Introduction
A ventricular septal defect is an abnormal opening
in the dividing wall between the ventricles, caused by a
hypoplastic ventricular septum in the embryonic period, which is a
common type of congenital heart defect. Ventricular septal defects
mainly occur in membranous and muscular intervals or at their
border. At present, simple membranous ventricular septal defects
are treated with interventional occlusion in the clinic, which is
the conventional method due to the small risk of trauma and minimal
complications. The short-term curative effect of occlusion has been
confirmed in the clinic; however, the medium-term and long-term
curative effects require further observation (1–8). In
the interventional therapy of ventricular septal defects, the size
and shape of the membranous ventricular septal defect, detected by
echocardiography, determines whether interventional surgery is
carried out. These parameters are also helpful for planning the
surgical procedure. The morphological observations of membranous
ventricular septal defects determine the type of occluder needed,
which is key to a successful interventional therapy. In addition to
these factors, there is a type of membranous ventricular septal
defect that is often complicated by a medium to large tricuspid
regurgitation volume (9–11). The surrounding tissues of a
membranous interventricular septum are complex, and include fibrous
tissues, tricuspid valve septa and its chordae tendineae, as well
as part of the anterior tricuspid valve septa and its chordae
tendineae. Fibrous tissues with peripheral defects are often
adhered to the tricuspid valve septa or its chordae tendineae and
form a lamellar shape. A number of fibrous tissues or chordae
tendineae may protrude across the mouth of the defect, dividing it
into a multiple ventricular septal defect, resulting in a shunt in
blood flow through two or more holes. A number of membranous
ventricular septal defects cause medium to large regurgitation of
the tricuspid valve due to these irregular adhesions (12,13).
In the clinic, it is not clear whether occlusion is suitable for
this type of membranous ventricular septal defect. Ventricular
septal defect occlusion in the treatment of membranous ventricular
septal defect complicated with tricuspid regurgitation often
involves complex peripheral tissues, and if unsuccessful, may cause
problems such as fracture or damage to the tricuspid chordae
tendineae (14,15). Furthermore, the implantation of an
occluder to treat membranous ventricular septal defect complicated
with tricuspid regurgitation may influence the occlusion of the
tricuspid valve since the implantation may cause the tricuspid
reverse flow to increase and affect right ventricular function.
With the development of occlusion therapy, sonographers are
requested to provide more imaging information to determine the
risks of ventricular septal defect occlusion in the treatment of
membranous ventricular septal defect complicated with tricuspid
regurgitation. In the current study, an ultrasound diagnostic
instrument with high resolution was used to carefully observe the
peripheral tissues of membranous ventricular septal defects. Color
Doppler ultrasonography was used to observe the morphology of
tricuspid regurgitation and quantitatively measure the
regurgitation volume. We explored the correlation between the
mechanism of tricuspid regurgitation and the form and size of the
ventricular septal defect. We observed the changes in tricuspid
regurgitation volume prior to and following interventional
occlusion, aiming to summarize the mechanism of membranous
ventricular septal defect complicated with tricuspid regurgitation
and the feasibility of applying occlusion in such cases.
Materials and methods
Subjects
We analyzed 43 patients with membranous ventricular
septal defect complicated with tricuspid regurgitation by
echocardiography. The group included 29 males and 14 females, aged
6–22 years old (average age, 12.6 years). There were 4 cases
showing a mild increase in pulmonary arterial pressure and 2 cases
with a moderate increase. This study was conducted in accordance
with the Declaration of Helsinki. The study was conducted with
approval from the Ethics Committee of the Air Force General
Hospital of PLA. Written informed consent was obtained from all
participants.
Methods
Prior to ventricular septal defect occlusion, the
size, shape, shunt flow and the form of the shunt in the membranous
ventricular septal defect were conventionally observed by
echocardiography and the defects were divided into funnel, pipe,
membranous tumor and irregular capsular types according to the
morphology of the right ventricular septal defect (3). We used Simpson’s method to record the
length, area and volume of tricuspid regurgitation and spectral
Doppler to measure the speed and differential pressure of the shunt
and tricuspid regurgitation.
The changes in length, area and volume of tricuspid
regurgitation 3 days and 1, 3 and 6 months after ventricular septal
defect occlusion were observed by echocardiography.
Statistical analysis
The various measurement data of tricuspid
regurgitation were expressed as the mean ± standard deviation. The
comparison prior to and following occlusion was analyzed by
variance analysis. P<0.05 was considered to indicate a
statistically significant difference. SPSS (SPSS Inc., Chicago, IL,
USA) software was used to analyze the data.
