Introduction
Spinal cord injury (SCI) in experimental animals,
particularly in adult mammalian models, is often associated with
varying degrees of spontaneous functional recovery. The ability of
the central nervous system to recover from injury is sometimes
remarkable and at other times frustratingly limited but in either
case it remains poorly understood (1–4).
Although recent studies have demonstrated axonal
regeneration across the transected spinal cord, it has been
suggested that the regenerated axons may not have been transected
completely. This could not be resolved by the normal transected
spinal cord model. We therefore established a method for shortening
the rat spine and spinal cord at the thoracic level to provide a
transected SCI model in which there was no doubt about whether
complete axonal transection was achieved.
While a great deal of attention has been paid to the
acute stage of SCI, there have been few studies concerning axonal
regeneration in the chronic period of complete paralysis following
SCI despite the majority of patients being chronic paralysis
patients.
The model developed in the present study was also
based on the assumption that glial scar tissue inhibits axonal
regeneration in chronic SCI.
Materials and methods
Animal care
The present study was undertaken at the Laboratory
for Experimental Studies of Yamaguchi University in accordance with
the Guidelines for Animal Experiments at Yamaguchi University
School of Medicine and the Law and Notification of the Government,
following the approval of the experimental design by the Committee
on the Ethics of Animal Experiments at Yamaguchi University School
of Medicine.
Adult, female Wistar rats (220–250 g) were used in
the study. The animals were provided with an ordinary laboratory
diet and water. The rats were housed in cages in rooms with
controlled lighting and temperature. Female rats were used due to
the ease of managing their bladder expression in SCI
experiments.
Surgical method
The surgery was performed under anesthesia by an
intramuscular injection of ketamine (60 mg/kg; Sankyo Co. Ltd.,
Tokyo, Japan) and xylazine (5 mg/kg; Bayer AG, Leverkusen,
Germany), with the assistance of a surgical microscope. Rats were
positioned on the special operating table in the prone position,
immobilized and the hair on the back area was shaved. Following a
