Introduction
Severe sepsis is a major cause of mortality in the
intensive care unit (ICU). Despite advances in supportive care and
diagnostic tools, severe sepsis and septic shock remain associated
with high mortality rates (25–54%) (1–3).
Although the induction of the inflammatory c–ascade in sepsis is
mainly associated with infectious agents, it exhibits great
similarity to ischemia/reperfusion (IR) injury in tissues. In
particular, the free oxygen radicals that are generated during
ischemia and the superoxide radicals that emerge during reperfusion
cause endothelial injury, increased capillary permeability and
tissue edema. The cytokines and adhesion molecules activated during
IR initiate systemic inflammatory response syndrome (SIRS) which is
also observed in sepsis (4–6).
Sepsis is one of the major extrapulmonary causes of
acute lung injury (ALI) and acute respiratory distress syndrome
(ARDS) (3). The pulmonary
endothelium becomes lined with cytokine-containing proteins as a
result of cytokine release, neutrophil activation and increased
capillary permeability and reactive oxygen species (ROS)
generation. ROS production at levels greater than the antioxidant
capacity causes lipid peroxidation and consequently tissue and cell
injury (7–9).
Another organ affected by sepsis is the kidney.
Sepsis/septic shock causes acute kidney injury (AKI) via the
modulation of renal inflammation by specific components of the
sepsis-induced inflammatory cascade. Tubular cell apoptosis in the
kidney may be significant in septic AKI (10,11).
Ischemic preconditioning (IP) consists of brief
ischemic periods that prepare the tissue or organs for a subsequent
long period of ischemia. The excitement of the endogenous
protective mechanisms against ischemia is the basis of IP. This
phenomenon was described by Murry et al for the first time
in 1986 (12). The protective
molecular or cellular mechanisms and mediators of IP remain
unclear. However, there is much evidence that IP modulates
adenosine receptors, activates K-ATP channels, decreases ROS
production and reduces the expression of TNFα and NFκB (13–15).
Remote IP is another method of conducting IP. The
basic mechanism of remote IP is preparing one organ for ischemic
insult by the application of IP to another site of the body
(generally a lower limb) (16–18).
Limb IP decreases the IR injuries in the lung, liver, myocardium
and kidney and ameliorates the hemodynamic response to tourniquet
application (19–27). These previous studies have shown
that limb IP is a promising method for the prevention of IR-induced
tissue injuries in certain clinical conditions. However, the effect
of limb IP on sepsis-induced tissue injury has not yet been
extensively studied. In only one study, the inflammatory response
was shown to be modulated by intestinal IP in an endotoxemic shock
model (28).
The cecal ligation and puncture (CLP) rodent model
is widely used as it mimics human polymicrobial sepsis more closely
than lipopolysaccharide (LPS) administration. According to
evidence-based data, CLP creates an inflammatory response after
inducing sepsis, by increasing TNFα, IL-8 and ROS production,
initiating apoptosis, decreasing mean arterial pressure and
increasing lactate production (29–33)., However, limb IP appears to be a
more feasible method for the treatment of the clinical conditions
of patients with sepsis. Thus, we designed an experimental study
using the CLP sepsis model in rats to evaluate the effects of limb
IP on histological and biochemical parameters in the lung and
kidney.
Materials and methods
Animals
A total of 21 adult male Wistar rats (body weight,
200–250 g) were used in the experiment. All animals were obtained
from the Multidisciplinary Experimental Animal Laboratory at Dokuz
Eylül University, School of Medicine, İzmir with the approval of
the Animal Experiment Ethical Committee of Dokuz Eylül University,
School of Medicine (İzmir, Turkey).
Animals were caged in groups of five with free
access to food and water and were maintained on a 12-h light-dark
cycle at a room temperature of 22±1°C.
The rats were anesthetized with a mixture of 50
mg/kg ketamine (Ketalar®, Pfizer Pharma GmbH, Berlin,
Germany) and 10 mg/kg xylazine hydrochloride (Alfazyne®,
2%; Alfasan International, Woerden, The Netherlands) that was
administered intraperitoneally. The doses were repeated for the
immobilization of the rats while maintaining spontaneous
ventilation.
Experimental sepsis model by CLP
The rats were subjected to CLP as previously
described (34,35). Briefly, under aseptic conditions, a
3-cm midline laparotomy was performed to allow the exposure of the
cecum and adjoining intestine. The cecum was tightly ligated with a
2.0-silk suture at its base, below the ileocecal valve, and was
perforated twice with an 18-gauge needle. The cecum was then gently
squeezed to extrude a small amount of feces from the puncture site.
The cecum was then returned to the peritoneal cavity and the
laparotomy was closed with 3.0-silk sutures. Sham-operated animals
underwent the same surgical procedure although the cecum was
neither ligated nor punctured. Saline (3 ml/100 g) was administered
to all rats intraperitoneally at the end of the procedure. All
animals were returned to their cages with free access to food and
water.
IP
Left-lower-limb IP was performed by applying a
rubber band tourniquet high around the left thigh for 10 min
followed by reperfusion for 10 min, as previously reported. The
cessation of the blood flow during the ischemic period was
confirmed with laser Doppler flowmetry. Three cycles of limb IP
were performed to achieve effective pre-conditioning (19).
Experimental groups and protocol
The three groups of animals used in the present
study were the sham-operated group (sham, n=7), which underwent a
laparotomy; the sepsis group (sepsis, n=7), which underwent CLP;
and the IP-treated group (IP+sepsis, n=7), which underwent CLP
immediately prior to the application of three cycles of IP.
The rats were kept at a constant environmental
temperature of 37°C to maintain body heat following the procedures.
At 6 h after the CLP, the rats were reanesthetized with the same
dose of ketamine and xylazine hydrochloride, then their abdomens
were opened and kidneys removed. Following a midline sternotomy,
the rats were exsanguinated by needle aspiration of the right
ventricle and their lungs were removed. Plasma samples and the left
lungs were immediately transferred to a biochemistry laboratory and
stored at −80°C for the determination of serum creatinine, blood
urea nitrogen (BUN), plasma neutrophil gelatinase-associated
lipocalin (NGAL) and lung tissue malondialdehyde (MDA) levels. The
right lungs and kidneys were fixed in 10% formalin solution for
histomorphological determination and apoptosis (cytokeratin 18 with
M30 immunostaining for lungs and caspase-3 immunostaining for
kidneys).
Serum creatinine and BUN levels
Serum creatinine and BUN were measured with a is
urine/CSF protein clinical chemistry kit (Abbott-Laboratories,
Abbott Park, IL, USA) on an Architect 16000 analyzer (Abbott
Laboratories, Chicago, IL, USA). BUN and serum creatinine results
are expressed as mg/dl.
Detection of lung tissue lipid
peroxidation
Tissue homogenates were prepared by the mechanical
disruption of tissue samples using a TissueLyser (Qiagen, Hilden,
Germany) for 5 min at 30 Hz in 0.1 M phosphate buffer, pH 7.5.