Results
General conditions
Preoperative two-dimensional echocardiography
revealed that the ventricular septal defects were 4.0–12.3 mm
(average 6.3±3.8 mm) in size, and included 8 funnel types, 11 pipe
types, 5 membranous tumor types and 19 irregular capsular types.
All caused tricuspid regurgitation. The defects had a length of
0.7-6.0 cm, an area of 0.7–7.6 cm2, a volume of 0.3–10.5
ml, a velocity of 2.6–5.3 m/sec and a differential pressure of
27–112 mmHg.
Regurgitation mechanism
The main causes of membranous ventricular septal
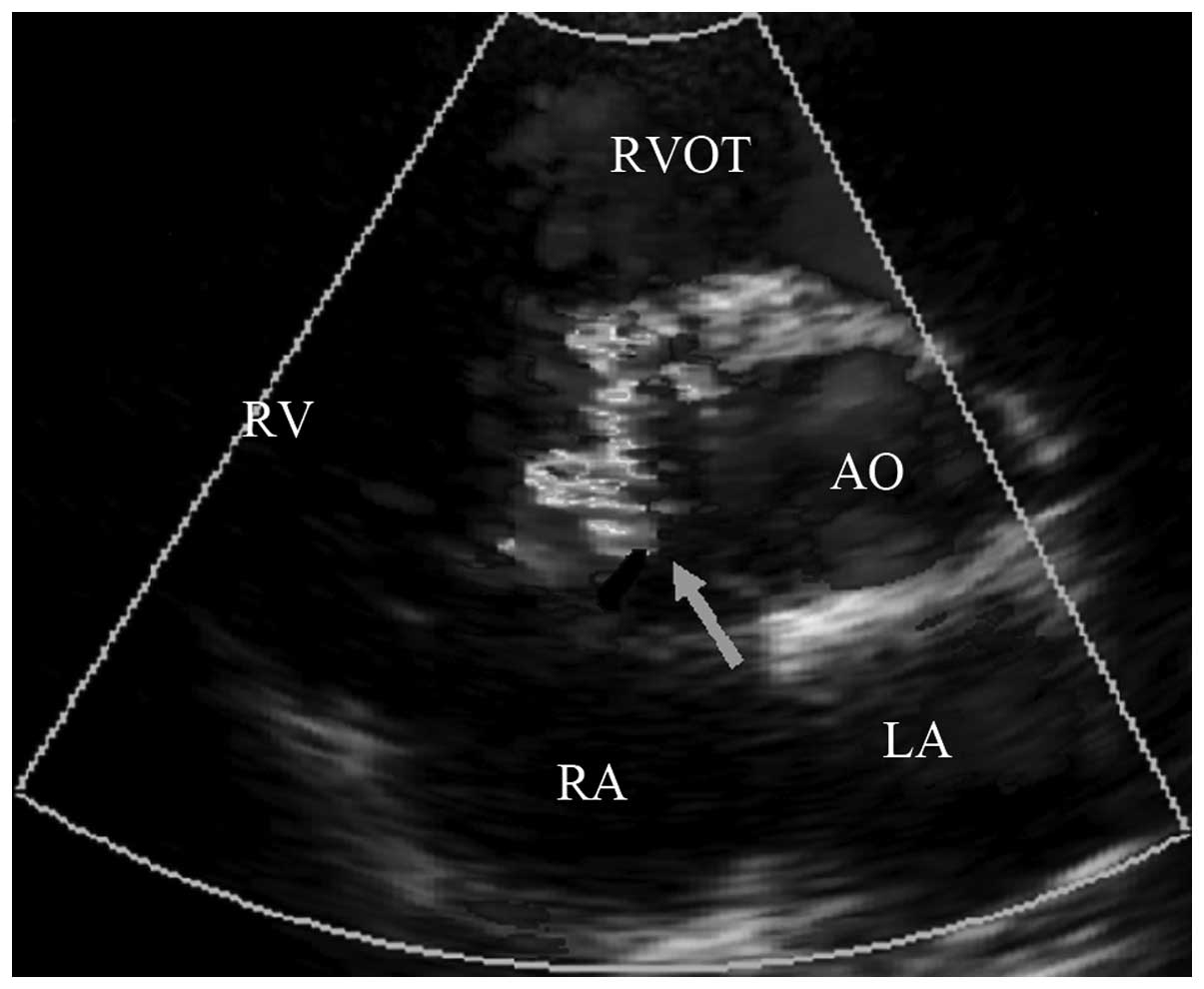
defect complicated with tricuspid regurgitation were: i) in the 7