Th5-12 midline skin incision and paravertebral muscle dissection,
the spinous processes and laminar arcs of Th7-9 were removed.
The 7th and 9th ribs were dissected from the pleura
to allow them to be passed by stitches (3/0 polyamide
multi-filament) for bringing together and wiring the Th7 spine to
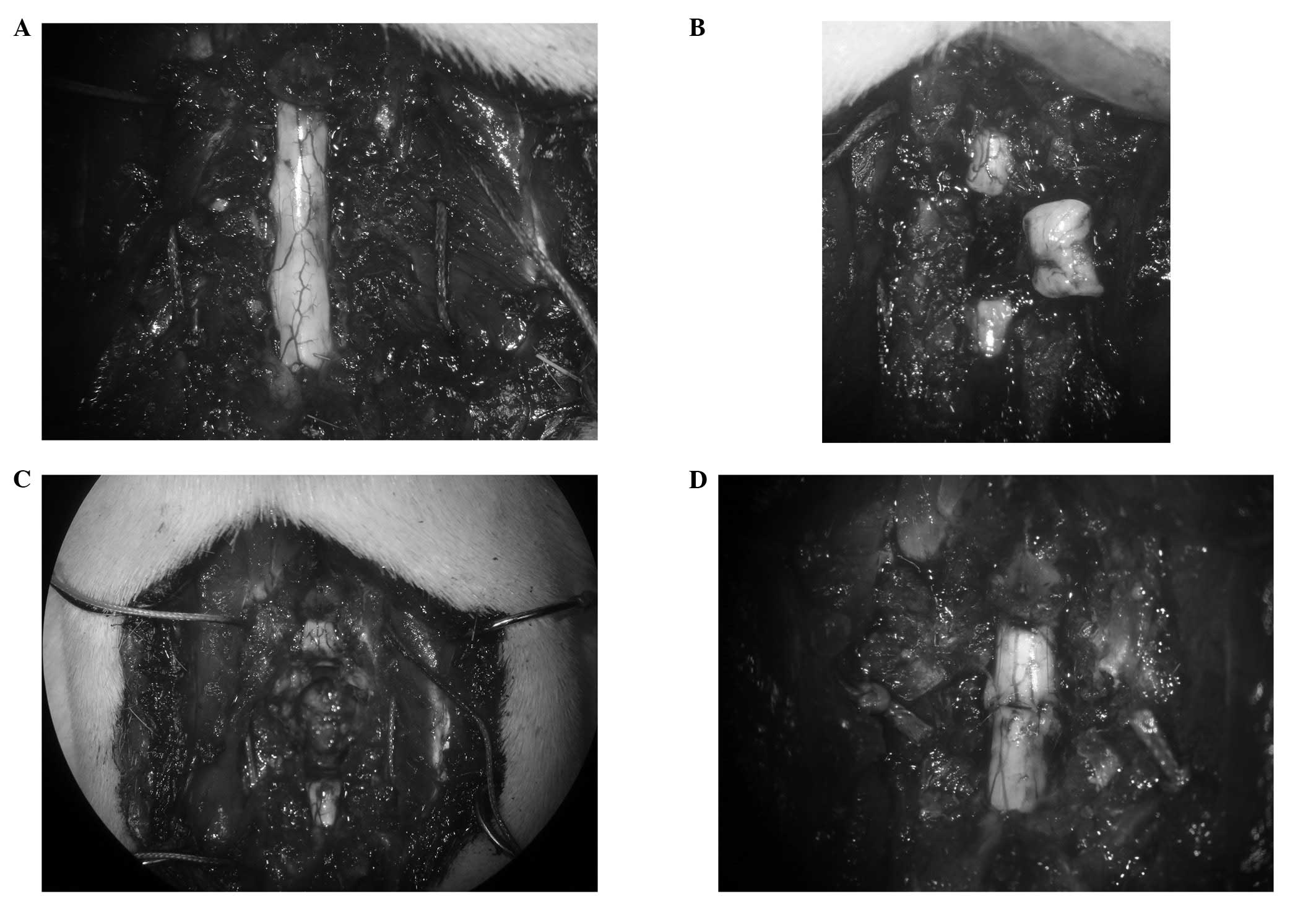
the Th9 spine (Fig. 1A). The
spinal cord was exposed and a 4-mm-long segment was removed at Th8
(Fig. 1B) using the edge of a
razor (FEATHER Safety Razor Co., Ltd., Osaka, Japan). Care was
taken to transect the spinal cord with as sharp a razor as possible
to minimize traumatic injury. The 8th rib was freed from the pleura
and dissected from the costovertebral joint. Subsequently, the
Th7/8 and Th8/9 discs were cut between the stumps of the spinal
cord using a sharp-pointed knife to remove the Th8 vertebra
(Fig. 1C). The stitches which had
been passed by the the 7th and 9th ribs bilaterally were tied
gradually to bring together the stumps of the spinal cord (Fig. 1D). Finally, the muscle and the
incision were sutured with a 3/0 polyamide multifilament.
Following the surgical procedure, the rats were
placed in a warming chamber for a number of days to maintain their
body temperature. Manual bladder expression was performed twice a
day until the voiding reflexes were reestablished.
Scaffold model
This model used a scaffold to bridge the defect of
the spinal cord. A total of 4,000 collagen filaments, each 20 μm in
diameter and made of highly purified type 1 atelocollagen, were
used to create the nerve scaffold (5–10). A
9-mm-long segment of the spinal cord was removed, producing a gap
of ∼5 mm in the spinal cord following the shortening of the spine.
A scaffold of almost the same size as the resected portion was then
implanted in the gap.
Histological analysis
After 12 weeks, the animals were deeply anesthetized
and an intracardiac perfusion was performed with isotonic saline
for 5 min, followed by 4% paraformaldehyde in 0.1 M
phosphate-buffered saline (PBS) for 5 min. Following the perfusion,
the thoracic spine and spinal cord were removed.
The spinal cord tissue was immersed in
paraformaldehyde in 0.1 M PBS at 4°C overnight. The samples were
cut longitudinally into 6-μm thick sections which were stained with
hematoxylin and eosin (H&E). The sections were then examined by
light microscopy.