Tissue homogenates were then centrifuged at 10,000 × g, 4°C for 5
min. The upper clear supernatants were transferred to a 2-ml
Eppendorf tube. The protein levels of the tissue samples were
measured with a quantitative kit using an Architect 16000 analyzer
(Abbott Laboratories). The concentrations of MDA in the samples
were determined using a high-pressure liquid chromatography method
as described by Hong et al(36), using a 5 μM C-18
reversed-phase column (250 × 4.6 mm I.D) and a mobile phase of
KH2PO4 (0.01 M) and 30% methanol with a
fluorescent detector. The MDA results are expressed in
μmol/g protein.
Serum neutrophil gelatinase-associated
lipocalin
Serum NGAL levels were quantified using a
commercially available NGAL ELISA kit (Boster Biological
Technology, Wuhan, China) according to the manufacturer’s
instructions. The plasma NGAL results are expressed in pg/ml.
Histomorphological procedures
Light microscopic tissue
preparation
The tissue samples were fixed in 10% formalin in
phosphate buffer for 24 h, then processed by routine histological
methods and embedded in paraffin blocks. Sections of 4–5 μm
thickness were obtained and stained with hematoxylin and eosin
(H&E), periodic acid-Schiff (PAS) and Masson’s trichrome
stains. The H&E-stained sections were used to evaluate the
general morphology; the PAS stain for the basal membrane and brush
border of the tubules; and the Masson’s trichrome stain for the
collagen content of the parenchyma. All histomorphological and
immunohistochemical assessments of the lungs and kidneys were
evaluated by two histologists blinded to the groups.
Histomorphological assessment of lung
tissue
Digital images were obtained from the H&E- and
Masson’s trichrome-stained sections using a digital camera (DP71;
Olympus, Tokyo, Japan) connected to a light microscope (Olympus
BX51). Three non-overlapping lung sections and a minimum of 30 lung
fields were examined per animal. A grading system was used to score
the general alveolar and parenchymal morphological changes,
including alveolar structure, inflammation, thickening of the
alveolar septum, alveolar macrophages, neutrophils, increased
capillary permeability, hemorrhage, edema and congestion. The
grading system was scored according to these findings by the
absence (score, 0), or presence (score, 1 for mild, 2 for moderate,
3 for marked and 4 for diffuse) of these changes in the alveolar
tissue (37).
The number of alveolar macrophages in the alveolar
septum and lumen was visualized digitally and counted in a given
area (macrophage count/0.016 mm2).
Histomorphological evaluation of
kidney tissue
The sections were stained with H&E and PAS
stains. Three non-overlapping kidney sections and a minimum of 30
kidney fields were examined per animal. The structural changes in
the kidney tissue sections were evaluated using light microscopy
and scored for proximal tubule damage (tubular atrophy, tubular
brush border loss, vacuolization, tubular dilation and cast
formation), mononuclear cell infiltration, erythrocyte
extravasation, interstitial structural changes, renal corpuscle
morphology and necrotic and apoptotic cells. The tubulointerstitial
damage in the obtained cross-sectional images was scored
semiquantitatively. The scoring system for these findings was: 0,
none; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100% (30).
Determination of apoptosis by
immunohistochemical study
To detect DNA fragmentation in epithelial cells in
the lung tissue, M30 with cytokeratin-18 was used, while active
caspase-3 immunohistochemistry was used to evaluate apoptosis in
the kidney tissue in the paraffin sections. Following routine
immunohistochemical procedures, the sections were incubated
overnight at 4°C with rat-specific anti-M30 antibody (1:100,
SC-32329; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
active form anti-caspase-3 antibody (1:100, AB3623; Millipore,
Temecula, CA, USA). The sections were then stained with DAB and
counterstained with Mayer’s hematoxylin. A grading system was used
to score the quantity of anti-M30 and anti-caspase-3 positive
staining in the sections (38).
The score was defined as follows: 0, no immunoreactivity; 1, little
positive staining; 2, moderate positive staining between grade 1
and grade 3; 3, marked positive staining evenly distributed across
the whole image.
Statistical analysis
The Statistical Package for the Social Sciences 15
(SPSS 15.0; SPSS, Inc., Chicago, IL, USA) software was used for
statistical analysis. Kruskal-Wallis and post hoc Mann-Whitney U
tests were used for the comparison of biochemical results. The
Chi-square test was used for comparison of the histopathological
results. P<0.05 was considered to indicate a statistically
significant difference. The results are expressed as the mean ±
SD.
Results
Evaluation of the kidney
Biochemical results
The serum creatinine and BUN levels were
significantly higher in the sepsis group compared with those in the
IP+sepsis and sham groups (P<0.05). No significant differences
were observed between the IP+sepsis and the sham groups (Table I).
 | Table ISerum creatinine, BUN, kidney injury
and apoptosis (caspase 3) scores in all experimental groups. |
Table I
Serum creatinine, BUN, kidney injury
and apoptosis (caspase 3) scores in all experimental groups.
| Group | Serum creatinine
(mg/dl) | BUN (mg/dl) | Kidney injury score
(0–4) | Caspase 3 score
(0–3) |
|---|
| Sham | 0.50±0.22 | 21.14±3.62 | 0.28±0.48 | 0.42±0.53 |
| Sepsis | 0.58±0.05a | 29.28±3.99a | 1.71±0.48a | 1.57±0.53a |
| IP+sepsis | 0.52±0.03b | 24.00±3.82b | 0.57±0.78b | 0.85±0.37b |
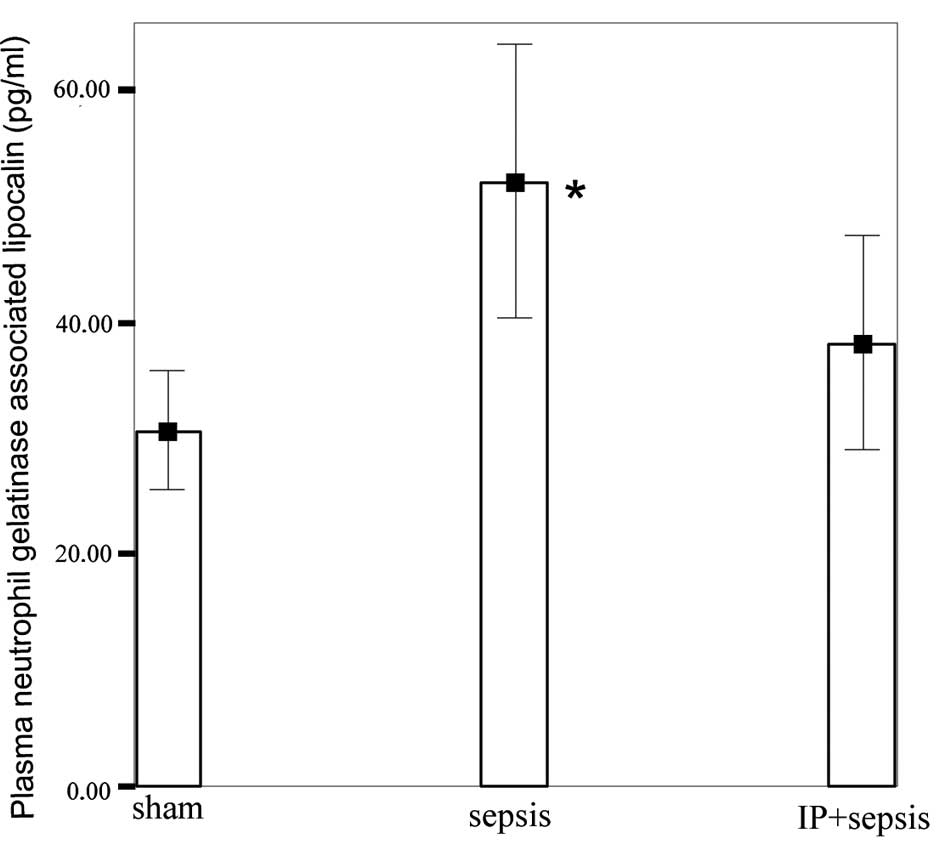
NGAL levels in the sepsis group (52.05±11.67 pg/ml)
were significantly higher than those in the sham (30.66±5.06 pg/ml)
and IP+sepsis (38.17±9.15 pg/ml) groups (P<0.05; Fig. 1). No significant differences were
observed between the sham and IP+sepsis groups.
