Introduction
Cardiorenal syndrome (CRS) is a complex problem.
Patients complicated with heart failure and chronic renal
insufficiencies are at a greater risk of poor prognosis. The
incidences of heart failure and renal failure are gradually
increasing, with poorer prognosis. CRS requires greater attention
and research to develop therapeutic measures in reference to the
fundamental mechanism of this disease. Currently, there are no
animal models for studying heart and renal dysfunction. Therefore,
it is necessary to establish an effective animal model of
heart-kidney interaction in order to increase our understanding of
this syndrome.
At present, there are a number of methods for
inducing heart (1,2) and renal insufficiency (3), which theoretically could be united to
establish a new disease model of heart-kidney interaction. However,
none of these methods completely represent the pathogenesis and
clinical features of human CRS. Therefore, an improved animal model
of heart-kidney interaction is required. The pathological changes
in an isoproterenol (ISO)-induced congestive heart failure (CHF)
model in Sprague-Dawley (SD) rats are characterized by repeated and
multiple focal myocardial necrosis, which is similar to ischemic
heart disease. There have been reports on varying dosages for
creating an ISO-induced CHF model in SD rats (4), in which subcutaneous injection of
>85 mg/kg ISO is considered a large dose. The large dose has a
high success rate of modeling, but with high mortality, while a
small dose has a low success rate of modeling, but with greater
longevity. The two models are detrimental to drug observation and
research. The most common method used to induce renal failure
reduces the survival of kidney tissue by different means, resulting
in varying extents of removal and severity of renal insufficiency.
Unilateral nephrectomy causes slight renal impairment, but without
significant urine protein increases and histological changes
(5). Renal sub-radical resection
leads to more serious renal insufficiency, as well as uremia and
complications of chronic kidney disease similar to humans (6,7). It
has also been reported that renal sub-radical resection causes
structural changes to the heart tissues (8–10).
This study aimed to explore a new model of CRS and its pathological
mechanism.
Materials and methods
Animals
Thirty SD male rats, weighing 180±20 g, provided by
the Laboratory Animal Center of Tongji Medical College of Huazhong
University of Science and Technology (China) were used in this
study. This study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol has been reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of Wuhan Puai Hospital.
Modeling
Thirty SD male rats were randomly divided into a
control group (n=15) and model group (n=15). After feeding for one
week, the rats in the model group were narcotized with 10% chloral
hydrate (0.3 ml/100 g intraperitoneal injection). The rats then
received resection of the lower pole of the left kidney in the
first week and radical resection of the right kidney after one week
according to the previously described protocol of twice undergoing
surgical resections (11). Rats in
the control group underwent surgery twice, although not kidney
resection. There was no death in the two groups after conventional
feeding for one week. Subcutaneous injections of ISO (100 mg/kg
body weight, injected twice) with an interval of 24 h were
administered to the rats in the model group, while rats in the
control group received two subcutaneous injections of normal saline
(100 mg/kg body weight) with an interval of 24 h. All 30 rats
received normal water and food for 4 weeks. The general conditions,
including diet, hair color, activity, cyanosis and edema were
observed and rats were weighed weekly.
Protein determination
After 4 weeks, the rats in the two groups were
placed into metabolism cages and urine was collected at 24 h. The
Bradford method (Coomassie brilliant blue G-250 staining) was used
to detect protein content in the urine and calculate the protein
excretion of urine at 24 h. Intraocular venous blood was then
collected to prepare serum and the levels of serum creatinine (Cr),
blood urea nitrogen (BUN), B-type natriuretic protein (BNP),
aldolase (ALD), angiotensin II (Ang II) and C-reactive protein
(CRP) was detected.
Hemodynamics
Approximately 24 h after blood collection, left
ventricular intubation was administered via the left common carotid
artery for left ventricular cardiac function testing. A neck
incision was made to expose and bluntly separate the right common
carotid artery. The left ventricular catheter was pre-charged with
10% heparin saline and was intubated against the right common
carotid artery into the left ventricle. The other end of the
catheter was connected to a pressure transducer (BL-420F biological
function experimental system) (Chengdu Technology & Market Co.