cases of short tricuspid valve septa during development, the porous
defect in the right ventricular surface directly opened onto the
short tricuspid valve septa, directing some of the ventricular
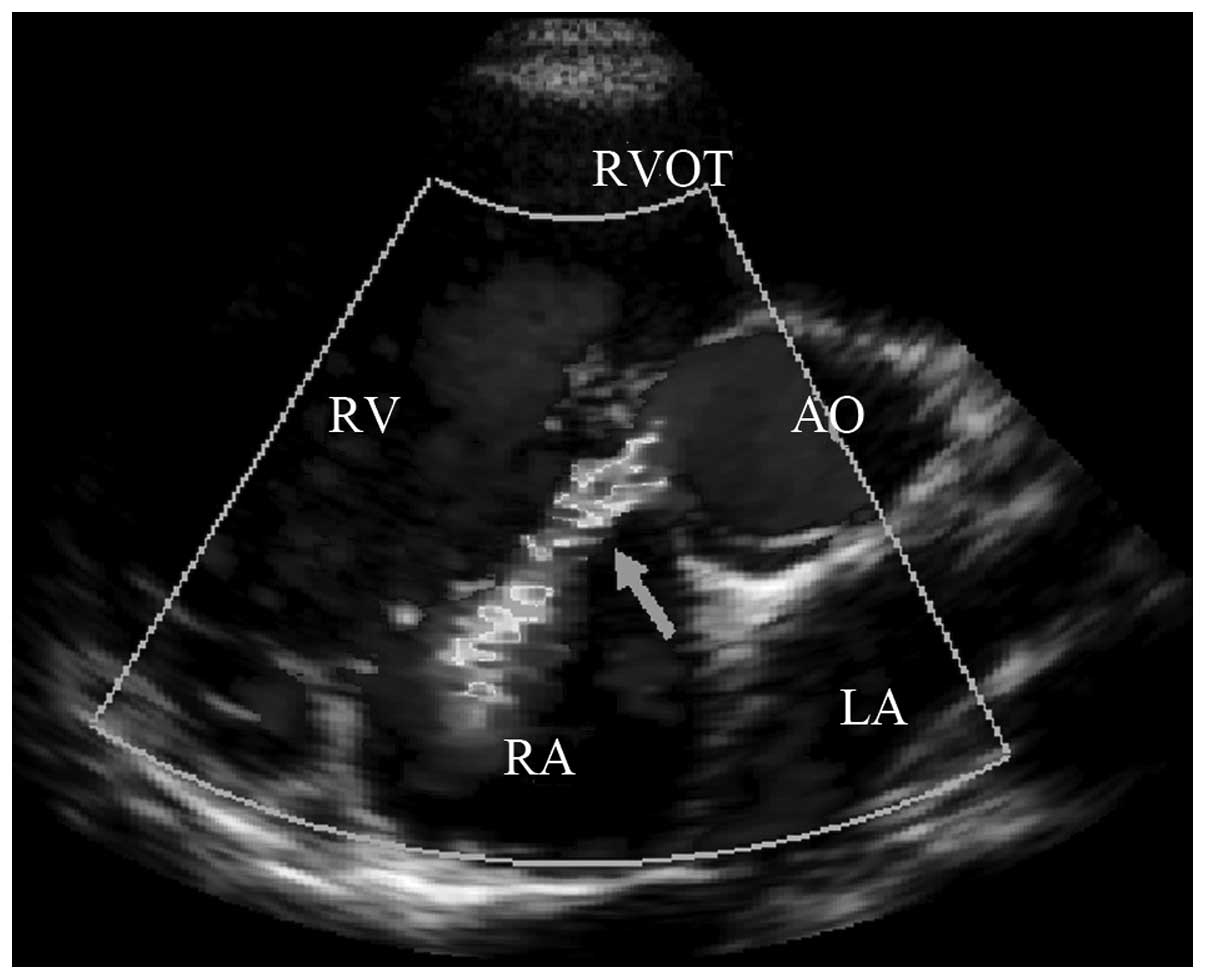
septal defect shunt flow into the right atrium (Fig. 1). ii) In the 16 cases of
interminable anterior tricuspid valve or abnormal attachment points
of chordae tendineae, the interminable anterior tricuspid valve or
abnormal attachment points of the chordae tendineae in the inferior
margin of the ventricular septal defect may cause the shunt flow to
hit the valvular leaf or chordae tendineae causing blood flow to
‘reflect’ into the right atrium (4) (Fig.