Histomorphological and
immunohistochemical results
The histological kidney injury and kidney tissue
immuonreacimmuonreactivity (caspase 3) scores in the sepsis group
were significantly increased compared with those in the sham and
IP+sepsis groups (P<0.05). The kidney injury and the kidney
tissue immunoreactivity (caspase 3) scores in the IP+sepsis group
were not significantly different from those in the sham group
(P>0.05; Table I).
Kidney photomicrographs
In the stained kidney tissue sections, the sham
group showed a normal kidney structure (Fig. 2Aa,Ba and Ca). In the sepsis group,
mononuclear cell infiltration around the glomeruli and capillaries,
vasodilation, scattered tubular degeneration and cast formation in
the tubules were observed (Fig.
2Ab). In the PAS-stained sections, loss of the brush border and
irregularity in the basal membrane were noted in the proximal
tubular cells of the sepsis group (Fig. 2Bb). In the IP+sepsis group, the
histomorphological changes were less evident compared with those in
the sepsis group (Fig. 2Ac and
Bc). In the active caspase-3 immunohistochemical staining, the
number of caspase-3 immunopositive cells was higher in the tissue
sections of the sepsis group than in those of the sham and
IP+sepsis groups (Fig. 2Cb and
Cc).
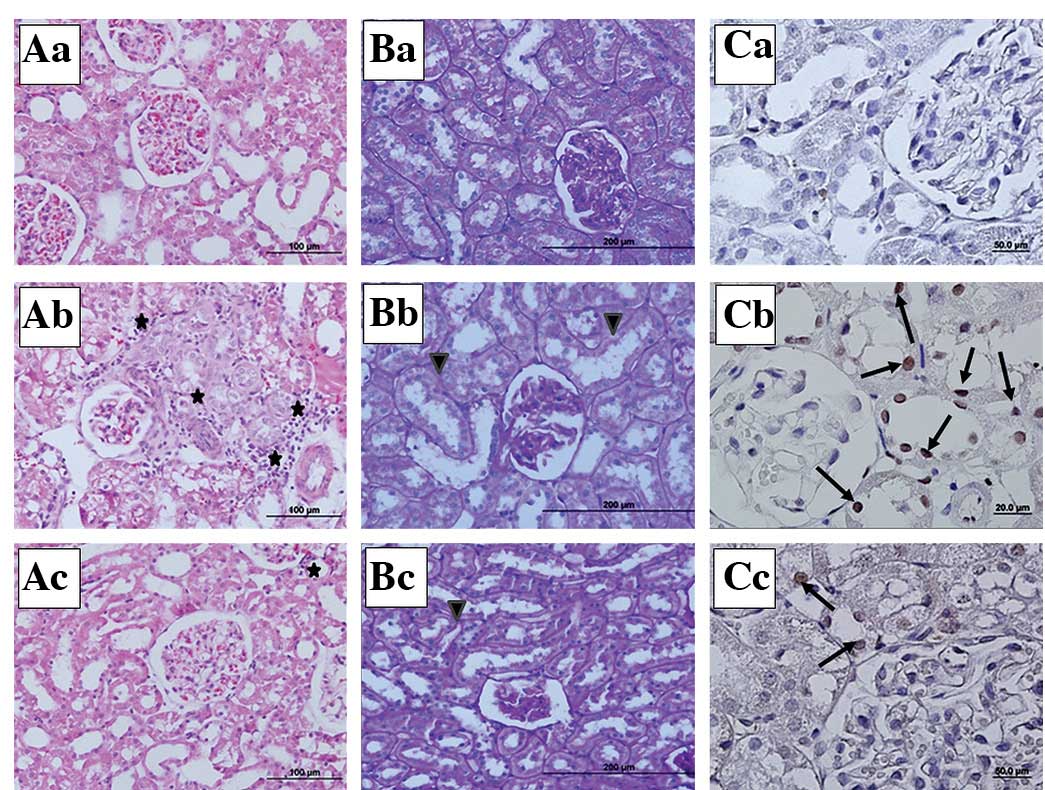 | Figure 2Representative micrographs of kidney
tissue sections stained histochemically and immunohistochemically
(H&E, PAS and active caspase-3 staining). (A) H&E
(magnification, ×20), (B) PAS (magnification, ×10) and (C) active
caspase-3 immunohistochemically stained sections (magnification,
×40) from the (a) sham, (b) sepsis and (c) IP+sepsis groups. ✶,
mononuclear cell infiltration; ▾, irregular basal membrane and
brush border loss in proximal tubular epithelium; →, active
caspase-3 immunpositive cells; H&E, hematoxylin and eosin; PAS,
periodic acid-Schiff; IP, ischemic preconditioning. |
Evaluation of the lung
Determination of lipid
peroxidation
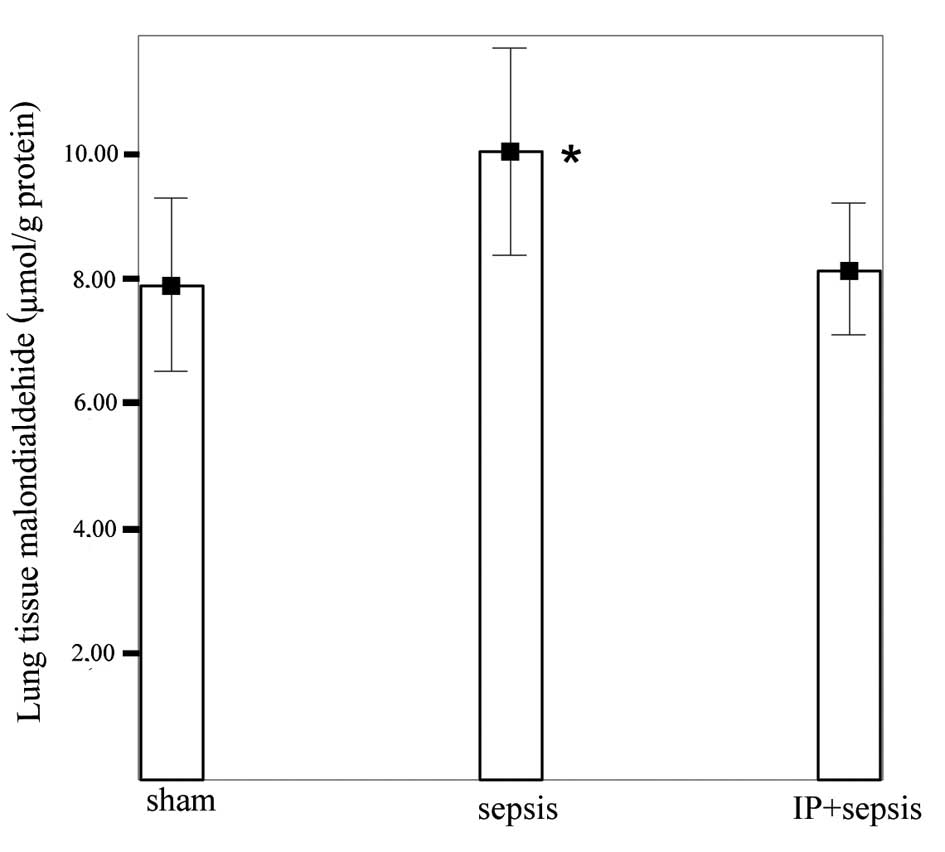
The lung tissue MDA levels were increased
significantly in the sepsis group (10.02±1.66 μmol/g
protein) compared with those in the sham (7.90±1.39 μmol/g
protein) and IP+sepsis (8.14±1.05 μmol/g protein) groups
(P<0.05). No significant differences were detected between the
IP+sepsis and sham groups (Fig.
