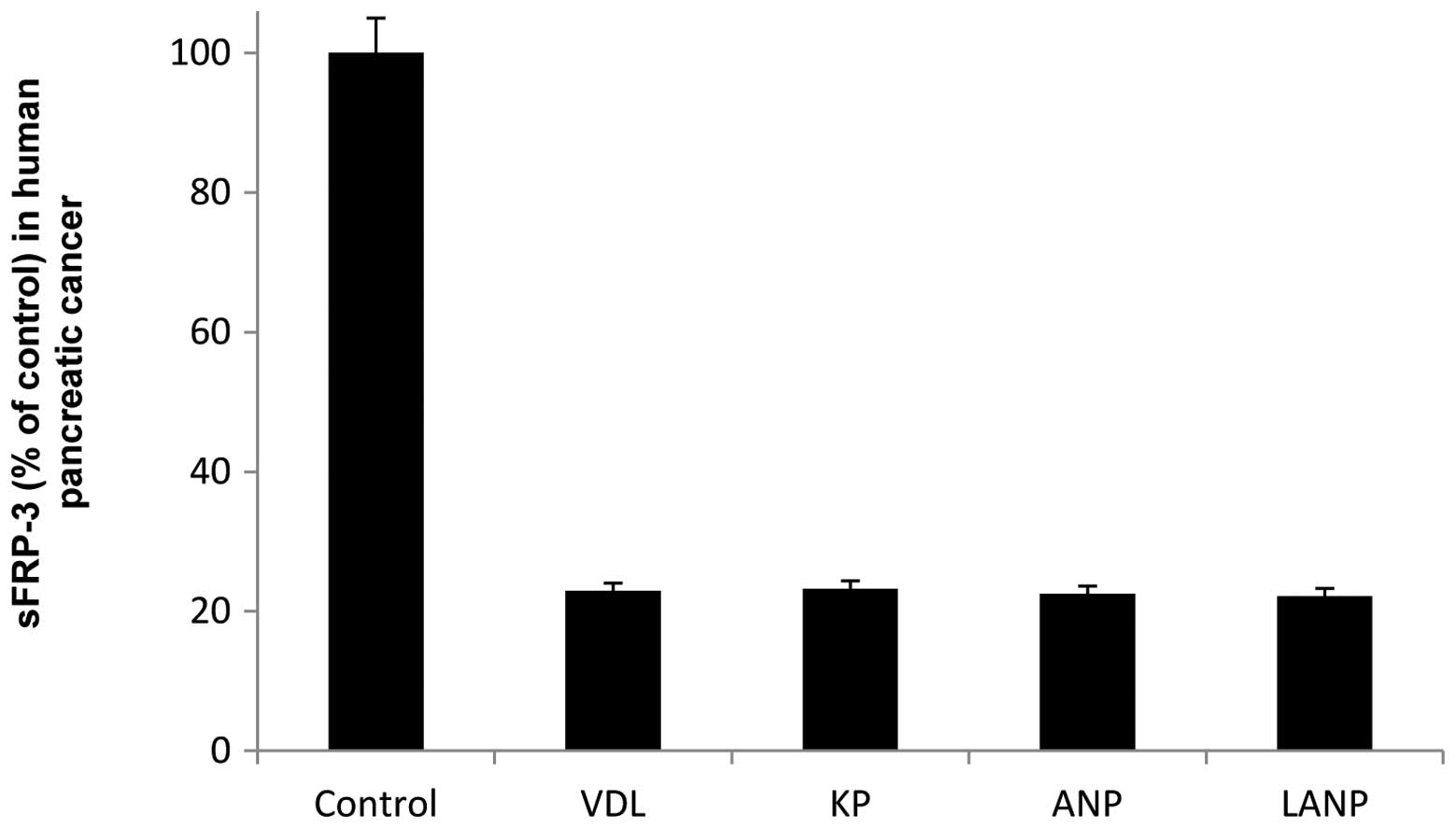
Vessel dilator maximally reduced the sFRP-3 level by
77% (P<0.0001) in the human pancreatic cancer cells at its 1 nM
concentration (Fig. 2). KP and ANP
also maximally reduced the sFRP-3 level in the human pancreatic
cancer cells by 77% (P<0.0001), the former at a concentration of
100 pM and the latter at concentrations of 100 nM and 1 nM
(Fig. 2). LANP reduced the sFRP-3
level in the human pancreatic cancer cells by 78% at its 1 nM
concentration (P<0.0001; Fig.
2). Each of the cardiac hormones had a similar marked ability
to reduce the sFRP-3 level in the human pancreatic cancer
cells.
