Introduction
Cancer is one of the leading causes of mortality
worldwide. Chemotherapy is one of the major therapeutic modalities
commonly used for the treatment of a variety of cancer types.
However, in numerous cases, chemotherapy cannot achieve a
satisfactory therapeutic outcome, namely the complete remission of
tumors, and induces severe side-effects at therapeutically
effective doses. Cyclophosphamide (CTX) has been used widely in
chemotherapy since the late 1950s and has been shown to have a high
therapeutic index and broad spectrum of activity against a variety
of types of cancer (1). However,
the use of CTX as an effective chemotherapeutic agent is often
restricted due to its wide toxicity and adverse side-effects, which
include leukopenia, myelosuppression and immunosuppression
(2,3). CTX is presently being used in
combination with various detoxifying and protective agents with the
purpose of reducing or eliminating its adverse toxic effects.
Panax quinquefolius L. has been used
worldwide for thousands of years in traditional herbal medicine
(4). Ginsenosides are considered
to be one of main bioactive constituents of Panax
quinquefolius L. and have been used clinically for the
treatment of cardiovascular diseases and stroke in China (5). The anticancer properties of Panax
quinquefolius L. and/or ginsenosides have been well documented
in vitro and in vivo(6–8).
Upon oral consumption, Panax quinquefolius L. or
ginsenosides are partly transformed into 20(S)-protopanaxadiol
(PPD) through a series of deglycosylations by acid hydrolysis and
intestinal bacterial actions (9).
PPD-type ginsenosides are generally considered to be the most
pharmacologically active components of Panax quinquefolius
L. At present, the valuable information concerning the
pharmacological and toxic effects of PPD combined with
chemotherapeutic agents is scarce. The present study aimed to
evaluate whether PPD is able to exert beneficial effects on
antitumor activity and toxicity of CTX in tumor-bearing mice
Materials and methods
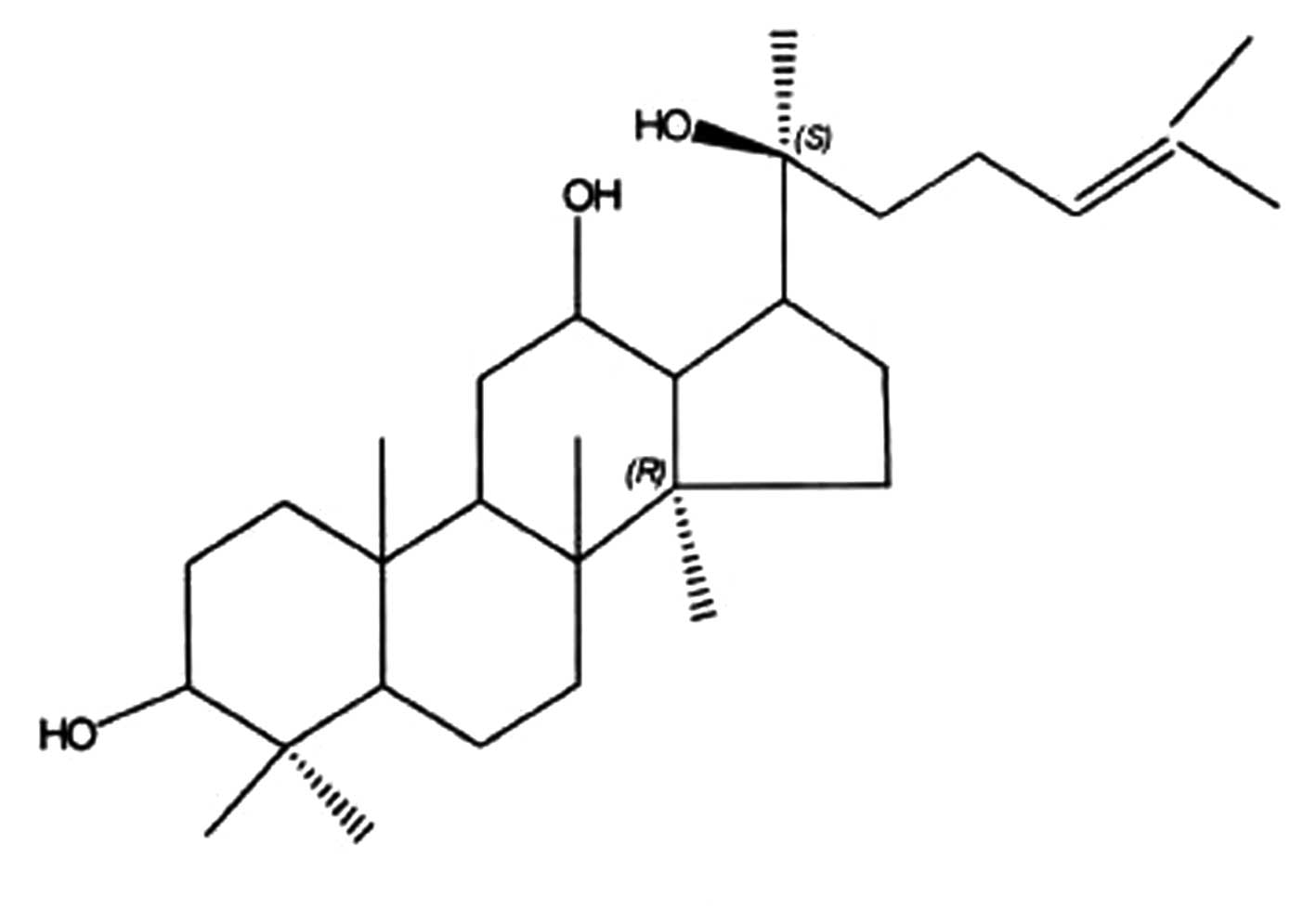
Preparation of PPD
PPD is extracted from protopanaxadiol Rh2
(Hainan Asia Pharmaceutical Co. Ltd., Haikou, China) that is
prepared from the roots and leaves of Panax quinquefolium L.