3).
Histomorphological and
immunohistochemical results
The histological lung injury and lung tissue
immunoreacimmunoreactivity (M30) scores in the sepsis group were
significantly increased compared with those in the sham and
IP+sepsis groups (P<0.05). There were no differences in the lung
tissue immunoreactivity (M30) scores between the IP+sepsis and sham
groups. The lung injury score in the IP+sepsis group was elevated
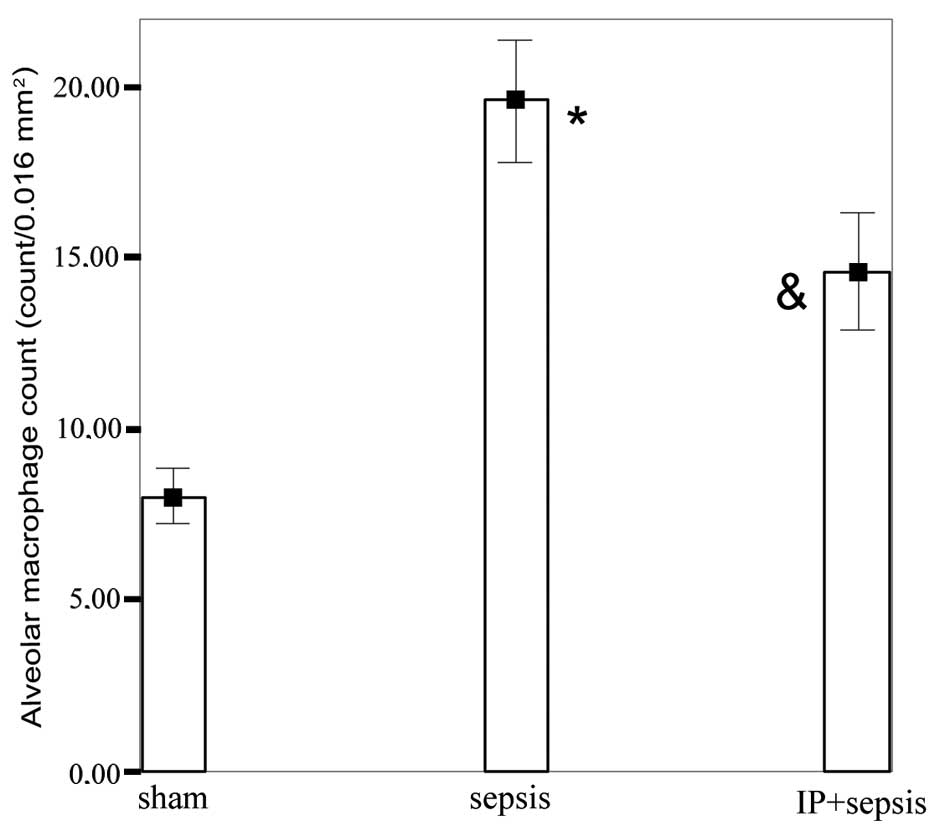
compared with that in the sham group (Table II). The alveolar macrophage count
in the sepsis group (19.57±1.81) was significantly increased
compared with those in the sham (8.00±0.81) and IP+sepsis
(14.57±1.71) groups (P<0.05) and was higher in the IP+sepsis
group than in the sham group (P<0.05; Fig. 4).
 | Table IIHistological lung injury and
apoptosis (M30) scores in all experimental groups. |
Table II
Histological lung injury and
apoptosis (M30) scores in all experimental groups.
| Group | Lung injury score
(0–4) | M30 score
(0–3) |
|---|
| Sham | 0.14±0.37 | 0.42±0.53 |
| Sepsis |
2.00±0.57ab |
2.00±0.57ab |
| IP+sepsis | 0.71±0.71a | 0.85±0.37 |
Lung photomicrographs
The staining of the lung tissue sections showed that
the sham group had normal lung histology (Fig. 5Aa, Bb and Cc). By contrast, there
was a significant increase in the lung injury score following
sepsis, with the development of alveolar wall thickening and
inflammatory infiltration (Fig. 5Ab
and Bb). When the sections from the sepsis group were observed,
inflammation and thickening of the alveolar septum, particularly
increases in alveolar macrophage and mononuclear-cell infiltration
which ensured that the alveolar septum increased capillary
permeability, hemorrhage, edema and congestion, were noted
(Fig. 5Bb). However, in the
IP+sepsis group, the structural lung injury was less severe
compared with that in the sepsis group (Fig. 5Ac and Bc). In the Masson’s
trichrome-stained sections, increased collagen content in the
parenchyma of the lung tissue sections of the sepsis group was
observed (Fig. 5Bb). Furthermore,
in the M30 immunohistochemistry scoring, the number of M30
immunopositive epithelial cells was increased in the sepsis group
compared with the numbers in the sham and IP+sepsis groups
(Fig. 5Cb and Cc).