Ltd., Chengdu, China) for hemodynamic detection of left ventricular
end-diastolic pressure (LVEDP) and left ventricular systolic
pressure (LVSP), as well as the maximum rates of increased and
decreased left ventricular pressure (±dP/dtmax). Changes
in cardiac function were calculated.
Hematoxylin and eosin (HE) staining
The left ventricular tissues were fixed in 10X PBS
or 4% neutral formaldehyde. The tissues paraffin-embedded and
sliced to produce 4 μm sections. The sections underwent HE
staining and then images were captured with a Leica microscope
(magnification, ×100).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Comparison of the mean values between the groups was
examined with the χ2 test and variance analysis. Data
were analyzed with statistical software SPSS 11.5 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
General information
Compared with the control group, rats in the model
exhibited darker hair, reduced feeding, decreased early weight,
gradually increased later weight, slow movement, cyanosis in the
mouth and nose, asthma, edema in the feet and paws, reduced
activity and a poor grab reaction. In the model group, 3 rats died
within 1 week and 4 rats died 4 weeks following the subcutaneous
injections of ISO, administered twice, with a survival rate of 73%.
No death was noted in the control group.
Cardiac and renal function
parameters
Compared with the control group, serum Cr, BUN and
urine protein in the model group were increased (P<0.01), which
indicates a successful modeling of renal failure (Table I). All the rats in the model group
demonstrated cardiac insufficiency, characterized by significantly
decreased LVSP, increased LVDP and LVEDP and decreased
dP/dtmax (P<0.05; Table
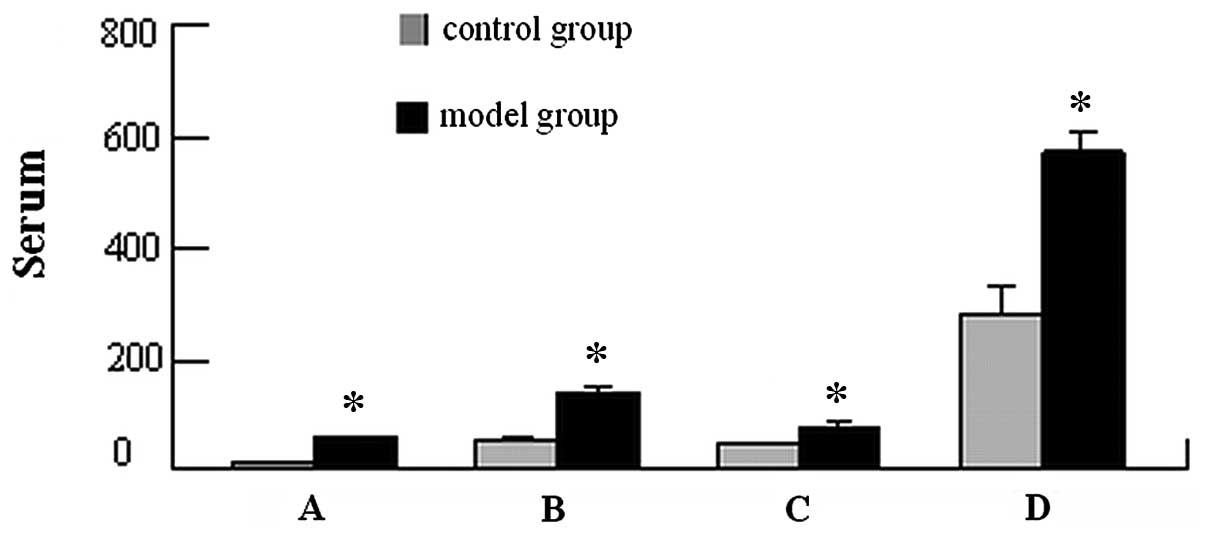
II). Compared with BNP (13.77±2.38 pg/ml), ALD (51.57±9.17
μg/l), Ang II (43.36±4.63 μg/l) and CRP (282.9±47.58