by the alkali hydrolysis of glucose at the C-3 position of the
dammarane structure at 25°C. At normal pressure, the reaction
medium, 4-butanediol caused the reaction temperature to increase to
180°C in alkali solution. The PPD was purified by silica gel column
chromatography and acetic ether re-crystallization. The yield of
the extract as a dried material was ∼1.8% by weight of the original
material. Further analysis by HPLC showed that the content of PPD
in the resultant extract was 98.6% (Fig. 1).
Animals
Male C57BL/6 mice (7–8 weeks old) were purchased
from the Institute of Zoology of the Chinese Academy of Sciences
(Beijing, China) and the certificate number was SCXK11-00-0006. The
mice were acclimated to laboratory conditions (22±2°C) and (55±5%
humidity) for 7 days, with a commercial standard mouse cube diet
(Experimental Animal Center of Jilin University, Changchun, China)
and water ad libitum prior to the experiment. All animal
experiments were conducted in compliance with the National
Institute of Health Guidelines for the Care and Use of Laboratory
Animals (publication 86-23, revised in 1986) and were approved by
the local Ethics Committee.
Materials
The culture medium RPMI-1640 and fetal calf serum
were from Gibco (Grand Island, NY, USA). The concanavalin A was
purchased from Sigma Chemical Co. (St. Louis, MO, USA). The
penicillin and streptomycin were from Huabei Pharmaceutical Co.
Ltd. (Shijiazhuang, China). Lewis lung carcinoma (LLC) cells were
purchased from the China Center for Type Culture Collection
(Beijing, China). The CTX was provided by the Jiangsu Hengrui
Company (Jiangsu, China). The ELISA kits of interleukin-2 (IL-2)
and interferon-γ (INF-γ) were purchased from Westang Biomedical
Technology Company (Shanghai, China).
Treatment and drug administration
LLC cells were cultured in RPMI-1640 complete medium
with 10% heat-inactivated FBS. LLC cells (0.2 ml, 1×107
cells/ml in sodium chloride) were implanted subcutaneously into the
mice. After implantation for 24 h, the mice bearing LLC cells were
randomly divided into four groups with 10 mice in each: the control
group (Control), PPD (50 mg/kg) alone group (PPD), CTX (20 mg/kg)
alone group (CTX) or PPD (50 mg/kg) in combination with CTX (20
mg/kg) group (PPD+CTX). PPD suspended in saline was orally
administered once a day for 14 consecutive days. CTX dissolved in
saline was intraperitoneally injected once a day on days 1, 3, 5
and 7 (total 4 injections) at a dose of 20 mg/kg body weight. The
mice in the PPD+CTX group received orally administered PPD (50
mg/kg) once a day for 14 consecutive days and were
intraperitoneally injected with CTX once a day on days 1, 3, 5 and
7. The mice in the control group received saline alone (20
ml/kg).
Antitumor activity of PPD in combination
with CTX
On day 15, the mice were anesthetized with sodium
pentobarbital (300 mg/kg, intraperitoneally) and were sacrificed by
cervical dislocation. The implanted sarcomas of each group were
then separated and weighed.
Peripheral white blood cell and bone
marrow cell counts
Blood and serum samples and femur bones were
obtained from the tumor-bearing mice. Blood was collected from the
retroorbital venous plexus in heparinized tubes. Bone marrow was
collected from the left femur bones by flushing thoroughly with
Hank’s balanced solution using a 28-gauge needle in a 1-ml syringe.
The cells were collected in a sterile tube and diluted to a total
volume of 5 ml. Peripheral white blood cell (WBC) and bone marrow
cell (BMC) counts were evaluated microscopically using a
hematocytometer (ABX micros 60; Horiba, Montpellier, France). The
serum IL-2 and INF-γ levels were assayed using commercial reagent
kits.
Spleen index and splenocyte proliferation
assay
After the last drug administration (24 h), the mice
were weighed and sacrificed by cervical dislocation. The spleens of
the mice were removed and weighed under sterile conditions. The
spleen index was calculated as spleen weight (mg)/body weight (g).