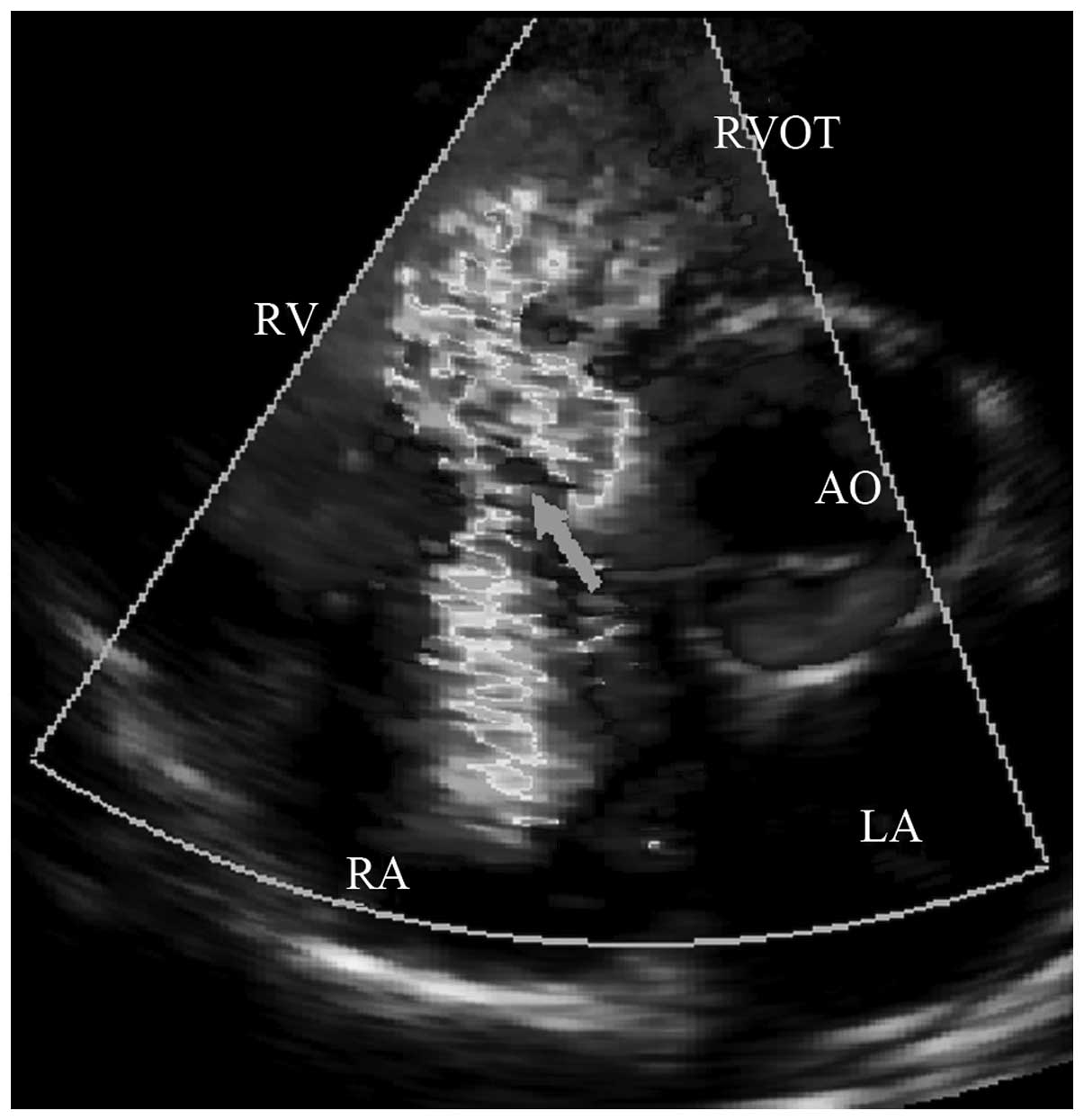
2). iii) In the 14 cases of irregular adhesion in the right
ventricular lateral defect, the irregular ‘tunnel’ caused by tissue
adhesions with peripheral defects, may direct the shunt flow into
the right atrium (Fig. 3). iv) In
the 6 cases of pulmonary arterial hypertension, the tricuspid
incompetence caused by the ventricular septal defect complicated
with pulmonary arterial hypertension may cause tricuspid
regurgitation.