μg/l) in the control group, the serum BNP (56.48±4.67
pg/ml), ALD (137.69±16.13 μg/l), Ang II (81.76±5.78
μg/l) and CRP (568.54±42.15 μg/l) were significantly
increased in the model group (P<0.05; Fig. 1). Serum Cr was positively
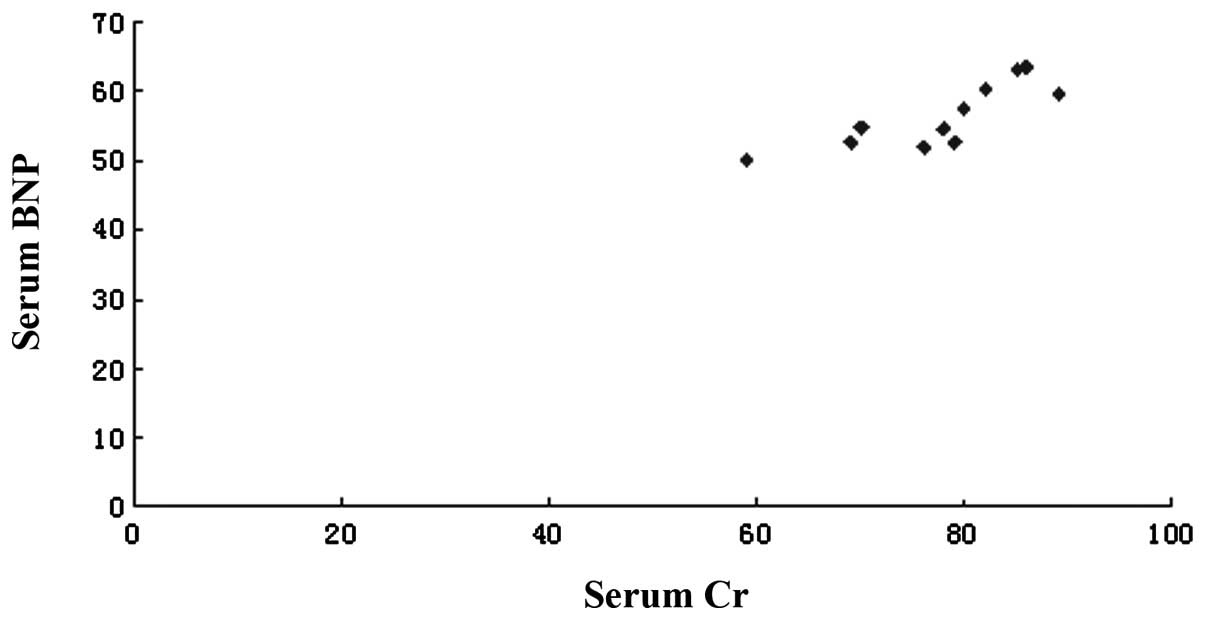
correlated with blood serum BNP levels in the model group (Fig. 2).
 | Table I.Comparison of urine protein, urea and
creatinine between the control and model groups. |
Table I.
Comparison of urine protein, urea and
creatinine between the control and model groups.
| Group | Serum creatinine
(μmol/l) | Urea nitrogen
(mmol/l) | Urine protein (mg/24
h) |
|---|
| Control | 40.6±10.8 | 5.89±2.26 | 15.6±5.36 |
| Model | 77.5±8.7a | 10.2±1.5a | 70.5±12.7a |
 | Table II.Comparison of left ventricular cardiac
function and blood dynamic parameters between the control and model
groups. |
Table II.
Comparison of left ventricular cardiac
function and blood dynamic parameters between the control and model
groups.
| Group | LVSP (mmHg) | LVDP (mmHg) | LVEDP (mmHg) | +dP/dtmax
(mmHg/sec) | −dP/dtmax
(mmHg/sec) |
|---|
| Control | 136.7±8.2 | 0.8±1.1 | 2.0±1.8 | 8060±892 | −6902±949 |
| Model | 110.9±8.7b | 1.8±0.6a | 4.6±1.0b | 5536±439b | −4036±413 |
Weight index
Compared with the control group, the weights of the
rats in the model group were significantly reduced, whereas weights
of the left ventricule and ventricular weight indices were
significantly increased in the model group (P<0.01). A
significant difference was detected between the weight of the left
ventricle and the left ventricular weight index (P<0.01;
Table III). Cardiac function
indices demonstrated that the left ventricle suffered clear
hypertrophy and reconstruction and entered the decompensatory stage
of heart failure in the model group.
 | Table III.Comparison of weight, left ventricular
mass and left ventricular mass index between the control and model
groups. |
Table III.