Results
Transected SCI model
The procedure did not require the aid of an
assistant. A few rats operated on initially died in the
intraoperative period and the deaths were attributed to
pneumothorax caused by the rupture of the pleura during the removal
of the Th8 vertebra. Almost all the rats survived until the end of
the experiment.
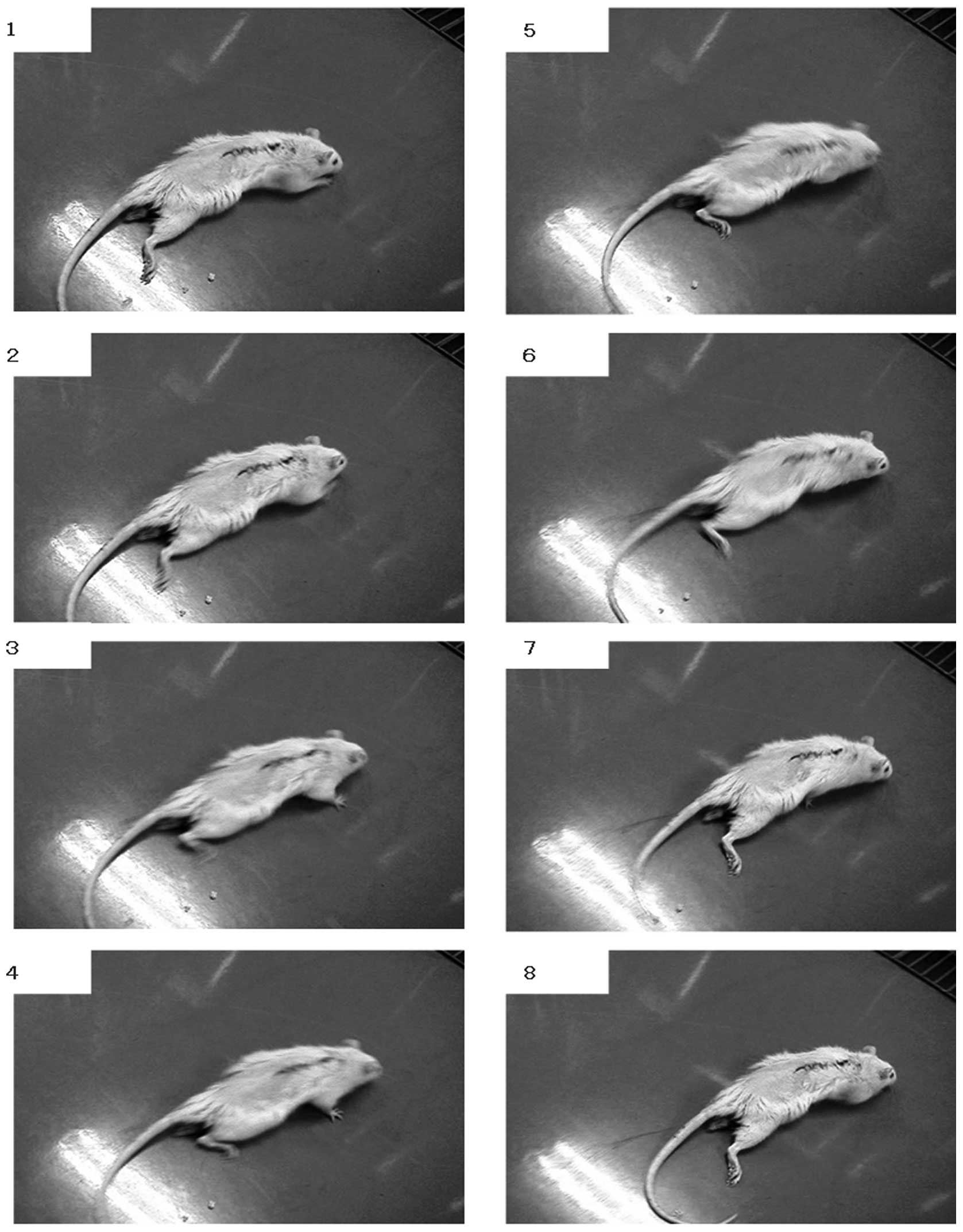
The rats had complete flaccid paraplegia immediately
after the cord resection. The hind limbs were flaccid in all rats 1
week after the surgery. The rats dragged themselves around with
their forelimbs and the hind limbs were stretched out behind. The
scratch reflex of the hind limbs was detected at 2–3 weeks after
surgery. Uncoordinated movements of the hind limbs in locomotion
were observed at 4 weeks after surgery (Fig. 2). However coordinated movements of
the hind limbs in locomotion were not observed until the end of the
experiment and the maximum Basso-Beattie-Bresnahan (BBB) score was
4.