Changes in regurgitation volume
Forty-three patients with membranous ventricular
septal defects successfully underwent surgical occlusion. Of these,
6 cases received successful surgical occlusion following the
reestablishment of the occlusion route due to the abnormal
development of anterior tricuspid valve chordae tendineae that was
circled by wire. Patients immediately underwent echocardiography
and the 43 occluders were observed to be in the normal position,
without residual shunt in the ventricular septal defect and various
indices of tricuspid regurgitation volume were significantly
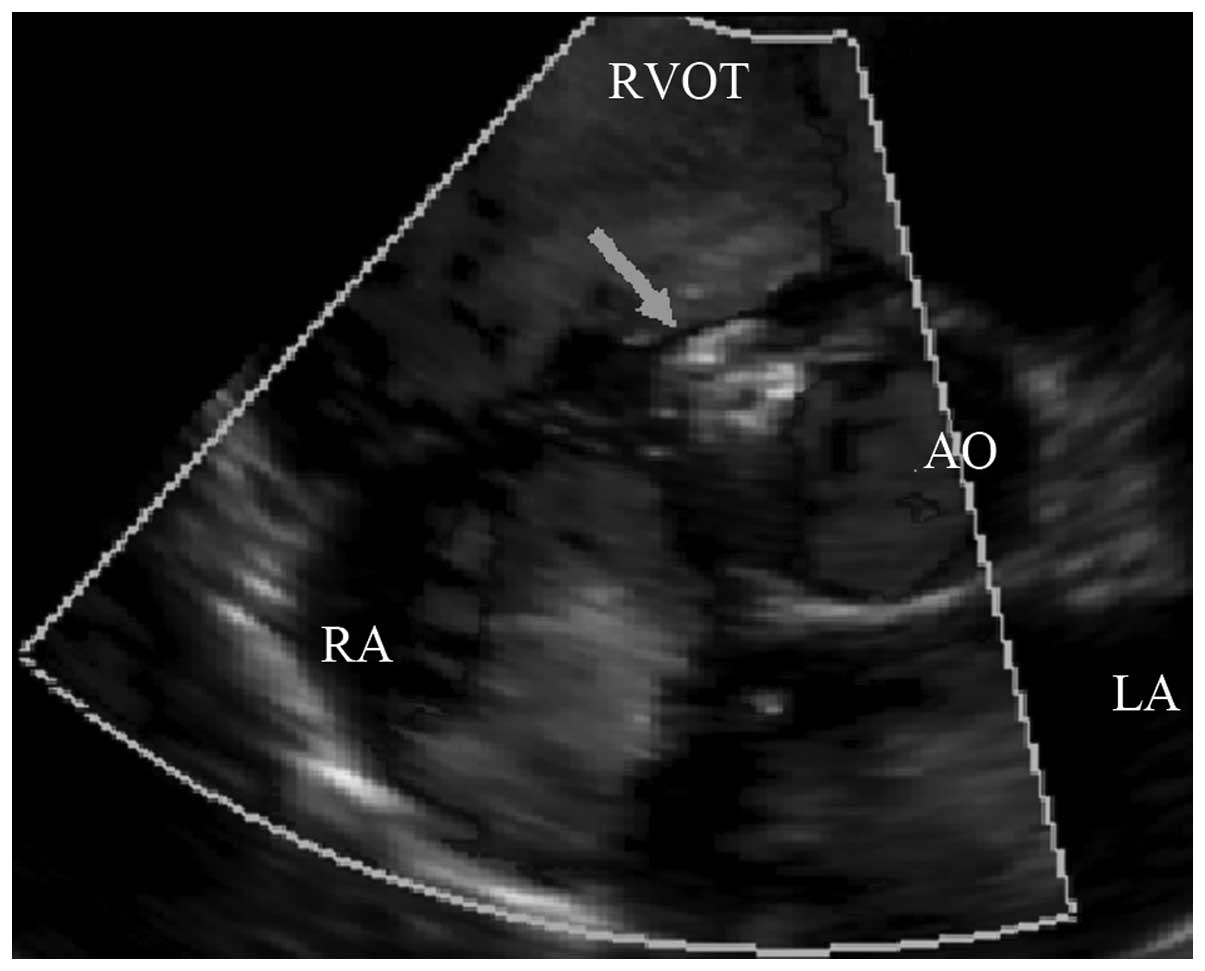
reduced compared with those before occlusion (Table I). The tricuspid regurgitation
volumes in the 37 cases of tricuspid regurgitation without
pulmonary arterial hypertension instantly disappeared (Fig. 4) or significantly reduced
(tricuspid regurgitation volume 0–0.6 ml). The tricuspid
regurgitation volumes in the 6 cases of tricuspid regurgitation
with pulmonary arterial hypertension were instantly reduced by
varying degrees; 2 cases that previously presented normal-mild
pulmonary arterial hypertension demonstrated a normal level
following right cardiac catheterization and 4 cases presenting mild
to moderate pulmonary arterial hypertension decreased in varying
degrees.