The fresh spleen sample was used to evaluate the splenocyte
proliferation. Splenocytes from the tumor-bearing mice were
prepared as previously described (10) and seeded into 96-well flat-bottom
microplates at 1×106 cell/ml in 100 μl complete medium.
Subsequently, concanavalin A (final concentration 5 μg/ml) or
RPMI-1640 medium were added, giving a final volume of 200 μl. The
plates were incubated at 37°C in a humid atmosphere with 5%
CO2. After 44 h, 50 μl of MTT solution (2 mg/ml) were
added to each well and incubated for further 4 h. The plates were
centrifuged (1400 x g, 5 min) and the untransformed MTT was removed
carefully by pipetting. To each well, 150 μl of a DMSO working
solution (180 μl DMSO with 20 μl 1 M HCl) was added and the
absorbance was evaluated using an ELISA reader (Synergy HT; BioTek,
Winooski, VT, USA) at 570 nm after 15 min. The splenocyte
proliferation was calculated based on the following formula:
splenocyte proliferation = absorbance value of concanavalin
A-stimulated cultures / absorbance value of non-stimulated
cultures.
Natural killer (NK) cell activity
Splenocytes were prepared as the effector cells for
the splenic NK cell activity assay as described previously
(11). YAC-1 cells were used as
the target cells. Briefly, effector cells (5×105
cells/well) in 96-well flat-bottom microplates were co-cultured in
triplicate with target cells at 37°C in a humid atmosphere of 5%
CO2 at a ratio of effector to target cells of 50:1.
Serum-free RPMI-1640 medium was used as a control. The NK cell
activity of the splenocytes was measured using the MTT assay after