Comparison of weight, left ventricular
mass and left ventricular mass index between the control and model
groups.
| Group | LVW (g) | BW (kg) | LVW/BW (g/kg) |
|---|
| Control | 0.40±0.04 | 0.29±0.01 | 1.38±0.14 |
| Model | 0.57±0.04a | 0.25±0.01a | 2.29±0.19a |
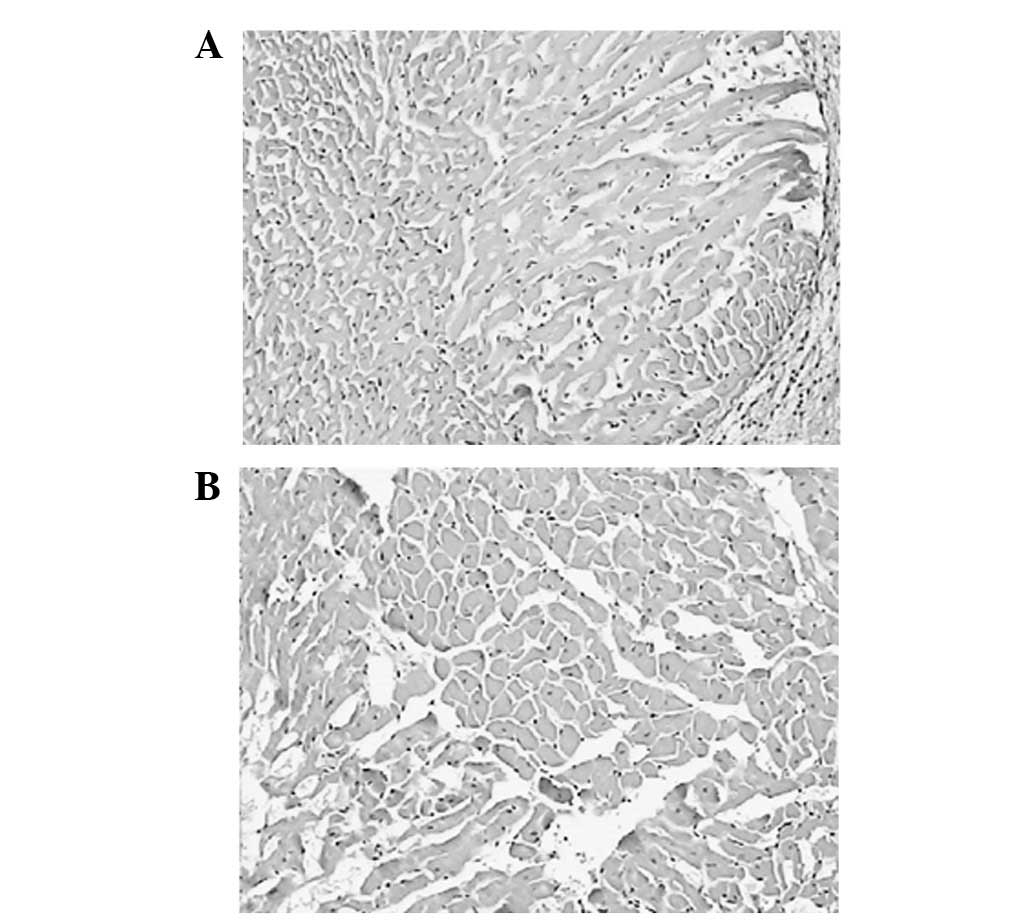
HE staining
Following HE staining, slices were examined and
compared with the control group. There was clear cardiomyocyte
hypertrophy in the model group, with coarsened myocardial cells in
an uneven arrangement and myocardial fibrosis. In the control
group, myocardial cells were in even distribution, with fewer and
inconspicuous extracellular matrices (Fig. 3).
Discussion
The incidences of heart failure and renal failure
has gradually increased. Renal insufficiency is an independent
predictor of heart failure, while myocardial hypertrophy and heart
function failure are serious complications of chronic renal
failure, closely associated with mortality (12–14).
In this study, a rat model of CRS was established to examine the
pathophysiological mechanism in order to identify effective drugs
and early intervention therapy.