 | Table I.Measurements of 43 perimembranous
ventricular septal defects complicated with tricuspid
regurgitation, before and after occlusion surgery. |
Table I.
Measurements of 43 perimembranous
ventricular septal defects complicated with tricuspid
regurgitation, before and after occlusion surgery.
| Before surgery | After surgery | 3 days after
surgery | 1 month after
surgery | 3 months after
surgery | 6 months after
surgery |
|---|
| Length (cm) | 2.67±0.17 | 0.66±0.18a | 0.67±0.18a | 0.56±0.13a | 0.55±0.15a | 0.56±0.18a |
| Area
(cm2) | 2.57±0.24 | 0.55±0.20a | 0.55±0.21a | 0.47±0.19a | 0.49±0.21a | 0.45±0.20a |
| Volume (ml) | 2.30±0.33 | 0.60±0.27a | 0.61±0.27a | 0.57±0.29a | 0.50±0.27a | 0.51±0.21a |
Follow-up
The patients were reviewed by echocardiography at 3
days and 1, 3 and 6 months after surgery. The length, area and
volume of tricuspid regurgitation in the 43 patients with
membranous ventricular septal defect complicated with tricuspid
regurgitation were significantly reduced compared with those before
occlusion (Table I). Patients were
reviewed by color Doppler ultrasound for 6 months after occlusion.
There were no significant changes in tricuspid regurgitation volume
in the 37 patients with tricuspid regurgitation without pulmonary
arterial hypertension compared with those immediately after
occlusion (P>0.05) and the tricuspid regurgitation volume in the
6 patients with tricuspid regurgitation with pulmonary arterial
hypertension were significantly reduced compared with those
immediately after occlusion (P<0.05; Table II).
 | Table II.Tricuspid reverse flow resulting from
various causes (volume, ml). |
Table II.
Tricuspid reverse flow resulting from
various causes (volume, ml).
| Cause | n | Before surgery | After surgery | 3 days after
surgery | 1 month after
surgery | 3 months after
surgery | 6 months after
surgery |
|---|
| Short partition
valve | 7 | 1.87±0.33 | 0.11±0.05a | 0.10±0.04a | 0.10±0.06a | 0.07±0.06a | 0.06±0.04a |
| Abnormal anterior
valve | 16 | 1.80±0.28 | 0.02±0.01a | 0.02±0.01a | 0.02±0.01a | 0.02±0.01a | 0.01±0.01a |
| Right ventricle side
adhesion | 14 | 1.72±0.43 | 0.08±0.04a | 0.08±0.04a | 0.06±0.03a | 0.06±0.04a | 0.06±0.03a |
| Pulmonary
hypertension | 6 | 6.96±1.30 | 5.36±1.47a | 5.10±1.38a | 4.69±1.52a | 3.60±1.45a | 2.83±1.48a |
Discussion
In recent years, with the development of a delivery
system and surgical technique for occlusion, ventricular septal
defect occlusion has been successfully applied in the clinic. The
surrounding tissues of the membranous interventricular septum are
complex. It is adjacent to the attachment points of the chordae
tendineae of the tricuspid valve septa and the anterior valve,
which may lead to the development a short valve, interminable
anterior valve and the attachment of the chordae tendineae in a
variant position (16–19). In addition, under prolonged
conditions of hemodynamic turbulence, caused by ventricular septal
defect shunt flow, the peripheral defect, often made part of the
bilateral defect of membranous ventricular septal defects, adheres
to the surrounding valve leaves and chordae tendineae tissues, to
form multiple types of right ventricular septal defects. Due to the
complex relationship between the right ventricle tissues of the
ventricular septal defect, the ventricular septal defect causes
various shunt flows (19,20) and part of the membranous
ventricular septal defect may cause varying degrees of tricuspid
regurgitation. In previous literature, ventricular septal defects
have been divided into 4 types, including funnel, membranous tumor,
pipe and irregular capsular type. It is important to distinguish
the size of the ventricular septal defect and the defective
condition of the right ventricular surface for occlusion.