Spinal analysis
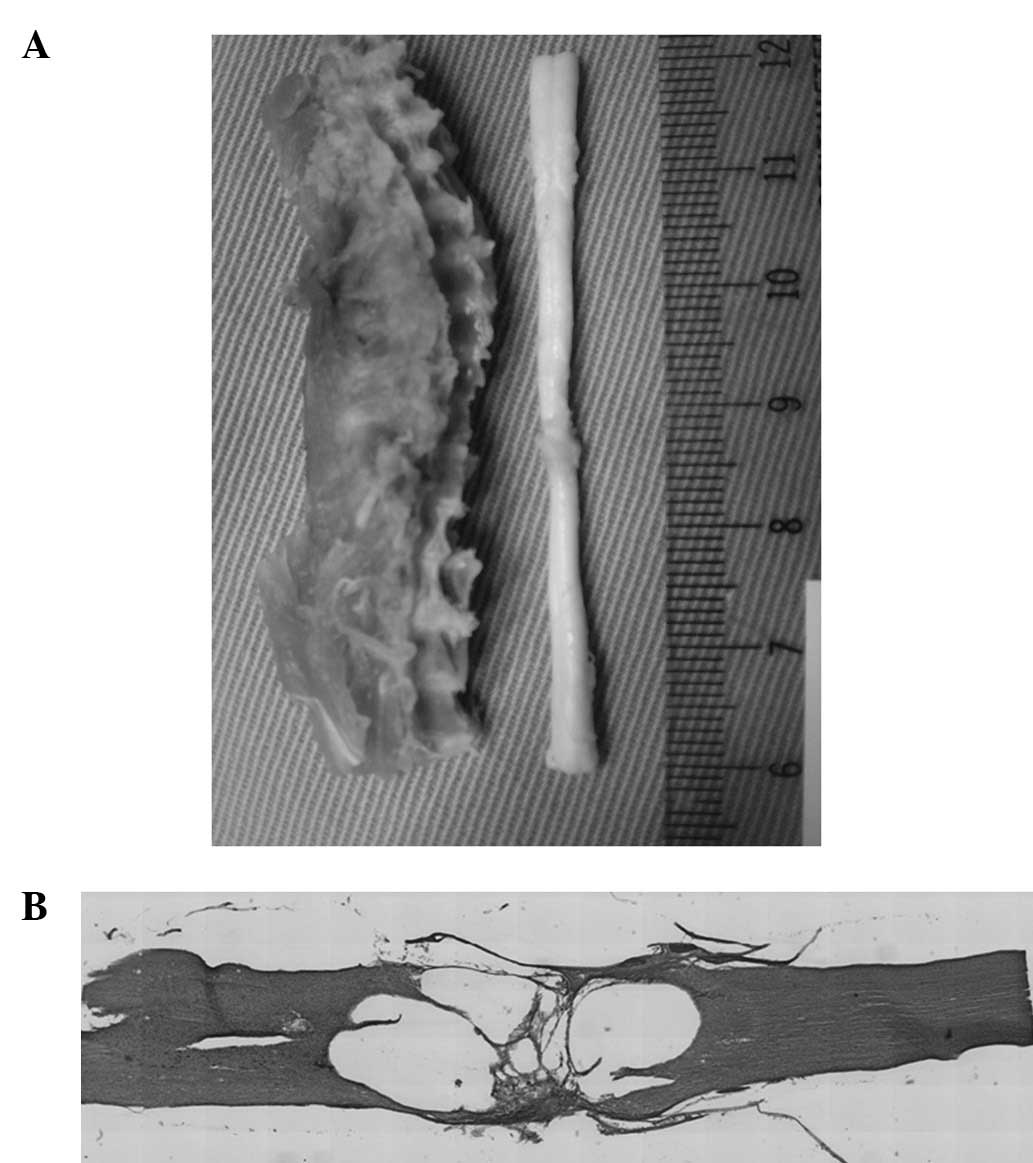
No signs of infection were observed in the thoracic
spine and spinal cord. The bone fusion of the Th7 and Th9 vertebrae
was observed to be complete in all specimens and the alignment of
the thoracic spine was maintained. The spinal canal was also
correctly reconstituted. Although the dura adhered to the bone at
the site of the surgery, the stumps of the spinal cord were
connected. The spinal cords were slightly atrophic around the
connection site (Fig. 3A).