A successful model of CRS, not only simulates the
clinical characteristics of CRS, including changes in the heart,
kidney, hemodynamics and neuroendocrinology, but also evaluates the
therapeutic effect. This model must include joint damages of kidney
and heart, characterized by progressive deterioration of
cardio-nephric function. The model must present systolic
dysfunction confirmed by echocardiography and haemodynamics,
resulting in decreased cardiac output. In addition, to further
explore the latest clinical findings, increased enddiastolic
pressure and venous congestion are also necessary conditions. At a
histological level, the model must present characteristics of
hypertrophy and fibrosis, particularly mismatched
myocardial/capillary. To successfully represent kidney damage, the
model must present characteristics of increased Cr and excretion of
albumin, as well as decreased progressive renal function caused by
a decrease in the glomerular filtration rate (GFR)/Cr clearance
ratio. This study aimed to explore the physiopathological mechanism
of a CRS model induced by three-quarters nephrectomy and
subcutaneous injection of ISO.
Compared with previous models of simple heart or
renal failure, rats in this study presented earlier renal and heart
failure, characterized by significantly increased Cr, urine protein
and left ventricular weight index, as well as decreased hemodynamic
index, including ±dP/dtmax and increased serum BNP. HE
staining of the myocardium revealed clear hypertrophy of myocardial
cells and myocardial fibrosis in the model group, indicating that
reconstruction of myocardial hypertrophy had begun. Compared with
heart failure caused only by ISO, the amount of subcutaneous
injection of ISO was reduced in this model group. In the report of
a CRS model by Van Dokkum et al and Windt et
al(5,7), there was reduced interactive
influence between heart and renal function. Dikow et
al(6) hypothesized that the
increase in Cr aggravated the left ventricular remodeling of
myocardial infarction. We consider that the differences in
experimental design may be associated with the varying degrees of
damage to the heart and kidney, as well as different induction
times. We analyzed indices of heart and kidney function in this
model and identified that serum BNP was positively correlated to Cr
in the model group, with a correlation coefficient of 0.81
(P<0.01), which is consistent with the study by Butler et
al(15) and the retrospective
study by Weinfeld et al(16). The hemodynamic variable associated
with worsening renal function was right atrial pressure. Heart and
renal function may influence each other in the occurrence and
development of diseases. This model simulates the process of heart
failure complicated with renal failure.
The possible mechanisms of heart-kidney interactions
that have previously been considered include hemodynamic changes,
endothelial dysfunction, inflammation, activation of the
renin-angiotensin aldosterone system (RAAS) and/or the sympathetic
system, any of which may cause cascade reactions of other factors,
leading to structural and functional damage to the heart and kidney
(17,18). The mechanism that causes and
maintains heart-kidney interactions remains unclear. In this study,
serum ALD and Ang II were significantly increased in the model
group compared with the control group, indicating that activation
of the RAAS is important in the occurrence of this model. At the
same time, serum CRP was significantly increased in the model group
compared with the control group (P<0.01), indicating that
inflammation also plays a promoting role in this model of CRS. We
also detected a marked change in urine protein, which is a strong
and independent risk factor for cardiovascular disease. When it
appears, proteinuria causes an accelerated atherosclerotic process,
endothelial dysfunction, increased risk of terminal organ damage,
serious cardiovascular events and mortality (19). Rats in this model suffer
heart-kidney interactions, then when CRS occurs, the prognosis
becomes significantly worse.
Due to the physiological changes of decreased renal
function caused by three-quarters nephrectomy, this model had an
increased sensitivity to adverse factors in chronic heart failure.
Inflammation and activation of the RAAS promotes the occurrence and
development of cardiorenal failure. Hemodynamic changes cause an
increase in Ang II release, vasoconstriction, contraction of the
efferent glomerular arteriole, cardiac remodeling and an increase
in aldosterone release, as well as water and sodium retention,
which promote myocardial fibrosis (20).
This study demonstrated that three-quarters
nephrectomy complicated with subcutaneous injection of ISO induces
concurrent heart and renal failure, with a high success rate,
providing a simple, reliable and easy animal model for clinical
discussion of the interactive mechanism of heart and renal
function. However, this study did not investigate the development
time of CRS, which is required to examine the illness and possible
preventive therapeutic measures. This model is likely to be useful
for further studies on the pathogenesis and development of
heart-renal interaction at different stages.