24 h of culture. The MTT solution (5 mg/ml) was added to each well.
After 4 h of incubation, the cells were lysed and the purple
formazan crystals were solubilized by DMSO for detection at 570 nm
using a microplate reader. The NK activity of the effector cells
was calculated using the following formula: cytotoxicity (%) =
(A+B−C) / A×100, where A is the absorbance of the well of target
cells, B is the absorbance of the well of effector cells and C is
the absorbance of the experimental well.
Statistical analysis
The data were presented as mean ± standard deviation
(SD). Data were analyzed by one-way analysis of variance (ANOVA),
followed by Student-Newman-Keuls tests. P<0.05 was considered to
indicate statistically significant differences.
Results
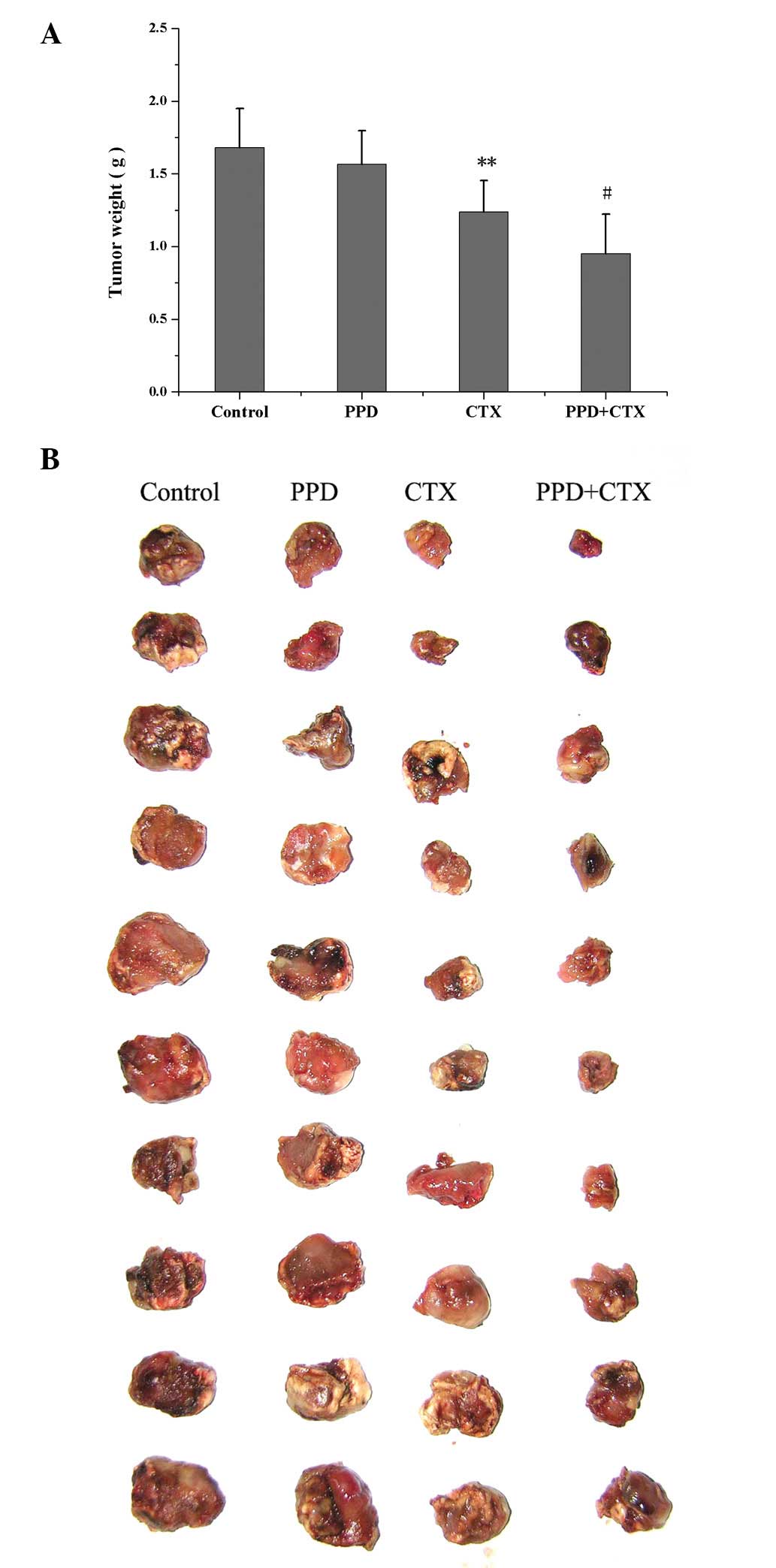
Effects of PPD on antitumor activity in
CTX-treated tumor-bearing mice
The effects of PPD on CTX-induced changes of tumor
weight in tumor-bearing mice are shown in Fig. 2. Compared with the control group,
PPD alone had no effects on tumor weight, while CTX alone
significantly reduced tumor weight (P<0.01). PPD in combination
with CTX significantly decreased tumor weight (P<0.05).
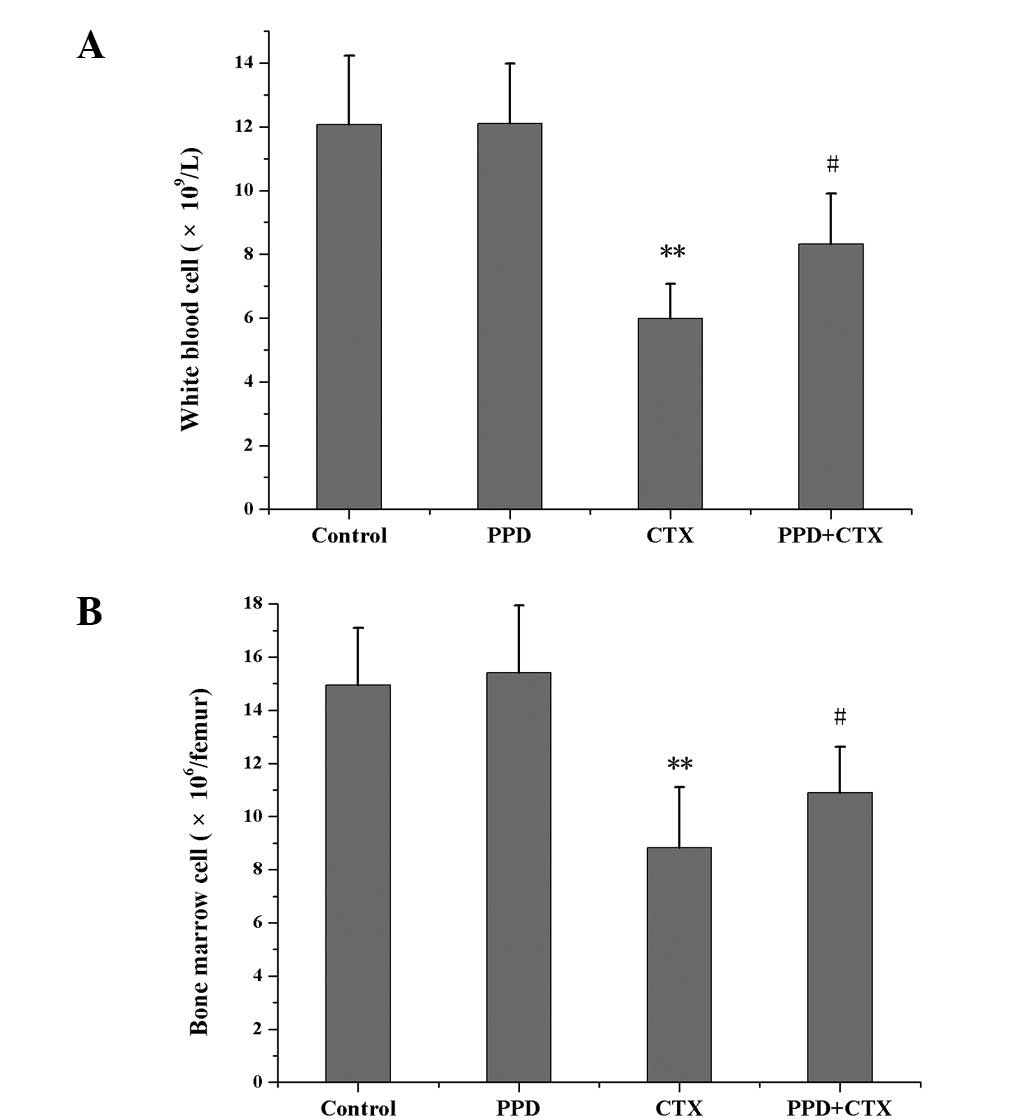
Effects of PPD on WBC and BMC counts in
CTX-treated tumor-bearing mice
Compared with the control group, PPD alone had no
effects on WBC and BMC counts, while CTX alone reduced WBC and BMC
(P<0.01) counts. However, compared with the CTX group, PPD in
combination with CTX significantly increased WBC and BMC counts
(P<0.05; Fig. 3).