Light microscopy of the cord showed that scar tissue
intervened at the connection site. Cavitation inhibiting the axonal
regeneration was also observed (Fig.
3B).
Scaffold model
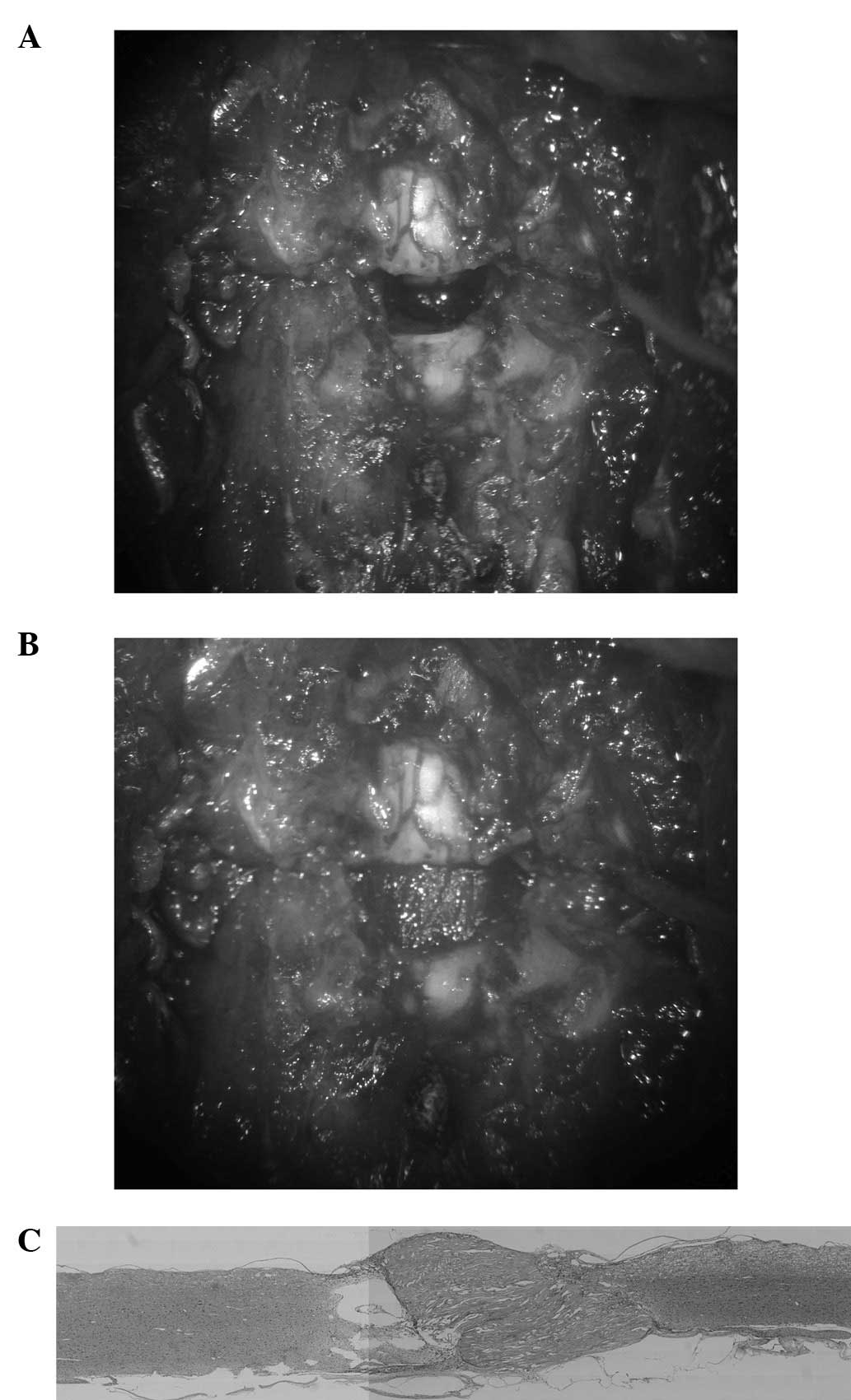
A 9-mm-long segment of the spinal cord was removed
to produce a gap of ∼5 mm after the shortening of the spine
(Fig. 4A). A nerve scaffold
created from collagen filaments was then implanted in the gap
(Fig. 4B). The implant firmly
connected the stumps of the spinal cord and the scar tissue and
cavitation were less than in previous models (Fig. 4C).