Additionally, these evaluations may aid in the prognosis of
tricuspid regurgitation and the prediction of complications,
wherein, echocardiography plays an important directive
function.
In the current study, we noted that any form of
membranous ventricular septal defect causes tricuspid regurgitation
(Table III), which indicates the
complexity of the tricuspid regurgitation mechanism. Observation of
the tissues surrounding the ventricular septal defect and analysis
of color Doppler blood flow of the ventricular septal defect shunt
flow revealed that tricuspid regurgitation mainly occurred when
there was short tricuspid valve septa in development, interminable
anterior tricuspid valve septa, abnormal attachment point of the
chordae tendineae, irregular adhesion of right ventricular septal
defect or pulmonary arterial hypertension. In this study, there
were 37 cases of tricuspid regurgitation without pulmonary arterial
hypertension, accounting for 86%. These particularly involved an
abnormal anterior tricuspid valve and adhesion in the right
ventricle, which accounted for 37 and 32% of cases, respectively.
Tricuspid regurgitation with pulmonary arterial hypertension
accounted for 14% of cases. Therefore, the complexity of the
tissues surrounding the membranous ventricular septal defect is the
main cause of membranous ventricular septal defect complicated with
tricuspid regurgitation.
 | Table III.Correlation between various types of
perimembranous ventricular septal defect and tricuspid reverse flow
resulting from different causes. |
Table III.
Correlation between various types of
perimembranous ventricular septal defect and tricuspid reverse flow
resulting from different causes.
| Defect type | Short partition
valve | Abnormal anterior
valve | Right ventricle side
adhesion | Pulmonary
hypertension |
|---|
| Funnel type | 2 | 5 | - | 1 |
| Pipe type | 2 | 4 | 2 | 3 |
| Membranous tumor
type | - | 1 | 2 | 2 |
| Irregular capsular
type | 3 | 6 | 10 | - |
The correlation between the membranous ventricular
septal defect and tricuspid valve tissues was observed by
echocardiography. The section of aortic short axis and parasternal
five chamber view was the most important section (Fig. 5). We observed the size of the
membranous ventricular septal defect and the situation of a
defecting right ventricle, as well as the adjacent tricuspid valve
and the correlaton between its valve apparatus and the peripheral
defect. Using color Doppler ultrasound we detected the shunt flow
of the ventricular septal defect and deduced the cause of
membranous ventricular septal defect complicated with tricuspid
regurgitation. We observed that the majority of membranous
ventricular septal defects complicated with tricuspid regurgitation
are without pulmonary arterial hypertension. If pulmonary arterial
pressure is estimated by the tricuspid regurgitation method, the
pulmonary artery systolic pressure would be overestimated. Due to
this the differential pressure of the tricuspid valve was not the
same as that of the right ventricle and right atrium, but instead
the same as the left ventricle and right atrium. Application of
color Doppler ultrasound avoids such mistakes by carefully
observing the morphology of shunt flow.
Theoretically, under the condition of an occluder
closing the membranous ventricular septal defect, particularly when
the left ventricular surface is completely closed without any
residual shunt, tricuspid regurgitation without pulmonary arterial
hypertension should disappear, consistent with the results observed
in this study. Continuous observation until 6 months post-surgery
revealed that tricuspid regurgitation disappeared or significantly
reduced. The reduction of tricuspid regurgitation with pulmonary
arterial hypertension is related to the decrease of pulmonary
arterial pressure following ventricular septal defect
occlusion.
The extensive development of ventricular septal
defect occlusion relies on the diagnosis of ventricular septal
defects by echocardiography. It is important to carefully observe
the size, form and adjacent relations with peripheral defects. As a
simple and safe means of detection, echocardiography forecasts
complications and relates them to the prognosis to ensure the
long-term curative effects of occlusion.
Acknowledgements
This study was supported by the Major
Twelfth Five projects of PLA (no. AKJ11J004).
References
|
1.
|
Look JE, Block PC, McKay RG, Baim DS and
Keane JF: Transcatheter closure of ventricular septal defects.
Circulation. 78:361–368. 1988. View Article : Google Scholar
|
|
2.
|
Kalra GS, Verma PK, Dhall A, Singh S and
Arora R: Transcatheter device closure of ventricular septal
defects: immediate results and intermediate-term follow-up. Am
Heart J. 138:339–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Janorkar S, Goh T and Wilkinson J:
Transcatheter closure of ventricular septal defects using the
Rashkind device: initial experience. Catheter Cardiovasc Interv.