References
|
1.
|
Balakumar P, Singh AP and Singh M: Rodent
models of heart failure. J Pharmacol Toxicol Methods. 56:1–10.
2007. View Article : Google Scholar
|
|
2.
|
Patten RD and Hall-Porter MR: Small animal
models of heart failure: development of novel therapies, past and
present. Circ Heart Fail. 2:138–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yang HC, Zuo Y and Fogo AB: Models of
chronic kidney disease. Drug Discov Today Dis Models. 7:13–19.
2010. View Article : Google Scholar
|
|
4.
|
Takeshita D, Shimizu J, Kitagawa Y, et al:
Isoproterenol-induced hypertrophied rat hearts: does short-term
treatment correspond to long-term treatment? J Physiol Sci.
58:179–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Van Dokkum RP, Eijkelkamp WB, Kluppel AC,
et al: Myocardial infarction enhances progressive renal damage in
an experimental model for cardio-renal interaction. J Am Soc
Nephrol. 15:3103–3110. 2004.PubMed/NCBI
|
|
6.
|
Dikow R, Schmidt U, Kihm LP, et al: Uremia
aggravates left ventricular remodeling after myocardial infarction.
Am J Nephrol. 32:13–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Windt WA, Henning RH, Kluppel AC, Xu Y, de
Zeeuw D and van Dokkum RP: Myocardial infarction does not further
impair renal damage in 5/6 nephrectomized rats. Nephrol Dial
Transplant. 23:3103–3110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Amann K, Wiest G, Zimmer G, Gretz N, Ritz
E and Mall G: Reduced capillary density in the myocardium of uremic
rats - a stereological study. Kidney Int. 42:1079–1085. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Amann K, Tyralla K, Gross ML, et al:
Cardiomyocyte loss in experimental renal failure: prevention by
ramipril. Kidney Int. 63:1708–1713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mall G, Rambausek M, Neumeister A, Kollmar
S, Vetterlein F and Ritz E: Myocardial interstitial fibrosis in
experimental uremia - implications for cardiac compliance. Kidney
Int. 33:804–811. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tsuruoka S, Nishiki K, Wakaumi M, et al:
Chronopharmacology of oxacalcitriol in 5/6 nephrectomized rats.
Life Sci. 75:809–822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu CY: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sarnak MJ, Levey AS, Schoolwerth AC, et
al: Kidney disease as a risk factor for development of
cardiovascular disease: a statement from the American Heart
Association Councils on Kidney in Cardiovascular Disease, High
Blood Pressure Research, Clinical Cardiology, and Epidemiology and
Prevention. Hypertension. 42:1050–1065. 2003. View Article : Google Scholar
|
|
14.
|
Wali RK, Wang GS, Gottlieb SS, et al:
Effect of kidney transplantation on left ventricular systolic
dysfunction and congestive heart failure in patients with end-stage
renal disease. J Am Coll Cardiol. 45:1051–1060. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Butler J, Geisberg C, Howser R, et al:
Relationship between renal function and left ventricular assist
device use. Ann Thorac Surg. 81:1745–1751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Weinfeld MS, Chertow GM and Stevenson LW:
Aggravated renal dysfunction during intensive therapy for advanced
chronic heart failure. Am Heart J. 138:285–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bongartz LG, Cramer MJ, Doevendans PA,
Joles JA and Braam B: The severe cardiorenal syndrome: ‘Guyton
revisited’. Eur Heart J. 26:11–17. 2005.
|
|
18.
|
Ronco C, Haapio M, House AA, Anavekar N
and Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol.
52:1527–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bongartz LG, Braam B, Verhaar MC, et al:
Transient nitric oxide reduction induces permanent cardiac systolic
dysfunction and worsens kidney damage in rats with chronic kidney
disease. Am J Physiol Regul Integr Comp Physiol. 298:R815–R823.
2010. View Article : Google Scholar
|
|
20.
|
Rajaram V and Joseph J: Role of adenosine
antagonism in the cardio-renal syndrome: pathophysiology and
therapeutic potential. Curr Heart Fail Rep. 4:153–157. 2007.
View Article : Google Scholar : PubMed/NCBI
|