Discussion
Although axonal regeneration is extremely limited in
the mammalian adult central nervous system, partial lesions of the
spinal cord may be followed by spontaneous functional improvements.
The mechanisms underlying this recovery are not fully
understood.
Certain studies have presented evidence that the
regeneration of axons in the spinal cord may occur following spinal
cord transection in young rats (11–16).
However, in a transection-regeneration model, the completeness of
the transection has always been a matter of dispute.
Studies have demonstrated that the injured spinal
cord spontaneously forms a new intraspinal circuit in adult rats.
Transected hind limb corticospinal tract axons have been reported
to sprout into the cervical gray matter to contact short and long
propriospinal neurons following incomplete SCI (17,18).
There is a great difference between complete transection and
incomplete transection in the rat model. If the spinal cord had not
been transected completely, it is possible that the sprouted axon
may have remained following the transection rather than having been
regenerated across the transected spinal cord. This could not be
resolved by any transected spinal cord model.
We therefore established a method for shortening the
rat spine and spinal cord to provide a SCI model in which there was
no doubt about whether the axonal transection was complete.
Thoracic spondylectomy in the rat model is difficult
due to the adhesion of the pleura and mediastinal organs to the
ribs and column (19).
Nevertheless the thoracic level was selected for several reasons:
i) the spinal cord at the thoracic level is adequate for the
evaluation of corticospinal tract axonal regeneration; ii)
mechanical stress was observed to be low due to stabilization by
the rib so the chance of secondary displacement was lower following
thoracic spondylectomy; and iii) the rib is utilized for the
shortening and fixation of the spine.
Axonal regeneration was not observed across the
transected spinal cord in this model. Scar tissue and cavitation
inhibited axonal regeneration at the connection site.
The sharpness of the transection was considered to
be one of the most important factors for successful axonal
regeneration. An extremely sharp transection produced edema-free
lesions and later formed neither cysts nor scars, whereas a
relatively blunt transection produced edema followed by scars and
cysts around the lesions. Consequently, the spinal cord was
transected using the edge of a razor which was as sharp as possible
to minimize traumatic injury. However, the stump of the spinal cord
resulted in edema since it took 10 or 20 min to bring together the
stumps of the spinal cord following tran-section. It is necessary
to minimize the damage to the spinal cord and the surgery time for
successful axonal regeneration to occur.
Less scar formation and cavitation was observed in
the collagen filament model. It is possible that the contact
guidance by the collagen filaments guided the regeneration of the
axons and resulted in reduced scar formation.
In future, this model may be applied to the chronic
period of complete paralysis following SCI. Our model may be useful
in the study of axonal regeneration in SCI.
In conclusion, we established a method for
shortening the rat spine and spinal cord to provide a SCI model in
which there was no doubt about whether the axonal transection was
complete. This model was based on the assumption that the glial
scar tissue inhibits axonal regeneration in chronic SCI. Axonal
regeneration was not observed across the transected spinal cord in
this model. For successful axonal regeneration to occur, damage to
the spinal cord and the surgery time should be minimized. Our model
may be useful in the study of axonal regeneration in SCI.