46:43–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mullasari AS, Umesan CV, Krishnan U,
Srinivasan S, Ravikumar M and Raghuraman H: Transcatheter closure
of post myocardial infarction ventricular septal defect with
Amplatzer septal occluder. Catheter Cardiovasc Interv. 54:484–487.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hijazi ZM, Hakim F, Haweleh AA, et al:
Catheter closure of perimembranous ventricular septal defects using
the new Amplatzer membranous VSD occluder: Initial clinical
experience. Catheter Cardiovasc Interv. 56:508–515. 2002.
View Article : Google Scholar
|
|
6.
|
Bass JL, Kalra GS, Arora R, et al: Initial
human experience with the Amplatzer perimembranous ventricular
septal occluder device. Catheter Cardiovasc Interv. 58:238–245.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pawelec-Wojtalik M, Masura J, Siwińska A,
et al: Transcatheter closure of perimembranous ventricular septal
defect using an Amplatzer occluder - early results. Kardiol Pol.
61:31–41. 2004.PubMed/NCBI
|
|
8.
|
Pinto RJ, Dalvi BV and Sharma S:
Transcatheter closure of perimembranous ventricular septal defects
using amplatzer asymmetric ventricular septal defect occluder:
preliminary experience with 18-month follow up. Catheter Cardiovasc
Interv. 68:145–152. 2006. View Article : Google Scholar
|
|
9.
|
Eshaghpour E, Kawai N and Linhart JW:
Tricuspid insufficiency associated with aneurysm of the ventricular
septum. Pediatrics. 61:586–592. 1978.PubMed/NCBI
|
|
10.
|
Ogus NT, Naseri E and Arsan S: Congenital
tricuspid insufficiency due to a cleft in tricuspid anterior
leaflet associated with perimembranous VSD. An unusual case report.
Turk J Pediatr. 40:627–628. 1998.PubMed/NCBI
|
|
11.
|
Hagler DJ, Squarcia U, Cabalka AK,
Connolly HM and O’Leary PW: Mechanism of tricuspid regurgitation in
paramembranous ventricular septal defect. Am Soc Echocardiogr.
15:364–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wu MH, Chang CI, Wang JK and Lue HC:
Characterization of aneurysmal transformation in perimembranous
ventricular septal defects: an adhered anterior leaflet of
tricuspid valve predisposes to the development of left
ventricular-to-right atrial shunt. Int J Cardiol. 47:117–125. 1994.
View Article : Google Scholar
|
|
13.
|
Desai RV, Seghatol-Eslami F, Nabavizadeh F
and Lloyd SG: Unusual mechanism of tricuspid regurgitation in
ventricular septal defect. Echocardiography. 28:E36–E38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mertens L, Meyns B and Gewillig M: Device
fracture and severe tricuspid regurgitation after percutaneous
closure of perimembranous ventricular septal defect: a case report.
Catheter Cardiovasc Interv. 70:749–753. 2007. View Article : Google Scholar
|
|
15.
|
Holzer R, de Giovanni J, Walsh KP, et al:
Transcatheter closure of perimembranous ventricular septal defects
using the amplatzer membranous VSD occluder: immediate and midterm
results of an international registry. Catheter Cardiovasc Interv.
68:620–628. 2006. View Article : Google Scholar
|
|
16.
|
Hornberger LK, Sahn DJ, Krabill KA, et al:
Elucidation of the natural history of ventricular septal defects by
serial Doppler color flow mapping studies. J Am Coll Cardiol.
13:1111–1118. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tandon RH and Edwards JE: Aneurysmlike
formations in relation to membranous ventricular septum.
Circulation. 47:1089–1097. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Magherini A, Urciuolo A, Tommassini CR, et
al: Restrictive tissue in the area of perimembranous ventricular
septal defect. Cross-sectional and Doppler echocardiographic study.
Eur Heart J. 11:601–610. 1990.
|
|
19.
|
Ramaciotti C, Keren A and Silverman NH:
Importance of (perimembranous) ventricular septal aneurysm in the
natural history of isolated perimembranous ventricular septal
defect. Am J Cardiol. 57:268–272. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Beerman LB, Park SC, Fischer DR, et al:
Ventricular septal defect associated with aneurysm of the
membranous septum. J Am Coll Cardiol. 5:118–123. 1985. View Article : Google Scholar : PubMed/NCBI
|