Inhibition of sFRP-3 in human renal
adenocarcinoma cells
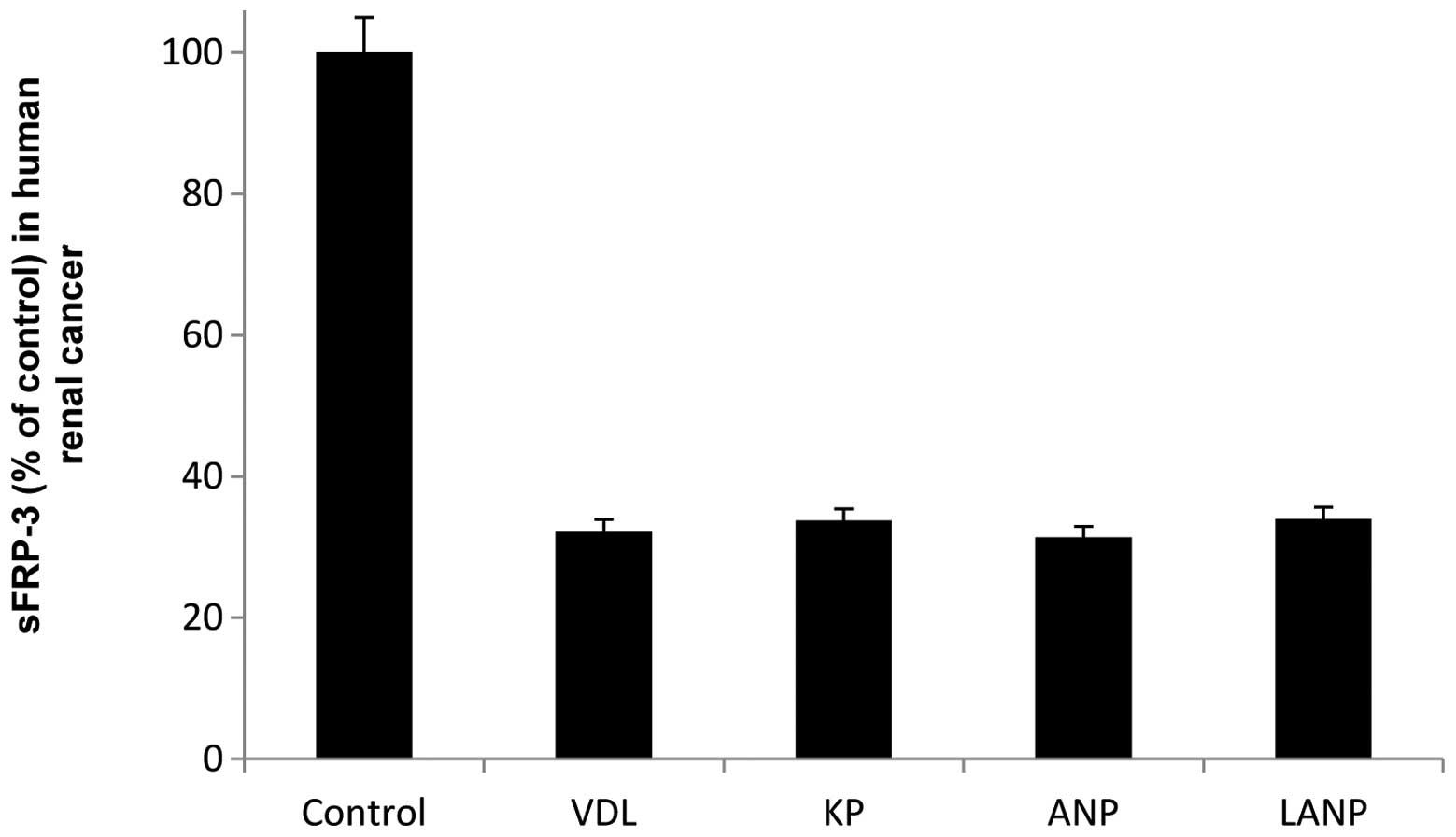
Vessel dilator decreased the sFRP-3 level in the
human renal cancer cells by 68% at a concentration of 1 μM
(P<0.0001), and KP maximally reduced the sFRP-3 level by 66%
(P<0.0001) at the same concentration. The maximal decrease in
the sFRP-3 level of the human renal cancer cells following
treatment with ANP was 68% (P<0.0001) at a concentration of 10
nM and following treatment with LANP was 66% (P<0.0001) at a
concentration of 100 nM (Fig. 3).
In the human renal cancer cells, the abilities of each of the
cardiac hormones to inhibit human sFRP were not significantly
different as each caused similar significant decreases in the
sFRP-3 level. The four cardiac hormones caused similar marked
decreases in the sFRP-3 levels of the human colorectal, pancreatic
and renal cancer cells (Figs.
1–3).