Acknowledgements
The authors would like to thank Dr
Satoru Yoshii for providing the collagen filaments.
References
|
1.
|
Schwab ME and Bartholdi D: Degeneration
and regeneration of axons in the lesioned spinal cord. Physiol Rev.
76:319–370. 1996.PubMed/NCBI
|
|
2.
|
Rossignol D, Drew T, Brustein E and Jiang
W: Locomotor performance and adaptation after partial or complete
spinal cord lesions in the cat. Prog Brain Res. 123:349–365. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wernig A and Müller S: Laufband locomotion
with body weight support improved walking in persons with severe
spinal cord injuries. Paraplegia. 30:229–238. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Dietz V, Wirz M, Curt A and Colombo G:
Locomotor pattern in paraplegic patients: training effects and
recovery of spinal cord function. Spinal Cord. 36:380–390. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yoshii S and Oka M: Peripheral nerve
regeneration along collagen filaments. Brain Res. 888:158–162.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yoshii S and Oka M: Collagen filaments as
a scaffold for nerve regeneration. J Biomed Mater Res. 56:400–405.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yoshii S, Oka M, Shima M, Taniguchi A and
Akagi M: 30 mm regeneration of rat sciatic nerve along collagen
filaments. Brain Res. 949:202–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yoshii S, Oka M, Shima M, Akagi M and
Taniguchi A: Bridging a spinal cord defect using collagen filament.
Spine (Phila Pa 1976). 28:2346–2351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yoshii S, Oka M, Shima M, Taniguchi A,
Taki Y and Akagi M: Restoration of function after apinal cord
transection using a collagen bridge. J Biomed Mater Res A.
70:569–575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yara T, Kato Y, Kataoka H, Kanchiku T,
Suzuki H, Gondo T, Yoshii S and Taguchi T: Environmental factors
involved in axonal regeneration following spinal cord transection
in rats. Med Mol Morphol. 42:150–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Iseda T, Nishino T, Kawaguchi S, Yamanoto
M, Kawasaki T and Wakisaka S: Spontaneous regeneration of the
corticospinal tract after transection in young rats: a key role of
reactive astrocytes in making favorable and unfavorable conditions
for regeneration. Neuroscience. 126:365–374. 2004. View Article : Google Scholar
|
|
12.
|
Hase T, Kawaguchi S, Hayashi H, Nishio T,
Mizoguchi A and Nakamura T: Spinal cord repair in neonatal rats:
correlation between axonal regeneration and functional recovery.
Eur J Neuroscience. 15:969–974. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Inoue T, Kawaguchi S and Kurisu K:
Spontaneous regeneration of pyramidal tract after transection in
young rats. Neurosci Lett. 274:151–154. 1998. View Article : Google Scholar
|
|
14.
|
Iwashita Y, Kawaguchi S and Murata M:
Restoration of function by replacement of spinal cord segments in
the rat. Nature. 367:167–170. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kikukawa S, Kawaguchi S, Mizoguchi A, Ide
C and Koshinaga M: Regeneration of dorsal column axons after spinal
cord injury in young rats. Neurosci Lett. 249:135–138. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kao CC, Chang LW and Bloodworth JM Jr:
Axonal regeneration across transected mammalian spinal cord: an
electron microscopic study of delayed microsurgical nerve grafting.
Exp Neurol. 54:591–615. 1977. View Article : Google Scholar
|
|
17.
|
Bareyre FM, Kerschensteiner M, Raineteau
O, Mettenleiter O, Weinmann O and Schwab ME: The injured spinal
cord spontaneously forms a new intraspinal circuit in adult rats.
Nat Neurosci. 7:269–277. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Courtine G, Song B, Roy RR, Zhong H,
Herrmann JE, Ao Y, Qi J, Edgerton VR and Sofroniew MV: Recovery of
supraspinal control of stepping via indirect propriospinal relay
connections after spinal cord injury. Nat Med. 14:69–74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
de Medinaceli L and Wyatt RJ: A method for
shortening of the rat spine and its neurologic consequences. J
Neural Transplant Plast. 4:39–52. 1993.PubMed/NCBI
|