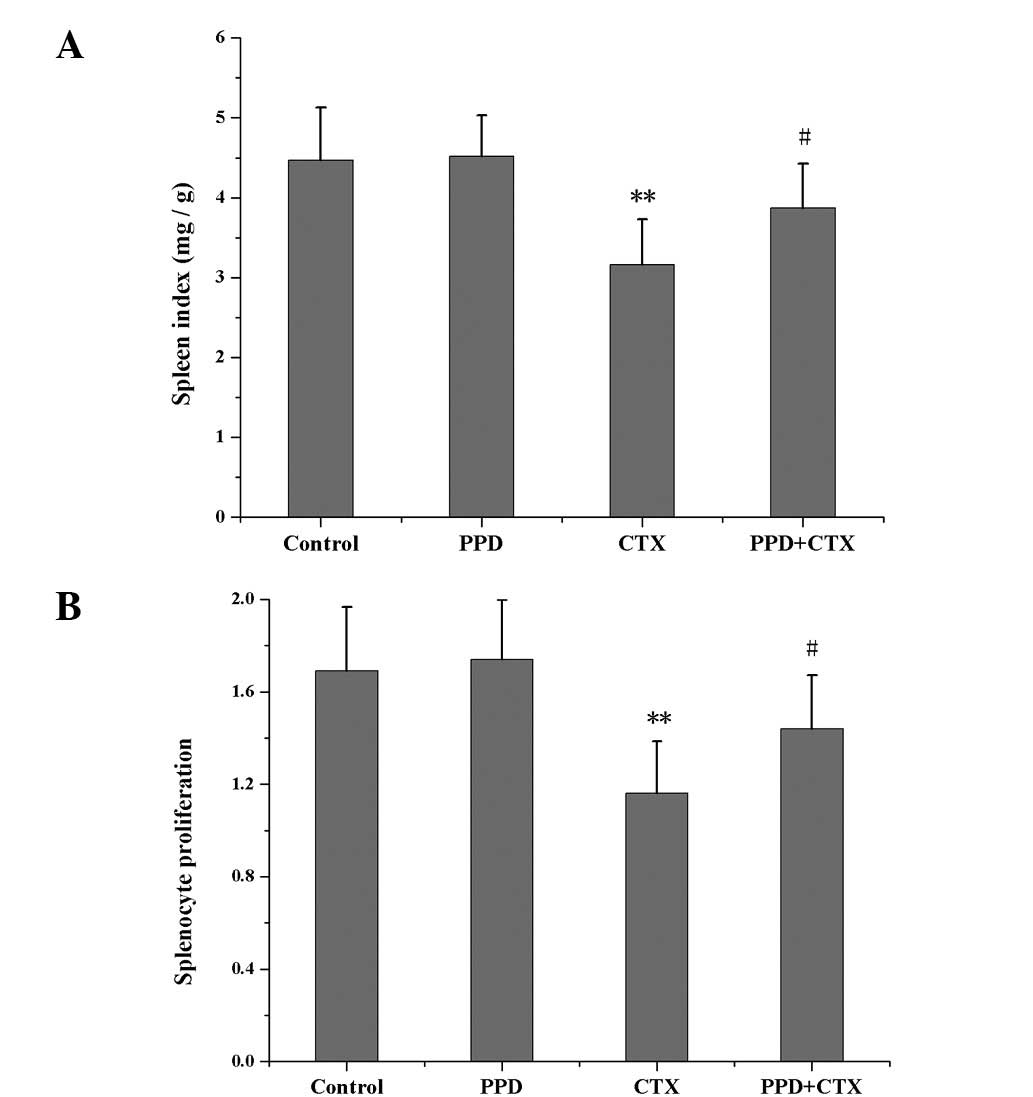
Effects of PPD on spleen index and
splenocyte proliferation in CTX-treated tumor-bearing mice
As shown in Fig. 4,
the spleen index and splenocyte proliferation values of the
CTX-treated group were much lower than those of the control group
(P<0.01). Treatment with PPD in combination with CTX increased
the spleen index and splenocyte proliferation in tumor-bearing mice
(P<0.05).