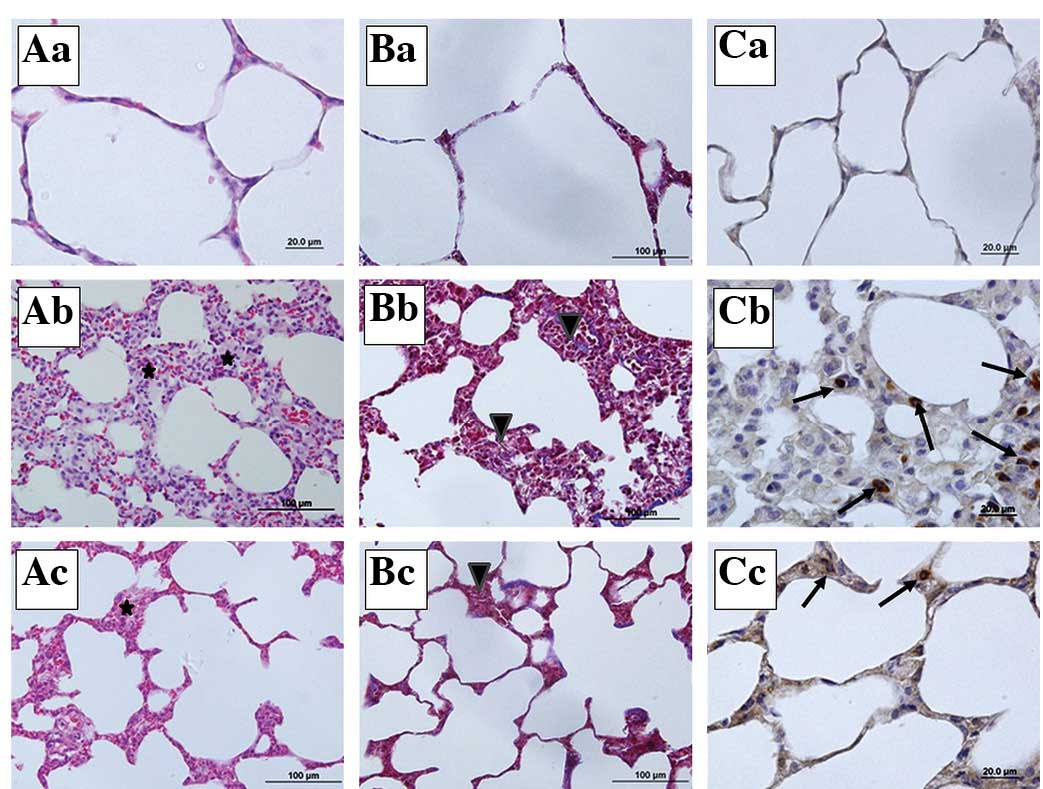 | Figure 5Representative micrographs of lung
tissue sections stained histochemically and immunohistochemically
(H&E, Masson’s trichrome and M30 staining). (A) H&E
(magnification, ×40), (B) Masson’s trichrome (magnification, ×20),
(C) M30 immunohistochemically stained sections (magnification, ×20)
from the (a) sham, (b) sepsis and (c) IP+sepsis groups. ✶,
mononuclear cell infiltration; ▾, collagen content of the
parenchyma; →, marked M30 immunopositive cells; H&E,
hematoxylin and eosin; IP, ischemic preconditioning. |
Discussion
In the present study, it was shown histologically
and biochemically that IP applied to the unilateral hind limb
attenuated septic lung and kidney injury. To the best of the
author’s knowledge, the present study is the first to evaluate
remote IP with regard to lung and kidney injury in a CLP sepsis
model. CLP was selected for the generation of sepsis as it is a
widely used experimental sepsis model that mimics human
polymicrobial sepsis (34,39).
Experimental and clinical studies have shown that IP
increases resistance against ischemic tissue injury to the organs,
including the heart, lung, kidney, intestine, liver and muscle
(20,25,28,40,41).
SIRS may be induced by infection as well as IR. The mechanisms by
which infections and IR trigger SIRS are almost the same.
Therefore, it has been hypothesized that either ischemic or
pharmacologically-simulated preconditioning may lead to new
possibilities in the treatment of critically ill patients with
sepsis and multiple organ dysfunction syndrome (MODS) (4,24).
The main ischemic conditioning methods are
preconditioning (prior to major ischemia), perconditioning (during
major ischemia) and postconditioning (during reperfusion) which are
named according to the application time (42). In the present study, IP was
initiated immediately following the CLP procedure and completed
within 1 h. It has been shown that sepsis occurs 5 h after CLP
(35,43). Therefore the present method of
ischemic conditioning was described as ischemic ‘preconditioning’.
The effectiveness of IP is associated with the number of ischemic
cycles and length of the ischemic period (17,44).
In the present study, three 10 min IP cycles were used, for which
effectiveness against IR had already been demonstrated (19), and the blood and tissue samples
were collected 6 h after the CLP procedure.
The kidney is the one of the main organs affected
during sepsis. Decreased renal perfusion and numbers of cortical
capillaries, peaking of renal tubular apoptosis and increased
leukocyte infiltration and ROS production have been observed in
experimental models 4–6 h after the induction of sepsis (29,32,45,46).
The protective effects of limb IP are transferred by
means of heat shock proteins, nitric oxide, adenosine, bradykinin
and neurogenic signals to the target organs (13,47).
The results of these interactions are reductions in ROS levels,
reductions in ATP depletion and increases in ATP production which
consequently increase the oxidative stress resistance of the cell
(13,15). The effect of limb IP on IR injury
to the kidney has been shown to reduce serum creatinine and BUN
levels (48). Er et
al(49) and Zimmerman et
al(25) demonstrated similar
results in clinical studies. In the present study, it was observed
that the BUN and serum creatinine levels were significantly higher
in the sepsis group than in the IP+sepsis group, while no
significant differences were observed between the IP+sepsis and
sham groups. These findings indicate that IP protects renal
function from sepsis-related kidney insult. This effect of limb IP
may be associated with the reduction of ROS production or an
increase in microvascular circulation.
NGAL is a highly sensitive, specific and predictive
early biomarker for AKI (50,51).
Bagshaw et al(52) observed
that patients with acute kidney sepsis have higher plasma and urine
NGAL levels compared with patients without acute septic kidney
injury. Furthermore, serum NGAL levels have been observed to be
elevated in SIRS, sepsis and septic shock (53,54).
NGAL is present in the kidney, liver, spleen, lung and trachea,
indicating that serum NGAL may not be a reliable marker of septic
AKI (55). By contrast, Chen et
al(14) showed that a major
cause of elevated serum NGAL in the kidney is ischemic renal injury
and the application of IP to the kidney decreases NGAL levels.
Therefore, it may be concluded that the elevation of the plasma
NGAL level in the present sepsis group may be associated not only
with the kidney, but also other NGAL-containing tissues such as the
lung, spleen and liver. However, the increase in the plasma NGAL
level of the sepsis group reflected the sepsis-induced NGAL release
from tissues which occurred in the present study. The present study
also showed that the sepsis-associated NGAL release from tissues
was efficiently inhibited by limb IP.
In sepsis, neutrophil accumulation in the lung is
followed by cytokine and ROS production. ROS production exceeding
the antioxidant capacity causes lipid peroxidation and consequent
tissue injury and cell death (9,56).
MDA is the end-product of fatty acid peroxidation and is generated
within inflammatory cells. The MDA content of the lungs increases
significantly following CLP-induced sepsis (43,57,58).
Numerous studies have shown that IP diminishes neutrophil
accumulation, lipid peroxidation and MDA levels in the lung
(19,23,59–61).
In the present study, the lung MDA levels were significantly higher
in the sepsis group than in the IP+sepsis and sham groups. We
conclude that remote IP has an antioxidant effect in CLP-induced
sepsis by decreasing lipid peroxidation levels in the lung
Previous studies have investigated lung and kidney
injuries using H&E staining and light microscopy. Notably high
histological lung and kidney injury scores have been observed in
septic rats (62–65). IP attenuates lung and kidney
histological injuries in ischemic conditions and Lee et
al(66) histologically showed
that hepatic IP is able to attenuate renal ischemic injury. It has
been demonstrated that IP acts via A1 adenosine receptor activation
(15,41). The activation of the A1 adenosine
receptor diminishes renal inflammation, apoptosis and necrosis in
renal ischemia (16,67).