Discussion
It has been reported that sFRP-3 promotes renal
cancer growth when injected into nude mice (8). sFRPs have also been linked to tumor
promotion in other types of cancer (9). It has been suggested (7) that the elevated sFRPs in various
types of cancers may be valuable therapeutic targets. The present
investigation demonstrates that vessel dilator, KP, ANP and LANP
decreased the levels of sFRP-3 by 77–78% in human pancreatic cancer
cells, 83–84% in human colorectal cancer cells and 66–68% in human
renal cancer cells. These significant reductions of sFRP-3 suggest
that it is a target of the four cardiac hormones in a variety of
types of cancer. With respect to the mechanism by which the
reduction of sFRP-3 levels by the cardiac hormones leads to their
anticancer effects, the ability to inhibit sFRP-3, the active CRD
of the Frizzled receptor (3),
blocks the propagation of the signal responsible for causing cancer
cell growth.
It is important to note that the reductions in
sFRP-3 levels (up to 84%) are similar in magnitude to the 80%
elimination of human pancreatic cancers in mice and 86% elimination
of human small-cell lung cancers growing in mice (10,11).
The decrease in FRP-3 is also similar in magnitude (% decrease) in
cell number of cancer cells in vitro(13–15).
These observations suggests that sFRP-3 is an important therapeutic
target of the cardiac hormones in mediating their anticancer
effects (9–11). Furthermore, this target is present
in more than one cancer type, and the present study demonstrates
that sFRP-3 is a treatment target in human pancreatic, renal and
colorectal cancers for each of the four agents evaluated.
Acknowledgements
The authors thank Karen Murphy for
excellent secretarial assistance. The present study was supported
in part by grants from the James and Esther King Florida Biomedical
Research Program, the Florida Department of Health and the Mama
Mare Breast Cancer Foundation.
References
|
1.
|
Dann CE, Hsieh JC, Rattner A, Sharma D,
Nathans J and Leahy DJ: Insights into Wnt binding and signalling
from the structures of two Frizzled cysteine-rich domains. Nature.
412:86–90. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Malbon CC: Frizzleds: new members of the
superfamily of G-protein-coupled receptors. Front Biosci.
9:1048–1058. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rattner A, Hsieh JC, Smallwood PM, Gilbert
DJ, Copeland NG, Jenkins NA and Nathans J: A family of secreted
proteins contains homology to the cysteine-rich ligand-binding
domain of frizzled receptors. Proc Natl Acad Sci U S A.
94:2859–2863. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lin K, Wang S, Julius MA, Kitajewski J,
Moos M Jr and Luyten FP: The cysteine-rich frizzled domain of
Frzb-1 is required and sufficient for modulation of Wnt signaling.
Proc Natl Acad Sci U S A. 94:11196–11200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xu YK and Nusse R: The Frizzled CRD domain
is conserved in diverse proteins including several receptor
tyrosine kinases. Curr Biol. 8:R405–R406. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bovolenta P, Esteve P, Ruiz JM, Cisneros E
and Lopez-Rios J: Beyond Wnt inhibition: new functions of secreted
Frizzled-related proteins in development and disease. J Cell Sci.
121:737–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hirata H, Hinoda Y, Ueno K, Majid S, Saini
S and Dahiya R: Role of secreted Frizzled-related protein 3 in
human renal cell carcinoma. Cancer Res. 70:1896–1905. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rubin JS, Barshishat-Kupper M,
Feroze-Merzoug F and Xi ZF: Secreted WNT antagonists as tumor
suppressors: pro and con. Front Biosci. 11:2093–2105. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Eichelbaum EJ, Sun Y, Alli AA, Gower WR Jr
and Vesely DL: Cardiac and kidney hormones cure up to 86% of human
small-cell lung cancers in mice. Eur J Clin Invest. 38:562–570.
2008.PubMed/NCBI
|
|
11.
|
Vesely DL, Eichelbaum EJ, Sun Y, Alli AA,
Vesely BA, Luther SL and Gower WR Jr: Elimination of up to 80% of
human pancreatic adenocarcinomas in athymic mice by cardiac
hormones. In Vivo. 21:445–451. 2007.
|
|
12.
|
Vesely DL, Vesely BA, Eichelbaum EJ, Sun
Y, Alli AA and Gower WR Jr: Four cardiac hormones eliminate up to
two-thirds of human breast cancers in athymic mice. In Vivo.
2:973–978. 2007.PubMed/NCBI
|
|
13.
|
Vesely BA, Eichelbaum EJ, Alli AA, Sun Y,
Gower WR Jr and Vesely DL: Urodilatin and four cardiac hormones
decrease human renal carcinoma cell numbers. Eur J Clin Invest.
36:810–819. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gower WR, Vesely BA, Alli AA and Vesely
DL: Four peptides decrease human colon adenocarcinoma cell number
and DNA synthesis via cyclic GMP. Int J Gastrointest Cancer.
36:77–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Vesely BA, McAfee Q, Gower WR Jr and
Vesely DL: Four peptides decrease the number of human pancreatic
adenocarcinoma cells. Eur J Clin Invest. 33:998–1005. 2003.
View Article : Google Scholar : PubMed/NCBI
|