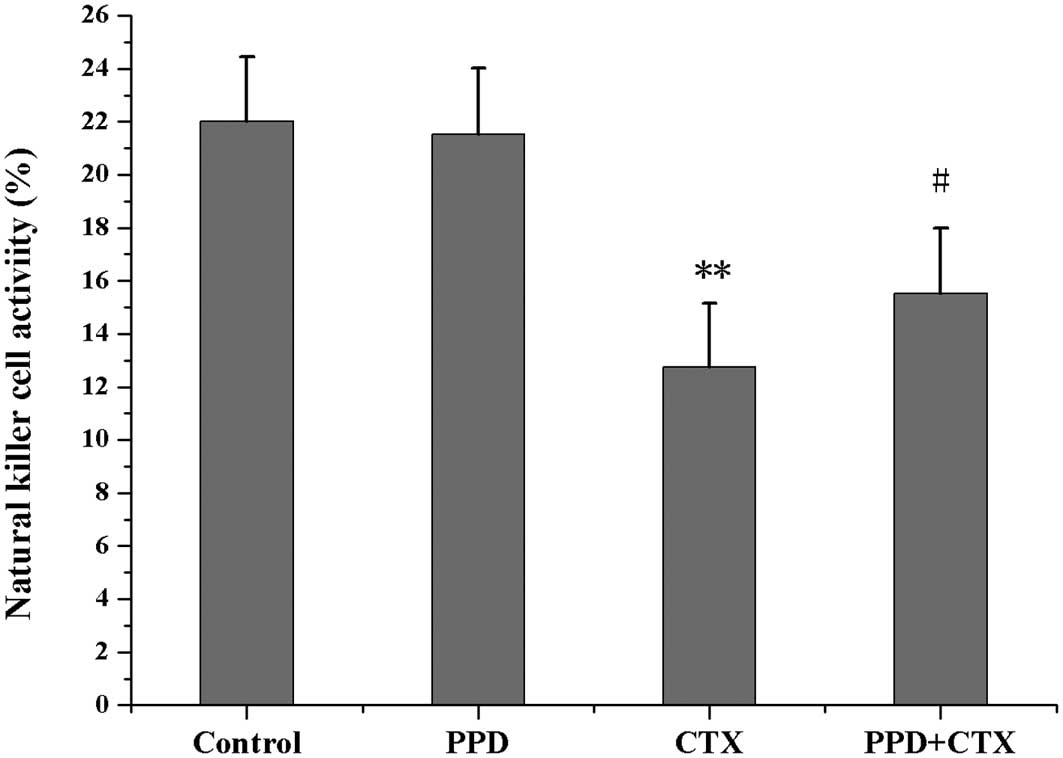
Effects of PPD on NK cell activity in
CTX-treated tumor-bearing mice
As shown in Fig. 5,
NK cell activity in CTX-treated tumor-bearing mice was markedly
decreased when compared with the control group (P<0.01).
However, NK cell activity in the tumor-bearing mice co-treated with
PPD and CTX was significantly higher than that of mice receiving
CTX treatment alone (P<0.05).
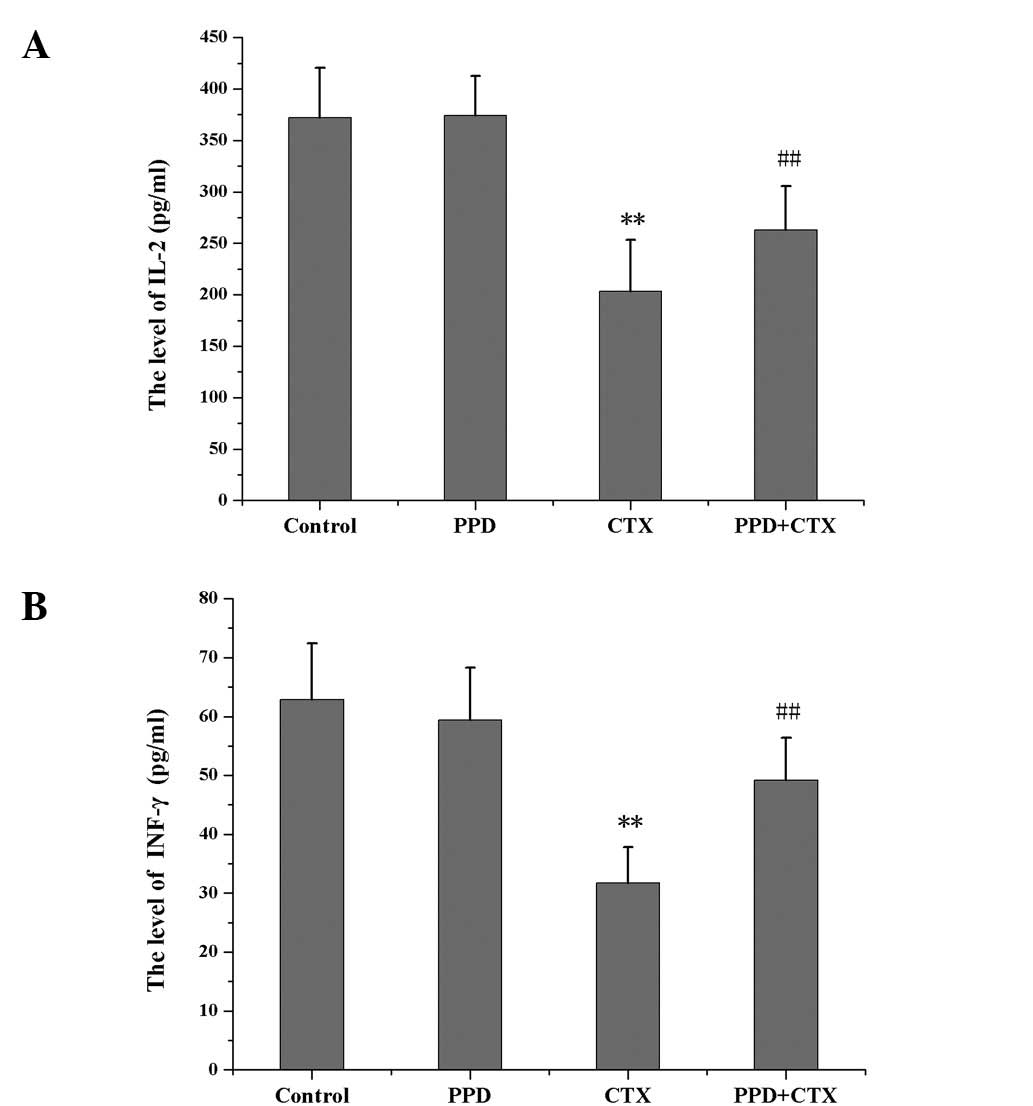
Effects of PPD on the levels of IL-2 and
INF-γ in CTX-treated tumor-bearing mice
IL-2 and INF-γ levels were significantly decreased
in CTX-treated tumor-bearing mice as compared with those of the
control group (P<0.01). Compared with the CTX group, the IL-2
and INF-γ levels in the PPD+CTX group were significantly increased
(P<0.01; Fig. 6).
Discussion
Although CTX is a drug widely applied in the
treatment of malignant and nonmalignant tumors, the clinical
outcomes of treatments with these agents are severely limited,
mostly due to its toxicity to normal tissues. Therefore, it is
necessary to develop adjuvant therapy which may be used in
combination with CTX to improve the efficacy of the treatment or
reduce the associated undesirable side-effects (12). The main objective of this study was
to evaluate the effect of PPD on the antitumor activity and
toxicity of CTX in tumor-bearing mice.
Following an administration of PPD alone to
LLC-bearing mice, the tumor weight was not reduced. By contrast,
the combination of PPD with CTX significantly reduced the tumor
weight when compared with that of the control and CTX alone groups.
Thereby, it is possible to conclude that PPD is able to enhance
antitumor activity of CTX.
The peripheral WBC and BMC counts are two frequently
studied clinical parameters which accurately reflect
chemotherapeutic injuries. In order to study the effect of PPD on
CTX-induced leukopenia and myelosuppression, the peripheral WBC and
BMC counts in CTX-treated LLC-bearing mice were measured. The
results showed that PPD significantly recovered the reduced WBC and
BMC counts in LLC-bearing mice treated with CTX, suggesting that
PPD may provide preferential protection against leukopenia and
myelosuppression induced by CTX.
The spleen is one of the immune organs that
generates immune cells, such as lymphocytes and macrophages, which