We have previously demonstrated that unilateral hind
limb IR causes lung injury and remote IP decreases the lung injury
score (19). Similarly, lung
injury has been mitigated using limb injury in the hemorrhagic
shock model (5). In the present
study it was observed that the lung and kidney histological injury
scores were significantly lower in the IP+sepsis group than in the
sepsis group. Therefore, we propose that IP reduces sepsis-induced
lung and kidney injury not only biochemically, but also
histologically.
The mononuclear phagocyte system is involved in
phagocytosis and numerous complex immunological and inflammatory
processes. Sepsis is associated with the increased production of
cellular proinflammatory and inflammatory mediators by
monocytes/macrophages. TNFα is the main mediator and has an
important role in acute kidney and lung injury in sepsis (68,69).
Remote IP decreases the levels of TNFα and other inflammatory
cytokines, including IL-6 and IL-8, in lung tissue and plasma,
(61,70). However, ROS activate the
transcription factor NFκB which stimulates the excessive production
of inflammatory cytokines. Takeshita et al(71) showed that direct IP reduced NFκB
activity and cytokine mRNA levels.
In the present study, a significantly higher
alveolar macrophage count was observed in the sepsis group than in
the sham and IP+sepsis groups. We were unable to measure the
cytokine levels, which was a limitation of the present study.
However, the CLP-induced alveolar macrophage count in the lung was
decreased by limb IP. This effect may be explained by the reduction
of TNFα levels and the inhibition of NFκB activity.
Apoptosis is a type of programmed cell death in
which DNA disintegration and cell death occur as a result of the
activation of death-inducing receptors or intracellular specific
serine proteases (caspases) (72,73).
The gold standard for the diagnosis of apoptosis is
morphological/ultrastructural evaluation. The determination of
apoptosis using H&E staining and light microscopy is sensitive
and specific, while also being the least expensive approach
(74,75). However, to evaluate acute injury
such as in CLP-induced sepsis, more early markers that indicate
apoptosis are required. Therefore, caspase 3 and M30 (caspase
cleaved cytokeratin 18 neo-epitope) staining were used in the
present study to show kidney and lung epithelial apoptosis,
respectively. The sensitivity of these two methods has been
demonstrated clinically and experimentally, including in
CLP-induced sepsis (11,57,76–78).
Apoptosis occurs through inflammatory cytokines and
TNFα, apoptosis-associated proteins and ROS-mediated pathways
during sepsis (69,79–82).
IP reduces apoptosis by regulating the genes which
encode these proteins and releasing the metabolites involved in the
apoptotic process (83). The
protective effect of IP on IR injury-induced apoptosis has been
demonstrated in the lung and kidney (80,83).
In the present study, significant increases were
observed in the M30-positive cell count of the sepsis group
compared with the sham group, indicating the presence of epithelial
cell-specific apoptosis in sepsis. Also, no significant changes
were noted in the number of pulmonary M30-positive cells in the
IP+sepsis group compared with the sham group, suggesting that limb
IP inhibits apoptosis in CLP-induced sepsis. Additionally, it was
also observed that number of caspase-3-stained renal tubular cells
was significantly lower in the IP+sepsis group than in the sepsis
group and not significantly different between the sham and
IP+sepsis groups. Jacobs et al(10) suggested that intra-renal
inflammation is decreased by caspase inhibition through unclear
mechanisms. It is possible that IP inhibits caspase. Together,
these results suggest that IP attenuates apoptosis in the kidney
and the lung during sepsis.
In conclusion, the present study presents evidence
for the effectiveness of IP in the management of sepsis. Remote IP
was observed to reduce lung lipid peroxidation, kidney and lung
injuries and apoptosis in a CLP-induced sepsis model. Further
investigations are required to fully elucidate the underlying
mechanisms of IP in sepsis.
References
|
1.
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Martin GS, Mannino DM, Eaton S and Moss M:
The epidemiology of sepsis in the United States from 1979 through
2000. N Engl J Med. 348:1546–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Vincent JL, Sakr Y, Sprung CL, et al
Sepsis Occurrence in Acutely Ill Patients Investigators: Sepsis in
European intensive care units: results of the SOAP study. Crit Care
Med. 34:344–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rock P and Yao Z: Ischemia reperfusion
injury, preconditioning and critical illness. Curr Opin
Anaesthesiol. 15:139–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jan WC, Chen CH, Tsai PS and Huang CJ:
Limb ischemic preconditioning mitigates lung injury induced by
haemorrhagic shock/resuscitation in rats. Resuscitation.
82:760–766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jan F, Burne M, O’Donnell M and Rabb H:
Pathophysiologic role of selectins and their ligands in ischemia
reperfusion injury. Front Biosci. 5:E103–E109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lange M, Szabo C, Traber DL, et al: Time
profile of oxidative stress and neutrophil activation in ovine
acute lung injury and sepsis. Shock. 37:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jacobs R, Honore PM, Joannes-Boyau O, et
al: Septic acute kidney injury: the culprit is inflammatory
apoptosis rather than ischemic necrosis. Blood Purif. 32:262–265.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lerolle N, Nochy D, Guérot E, et al:
Histopathology of septic shock induced acute kidney injury:
apoptosis and leukocytic infiltration. Intensive Care Med.
36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar
|
|
13.
|
Szijártó A, Czigány Z, Turóczi Z and
Harsányi L: Remote ischemic perconditioning - a simple, low-risk
method to decrease ischemic reperfusion injury: models, protocols
and mechanistic background. A review. J Surg Res. 178:797–806.
2012.PubMed/NCBI
|
|
14.
|
Chen X, Liu X, Wan X, Wu Y, Chen Y and Cao
C: Ischemic preconditioning attenuates renal ischemia-reperfusion
injury by inhibiting activation of IKKbeta and inflammatory
response. Am J Nephrol. 30:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Riksen NP, Smits P and Rongen GA:
Ischaemic preconditioning: from molecular characterisation to
clinical application - part I. Neth J Med. 62:353–363.
2004.PubMed/NCBI
|
|
16.
|
Souza Filho MV, Loiola RT, Rocha EL, et
al: Hind limb ischemic preconditioning induces an anti-inflammatory
response by remote organs in rats. Braz J Med Biol Res. 42:921–929.
2009.PubMed/NCBI
|
|
17.
|
Kharbanda RK, Mortensen UM, White PA, et
al: Transient limb ischemia induces remote ischemic preconditioning
in vivo. Circulation. 106:2881–2883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takaoka A, Nakae I, Mitsunami K, et al:
Renal ischemia/reperfusion remotely improves myocardial energy
metabolism during myocardial ischemia via adenosine receptors in
rabbits: effects of ‘remote preconditioning’. J Am Coll Cardiol.
33:556–564. 1999.PubMed/NCBI
|
|
19.
|
Olguner C, Koca U, Kar A, et al: Ischemic
preconditioning attenuates the lipid peroxidation and remote lung
injury in the rat model of unilateral lower limb ischemia
reperfusion. Acta Anaesthesiol Scand. 50:150–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Harkin DW, Barros D’Sa AA, McCallion K,
Hoper M and Campbell FC: Ischemic preconditioning before lower limb
ischemia - reperfusion protects against acute lung injury. J Vasc
Surg. 35:1264–1273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Şahin E, Olguner C, Bodur HA, Koca U,
Tuncel P, Örmen M, et al: Comparison of the effects of the remote
and direct ischemic preconditioning in the liver
ischemia-reperfusion injury. Türkiye Klinikleri. Tıp Bilimleri
Dergisi. 29:381–387. 2009.(In Turkish).
|
|
22.
|
Lai IR, Chang KJ, Chen CF and Tsai HW:
Transient limb ischemia induces remote preconditioning in liver
among rats: the protective role of heme oxygenase-1.
Transplantation. 81:1311–1317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Granfeldt A, Jiang R, Wang NP, et al:
Neutrophil inhibition contributes to cardioprotection by
postconditioning. Acta Anaesthesiol Scand. 56:48–56. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Xiong J, Liao X, Xue FS, Yuan YJ, Wang Q
and Liu JH: Remote ischemia conditioning-an endogenous
cardioprotective strategy from outside the heart. Chin Med J
(Engl). 124:2209–2215. 2011.PubMed/NCBI
|
|
25.
|
Zimmerman RF, Ezeanuna PU, Kane JC, et al:
Ischemic preconditioning at a remote site prevents acute kidney
injury in patients following cardiac surgery. Kidney Int.
80:861–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Van M, Olguner C, Koca U, et al: Ischaemic
preconditioning attenuates haemodynamic response and lipid
peroxidation in lower-extremity surgery with unilateral pneumatic
tourniquet application: a clinical pilot study. Adv Ther.
25:355–366. 2008. View Article : Google Scholar
|
|
27.
|
Lin LN, Wang LR, Wang WT, et al: Ischemic
preconditioning attenuates pulmonary dysfunction after unilateral
thigh tourniquet-induced ischemia-reperfusion. Anesth Analg.
111:539–543. 2010. View Article : Google Scholar
|
|
28.
|
Tamion F, Richard V, Renet S and Thuillez
C: Intestinal preconditioning prevents inflammatory response by
modulating heme oxygenase-1 expression in endotoxic shock model. Am
J Physiol Gastrointest Liver Physiol. 293:G1308–G1314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Seely KA, Holthoff JH, Burns ST, et al:
Hemodynamic changes in the kidney in a pediatric rat model of
sepsis-induced acute kidney injury. Am J Physiol Renal Physiol.
301:F209–F217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yasuda H, Yuen PS, Hu X, Zhou H and Star
RA: Simvastatin improves sepsis-induced mortality and acute kidney
injury via renal vascular effects. Kidney Int. 69:1535–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wu L, Gokden N and Mayeux PR: Evidence for
the role of reactive nitrogen species in polymicrobial
sepsis-induced renal peritubular capillary dysfunction and tubular
injury. J Am Soc Nephrol. 18:1807–1815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Messaris E, Memos N, Chatzigianni E, et
al: Apoptotic death of renal tubular cells in experimental sepsis.
Surg Infect (Larchmt). 9:377–388. 2008. View Article : Google Scholar
|
|
33.
|
Collin S, Sennoun N, Dron AG, et al:
Vascular ATP-sensitive potassium channels are over-expressed and
partially regulated by nitric oxide in experimental septic shock.
Intensive Care Med. 37:861–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Wichterman KA, Baue AE and Chaudry IH:
Sepsis and septic shock - a review of laboratory models and a
proposal. J Surg Res. 29:189–201. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Otero-Antón E, González-Quintela A,
López-Soto A, López-Ben S, Llovo J and Pérez LF: Cecal ligation and
puncture as a model of sepsis in the rat: influence of the puncture
size on mortality, bacteremia, endotoxemia and tumor necrosis
factor alpha levels. Eur Surg Res. 33:77–79. 2001.PubMed/NCBI
|
|
36.
|
Hong YL, Yeh SL, Chang CY and Hu ML: Total
plasma malondialdehyde levels in 16 Taiwanese college students
determined by various thiobarbituric acid tests and an improved
high-performance liquid chromatography-based method. Clin Biochem.
33:619–625. 2000. View Article : Google Scholar
|
|
37.
|
Albaiceta GM, Gutiérrez-Fernández A, Parra
D, et al: Lack of matrix metalloproteinase-9 worsens
ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol.
294:L535–L543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Tüzün F, Gencpinar P, Ozbal S, et al:
Neuroprotective effect of neotrofin in a neonatal rat model of
periventricular leukomalacia. Neurosci Lett. 520:6–10.
2012.PubMed/NCBI
|
|
39.
|
Holly MK, Dear JW, Hu X, et al: Biomarker
and drug-target discovery using proteomics in a new rat model of
sepsis-induced acute renal failure. Kidney Int. 70:496–506.
2006.PubMed/NCBI
|
|
40.
|
Peralta C, Closa D, Xaus C, Gelpí E,
Roselló-Catafau J and Hotter G: Hepatic preconditioning in rats is
defined by a balance of adenosine and xanthine. Hepatology.
28:768–773. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Riksen NP, Smits P and Rongen GA:
Ischaemic preconditioning: from molecular characterisation to
clinical application - part II. Neth J Med. 62:409–423.
2004.PubMed/NCBI
|
|
42.
|
Vinten-Johansen J and Shi W:
Perconditioning and postconditioning: current knowledge, knowledge
gaps, barriers to adoption, and future directions. J Cardiovasc
Pharmacol Ther. 16:260–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Hsu DZ and Liu MY: Effects of sesame oil
on oxidative stress after the onset of sepsis in rats. Shock.
22:582–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Saita Y, Yokoyama K, Nakamura K and Itoman
M: Protective effect of ischaemic preconditioning against
ischaemia-induced reperfusion injury of skeletal muscle: how many
preconditioning cycles are appropriate? Br J Plast Surg.
55:241–245. 2002. View Article : Google Scholar
|
|
45.
|
Legrand M, Bezemer R, Kandil A, Demirci C,
Payen D and Ince C: The role of renal hypoperfusion in development
of renal microcirculatory dysfunction in endotoxemic rats.
Intensive Care Med. 37:1534–1542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Wang Z, Holthoff JH, Seely KA, et al:
Development of oxidative stress in the peritubular capillary
microenvironment mediates sepsis-induced renal microcirculatory
failure and acute kidney injury. Am J Pathol. 180:505–516. 2012.
View Article : Google Scholar
|
|
47.
|
Shihab FS: Preconditioning: from
experimental findings to novel therapies in acute kidney injury.
Minerva Urol Nefrol. 61:143–157. 2009.PubMed/NCBI
|
|
48.
|
Kadkhodaee M, Seifi B, Najafi A and
Sedaghat Z: First report of the protective effects of remote per-
and postconditioning on ischemia/reperfusion-induced renal injury.
Transplantation. 92:e552011. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Er F, Nia AM, Dopp H, et al: Ischemic
preconditioning for prevention of contrast medium-induced
nephropathy: randomized pilot RenPro Trial (Renal Protection
Trial). Circulation. 126:296–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Devarajan P: Emerging biomarkers of acute
kidney injury. Contrib Nephrol. 156:203–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Dent CL, Ma Q, Dastrala S, et al: Plasma
neutrophil gelatinase-associated lipocalin predicts acute kidney
injury, morbidity and mortality after pediatric cardiac surgery: a
prospective uncontrolled cohort study. Crit Care. 11:R1272007.