phagocytose and destroy bacteria and dead tissue in order to remove
them from the circulating blood (13). The most sensitive indicator of
immunosuppression, particularly in short-term studies, is a
decrease in the relative spleen weight. The proliferation of
splenocytes is known to be a response to stimulation induced by
antigens or mitogens, which is a typical non-specific immune
reaction with a well-understood mechanism. Moreover, this assay has
been extensively used as an immune parameter to investigate
lymphocyte responsiveness due to its high sensitivity. In the
present experiment, CTX not only caused spleen atrophy but also
decreased splenocyte proliferation. However, the results showed
that treatment with PPD inhibited spleen atrophy and promoted the
recovery of splenocyte proliferation in CTX-treated tumor-bearing
mice.
The NK cell is an important part of the innate
immune system and is key to the first-line defense against
malignancies. Therefore, the use of NK cells in human cancer
immunotherapy has been suggested and treatments using these cells
have recently entered clinical trials (14). A number of treatment strategies
have also been exploited to activate endogenous NK cells, promote
NK cell proliferation or induce more potent NK cell-mediated
antitumor responses (15). One
major strategy is the systemic administration of cytokines involved
in NK cell differentiation and activation, such as IL-2 and
interferons (16). Cytokines
regulate the innate immune system and increase NK cell activity. NK
cells also regulate the adaptive immune system and responses to
produce cytokines (17). Cytokines
have been used successfully to treat several human cancers through
the direct or indirect activation of NK cells (18). IL-2 is an autocrine growth factor
from T lymphocytes and the transcription of IL-2 is an important
step in T cell activation. IFN-γ is produced predominantly by T
lymphocytes and NK cells following activation with immune and
inflammatory stimuli rather than viral infection (19). The present findings showed that CTX
caused significant decreases in NK cell activity and the levels of
IL-2 and IFN-γ, which are consistent with previous studies
(20). However, PPD significantly
increased the NK cell activity and levels of IL-2 and IFN-γ,
suggesting that PPD may improve cellular immune function in
CTX-treated tumor-bearing mice.