View Article : Google Scholar
|
|
52.
|
Bagshaw SM, Bennett M, Haase M, et al:
Plasma and urine neutrophil gelatinase-associated lipocalin in
septic versus non-septic acute kidney injury in critical illness.
Intensive Care Med. 36:452–461. 2010. View Article : Google Scholar
|
|
53.
|
Han M, Li Y, Liu M and Cong B: Renal
neutrophil gelatinase associated lipocalin expression in
lipopolysaccharide-induced acute kidney injury in the rat. BMC
Nephrol. 13:252012. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Mårtensson J, Bell M, Oldner A, Xu S,
Venge P and Martling CR: Neutrophil gelatinase-associated lipocalin
in adult septic patients with and without acute kidney injury.
Intensive Care Med. 36:1333–1340. 2010.PubMed/NCBI
|
|
55.
|
Paragas N, Qiu A, Zhang Q, et al: The Ngal
reporter mouse detects the response of the kidney to injury in real
time. Nat Med. 17:216–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Demirbilek S, Sizanli E, Karadag N, et al:
The effects of methylene blue on lung injury in septic rats. Eur
Surg Res. 38:35–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Ozdulger A, Cinel I, Koksel O, et al: The
protective effect of N-acetylcysteine on apoptotic lung injury in
cecal ligation and puncture-induced sepsis model. Shock.
19:366–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Ma H, Kou J, Zhu D, Yan Y and Yu B:
Liu-Shen-Wan, a traditional Chinese medicine, improves survival in
sepsis induced by cecal ligation and puncture via reducing
TNF-alpha levels, MDA content and enhancing macrophage
phagocytosis. Int Immunopharmacol. 6:1355–1362. 2006. View Article : Google Scholar
|
|
59.
|
Kahraman S, Kilinç K, Dal D and Erdem K:
Propofol attenuates formation of lipid peroxides in
tourniquet-induced ischaemia-reperfusion injury. Br J Anaesth.
78:279–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Soncul H, Oz E and Kalaycioglu S: Role of
ischemic preconditioning on ischemia-reperfusion injury of the
lung. Chest. 115:1672–1677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Xu B, Gao X, Xu J, et al: Ischemic
postconditioning attenuates lung reperfusion injury and reduces
systemic proinflammatory cytokine release via heme oxygenase 1. J
Surg Res. 166:e157–e164. 2011. View Article : Google Scholar
|
|
62.
|
Cinel I, Ark M, Dellinger P, et al:
Involvement of Rho kinase (ROCK) in sepsis-induced acute lung
injury. J Thorac Dis. 4:30–39. 2012.PubMed/NCBI
|
|
63.
|
Kono Y, Inomata M, Hagiwara S, Shiraishi
N, Noguchi T and Kitano S: A newly synthetic vitamin E derivative,
E-Ant-S-GS, attenuates lung injury caused by cecal ligation and
puncture-induced sepsis in rats. Surgery. 151:420–426. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Wu YX, Wu DW, Peng MM, Chen C, Lu HN and
Zhao LK: The effect of low-dose hydrocortisone on the expression of
glucocorticoid receptor alpha of the septic kidney and its
protective effect on kidney in rat. Zhongguo Wei Zhong Bing Ji Jiu
Yi Xue. 23:426–429. 2011.(In Chinese).
|
|
65.
|
Yan GT, Xue H, Lin J, Hao XH, Zhang K and
Wang LH: Leptin protects sepsis-induced renal injury and research
for its mechanism. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
18:665–667. 2006.(In Chinese).
|
|
66.
|
Lee JA, Choi JW, In JH, et al: Hepatic
ischemic preconditioning provides protection against distant renal
ischemia and reperfusion injury in mice. J Korean Med Sci.
27:547–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Lee HT, Gallos G, Nasr SH and Emala CW: A1
adenosine receptor activation inhibits inflammation, necrosis, and
apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc
Nephrol. 15:102–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
van Lanschot JJ, Mealy K, Jacobs DO, Evans
DA and Wilmore DW: Splenectomy attenuates the inappropriate
diuresis associated with tumor necrosis factor administration. Surg
Gynecol Obstet. 172:293–297. 1991.PubMed/NCBI
|
|
69.
|
Cunningham PN, Dyanov HM, Park P, Wang J,
Newell KA and Quigg RJ: Acute renal failure in endotoxemia is
caused by TNF acting directly on TNF receptor-1 in kidney. J
Immunol. 168:5817–5823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Percival TJ and Rasmussen TE: Reperfusion
strategies in the management of extremity vascular injury with
ischaemia. Br J Surg. 99(Suppl 1): S66–S74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Takeshita M, Tani T, Harada S, et al: Role
of transcription factors in small intestinal ischemia-reperfusion
injury and tolerance induced by ischemic preconditioning.
Transplant Proc. 42:3406–3413. 2010. View Article : Google Scholar
|
|
72.
|
Oberholzer C, Oberholzer A, Clare-Salzler
M and Moldawer LL: Apoptosis in sepsis: a new target for
therapeutic exploration. FASEB J. 15:879–892. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Mainous MR, Ertel W, Chaudry IH and Deitch
EA: The gut: a cytokine-generating organ in systemic inflammation?
Shock. 4:193–199. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Holubec H, Payne CM, Bernstein H, et al:
Assessment of apoptosis by immunohistochemical markers compared to
cellular morphology in ex vivo-stressed colonic mucosa. J Histochem
Cytochem. 53:229–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Gobe G: Identification of apoptosis in
kidney tissue sections. Methods Mol Biol. 466:175–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Wu HH, Hsiao TY, Chien CT and Lai MK:
Ischemic conditioning by short periods of reperfusion attenuates
renal ischemia/reperfusion induced apoptosis and autophagy in the
rat. J Biomed Sci. 16:192009. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Perl M, Chung CS, Perl U, et al:
Fas-induced pulmonary apoptosis and inflammation during indirect
acute lung injury. Am J Respir Crit Care Med. 176:591–601. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Oliver L and Vallette FM: The role of
caspases in cell death and differentiation. Drug Resist Updat.
8:163–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Jo SK, Cha DR, Cho WY, et al: Inflammatory
cytokines and lipopolysaccharide induce Fas-mediated apoptosis in
renal tubular cells. Nephron. 91:406–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Messmer UK, Briner VA and Pfeilschifter J:
Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic
cell death in bovine glomerular endothelial cells. Kidney Int.
55:2322–2337. 1999. View Article : Google Scholar
|
|
81.
|
Du C, Guan Q, Yin Z, Zhong R and Jevnikar
AM: IL-2-mediated apoptosis of kidney tubular epithelial cells is
regulated by the caspase-8 inhibitor c-FLIP. Kidney Int.
67:1397–1409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Jun N, Ke J, Gang C, Lin C, Jinsong L and
Jianjun W: The protective effect of ischemic preconditioning
associated with altered gene expression profiles in rat lung after
reperfusion. J Surg Res. 168:281–293. 2011. View Article : Google Scholar : PubMed/NCBI
|