In summary, the results of the present study
demonstrated that PPD synergistically enhanced the antitumor
activity of CTX. PPD significantly increased WBC count, BMC count
and the levels of IL-2 and IFN-γ in CTX-treated tumor-bearing mice.
The lowered levels of spleen index, splenocyte proliferation and NK
cell activity in tumor-bearing mice following CTX treatment were
also increased by PPD administration. Therefore, PPD may be a
beneficial supplement during CTX chemotherapy to enhance the
antitumor efficacy and reduce the toxicity of CTX.
Acknowledgements
The present study was supported by the
Foundation of Science and Technology for Key Projects of Jilin
province, China (grant no. 2002JL204A07). The authors would like to
thank Dr. Yongri King and Xuwen Li for their technical assistance
and helpful comments during the preparation of this manuscript.
References
|
1.
|
Pass GJ, Carrie D, Boylan M, et al: Role
of hepatic cytochrome p450s in the pharmacokinetics and toxicity of
cyclophosphamide: studies with the hepatic cytochrome p450
reductase null mouse. Cancer Res. 65:4211–4217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Papaldo P, Lopez M, Marolla P, et al:
Impact of five prophylactic filgrastim schedules on hematologic
toxicity in early breast cancer patients treated with epirubicin
and cyclophosphamide. J Clin Oncol. 23:6908–6918. 2005. View Article : Google Scholar
|
|
3.
|
Pratheeshkumar P and Kuttan G:
Ameliorative action of Vernonia cinerea L. on
cyclophosphamide-induced immunosuppression and oxidative stress in
mice. Inflammopharmacology. 18:197–207. 2010.
|
|
4.
|
Iyer R, Evans A, Qi X, Ho R, Minturn J,
Zhao H, Balamuth N, Maris J and Brodeur G: Lestaurtinib enhances
the antitumor efficacy of chemotherapy in murine xenograft models
of neuroblastoma. Clin Cancer Res. 16:1478–1485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang T, Yu X, Qu S, Xu H, Han B and Sui D:
Effect of ginsenoside Rb3 on myocardial injury and heart function
impairment induced by isoproterenol in rats. Eur J Pharmacol.
636:121–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Helms S: Cancer prevention and
therapeutics: Panax ginseng. Altern Med Rev. 9:259–274.
2004.
|
|
7.
|
Yun TK: Panax ginseng - a
non-organ-specific cancer preventive? Lancet Oncol. 2:49–55. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang CZ, Du GJ, Zhang Z, et al:
Ginsenoside compound K, not Rb1, possesses potential
chemopreventive activities in human colorectal cancer. Int J Oncol.
40:1970–1976. 2012.PubMed/NCBI
|
|
9.
|
Kaneko H and Nakanishi K: Proof of the
mysterious efficacy of ginseng: basic and clinical trials: clinical
effects of medical ginseng, korean red ginseng: specifically, its
anti-stress action for prevention of disease. J Pharmacol Sci.
95:158–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang T, Fu F, Zhang L, Han B, Zhu M and
Zhang X: Effects of escin on acute inflammation and the immune
system in mice. Pharmacol Rep. 61:697–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tsai YC and Won SJ: Effects of tramadol on
T lymphocyte proliferation and natural killer cell activity in rats
with sciatic constriction injury. Pain. 92:63–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Robak T, Lech-Maranda E and Robak P:
Rituximab plus fludarabine and cyclophosphamide or other agents in
chronic lymphocytic leukemia. Expert Rev Anticancer Ther.
10:1529–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mebius RE and Kraal G: Structure and
function of the spleen. Nat Rev Immunol. 5:606–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Koehn T, Trimble L, Alderson K, Erbe A,
McDowell K, Grzywacz B, Hank J and Sondel P: Increasing the
clinical efficacy of NK and antibody-mediated cancer immunotherapy:
potential predictors of successful clinical outcome based on
observations in high-risk neuroblastoma. Front Pharmacol. 3:912012.
View Article : Google Scholar
|
|
15.
|
Ljunggren HG and Malmberg KJ: Prospects
for the use of NK cells in immunotherapy of human cancer. Nat Rev
Immunol. 7:329–339. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Smyth MJ, Cretney E, Kershaw MH and
Hayakawa Y: Cytokines in cancer immunity and immunotherapy. Immunol
Rev. 202:275–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang G, Zhao J, Liu J, Huang Y, Zhong JJ
and Tang W: Enhancement of IL-2 and IFN-gamma expression and NK
cells activity involved in the anti-tumor effect of ganoderic acid
Me in vivo. Int Immunopharmacol. 7:864–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee J and Lim KT: SJSZ glycoprotein (38
kDa) modulates expression of IL-2, IL-12, and IFN-γ in
cyclophosphamide-induced Balb/c. Inflamm Res. Jul 20–2012.(Epub
ahead of print).
|