Introduction
Otitis media with effusion (OME) is a common
disorder characterized by inflammation of the middle ear (ME), with
accumulation of fluid. OME is a worldwide problem that leads to
conductive hearing loss in children, resulting in developmental
problems in speech, language and the acquisition of social skills
(1,2). Previous studies have suggested that
intrinsic or acquired Eustachian tube (ET) obstruction, nasal
allergies and/or bacterial infection of the ME may be involved in
the development and persistence of ME mucosal inflammation;
however, the exact etiology of OME remains unclear (3–5).
Aquaporins (AQPs) are integral membrane proteins
that serve as channels for the transfer of water and, in certain
cases, small solutes across the membrane (6). AQPs play a significant role in water
balance (local homeostasis) and deficits in AQP expression and/or
function have been associated with several diseases of
dysfunctional water regulation (7,8). The
first identified AQP subtype, AQP1, increases osmotic water
permeability by ∼20-fold (9). AQP1
is widely distributed in the ME mucosa and has previously been
implicated in the accumulation of effusion in the ME cavity
(10).
Glucocorticoids are used clinically as
anti-inflammatory drugs to suppress a wide variety of inflammatory
and immune responses, although their use as a medical treatment for
OME is controversial. Glucocorticoids have been shown to regulate
the water balance in several tissues and organs, including the
lung, peritoneum and inner ear (11–13).
Consequently, an understanding of the effects of glucocorticoids on
AQP1 in the ME cavity may provide new insights into the molecular
mechanisms involved in transcellular water transport in OME.
In this study, we investigated the expression
pattern of aquaporin 1 (AQP1) in a guinea pig model of OME, induced
by reversible ET obstruction, in order to analyze the effect of
glucocorticoids on AQP1.
Materials and methods
Animals and surgery
Male guinea pigs (n=26) weighing between 300 and 450
g were used in this study. Their ears were examined by
otomicroscopy and tympanometry to document that the ME was
disease-free bilaterally. Then, left ET obstruction was created
surgically in all animals. All animals were handled according to
the guidelines of the Animal Care and Use Committee of the
Affiliated Drum Tower Hospital of Nanjing University School of
Medicine (Nanjing, China). The guinea pigs were anesthetized by
intraperitoneal injection of a mixture of ketamine (50 mg/kg) and
diazepam (5 mg/kg). To create the reversible OME model, the
animal’s mouth was propped open and the nasal orifice of the left
ET was approached via a transpalatal incision and obstructed with
polyvinyl acetal material. The right ear was used as a control.
Following surgery, all ears were evaluated daily by otomicroscopy.
After effusion was observed in 22 of the animals, two animals were
sacrificed for immunohistochemical examination. The others were
divided randomly into the OME and dexamethasone (dexa) groups. The
dexa group received dexa (5 mg/kg/day) via intraperitoneal
injection for 7 days starting on the seventh postoperative day. All
animals were sacrificed under deep anesthesia on the fourteenth
postoperative day and the left and right temporal bones were
removed rapidly for the subsequent experiments.
Immunohistochemistry
Two guinea pigs with OME were anesthetized as
described above and perfused transcardially with physiological
saline and 4% paraformaldehyde to fix the tissues in situ.
The bullae were harvested, fixed with 4% paraformaldehyde at 4°C
for 24 h and decalcified with a 10% ethylenediamine tetraacetic
acid (EDTA) solution (pH 7.0) at 4°C for 14–17 days. The EDTA
solution was changed every day. The specimens were then dehydrated
and embedded in paraffin. The tissues were cut into 6-μm
sections, deparaffinized and hydrated in phosphate-buffered saline
(PBS, pH 7.4). The sections were treated with 3%
H2O2 at room temperature for 30 min to block
the endogenous peroxidase activity and then incubated with a rabbit
polyclonal antibody against AQP1 (Abcam, Cambridge, MA, USA; 1:400
dilution) at 4°C for 18 h. The sections were then washed with PBS
and incubated with a biotin-conjugated secondary antibody against
rabbit IgG (PowerVision™ Two-Step Histostaining Reagent; Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China) at room
temperature for 30 min. Finally, the sections were incubated with
0.05% 3,3′-diaminobenzidine and then counterstained with Mayer’s
hematoxylin. Negative control staining was performed using 0.01 M
PBS in place of the primary antibody.
Protein extraction and western
blotting
Protein extraction and western blotting were
performed as previously described (10). The ME membranes of the OME and dexa
group animals were microdissected and rapidly immersed and
homogenized in cold buffer [50 mM Tris-HCl, 0.1% sodium dodecyl
sulfate (SDS), 1.0 mM EDTA, 150 mM sodium chloride, 1% Triton
X-100, 1% sodium deoxycholate and 1 mM phenylmethylsulfonyl
fluoride (PMSF)]. The homogenates were then centrifuged at 12,000 ×
g at 4°C for 5 min. The supernatants were transferred to new tubes
and normalized to a protein content of 5 mg/ml using the Bradford
assay; each sample thus prepared was treated with 10X sample buffer
and electrophoresed on 10% SDS-polyacrylamide gels. The proteins
were transferred to a polyvinyldifluoride membrane, which was
incubated first with an affinity-purified polyclonal antibody
against AQP1 (rabbit anti-AQP1 affinity-purified polyclonal
antibody, 1 mg/ml; species reactivity, rat; Abcam) and then with
horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The resulting bands were
visualized using an enhanced chemiluminescence (ECL) system
(Amersham Pharmacia Biotech, Little Chalfont, UK). The labeling
density was quantified using ImageQuant software (LabWorks,
Portland, OR, USA). The optical densities of the bands from the two
groups relative to the densities of the β-actin bands from the same
samples were calculated to represent the relative protein
abundance.
Statistical analysis
Association analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The protein
expression of AQP1 was compared between the two groups using the
unpaired Student’s t-test with equal variance. Data are expressed
as the mean ± standard error. P<0.05 was considered to indicate
a statistically significant difference.
Results
Animals
Of the 26 guinea pigs, one died due to systemic
failure on the fifth postoperative day and another was observed to
have purulent otorrhea in the left ear on the eighth postoperative
day. These animals were excluded from further analysis. Of the 24
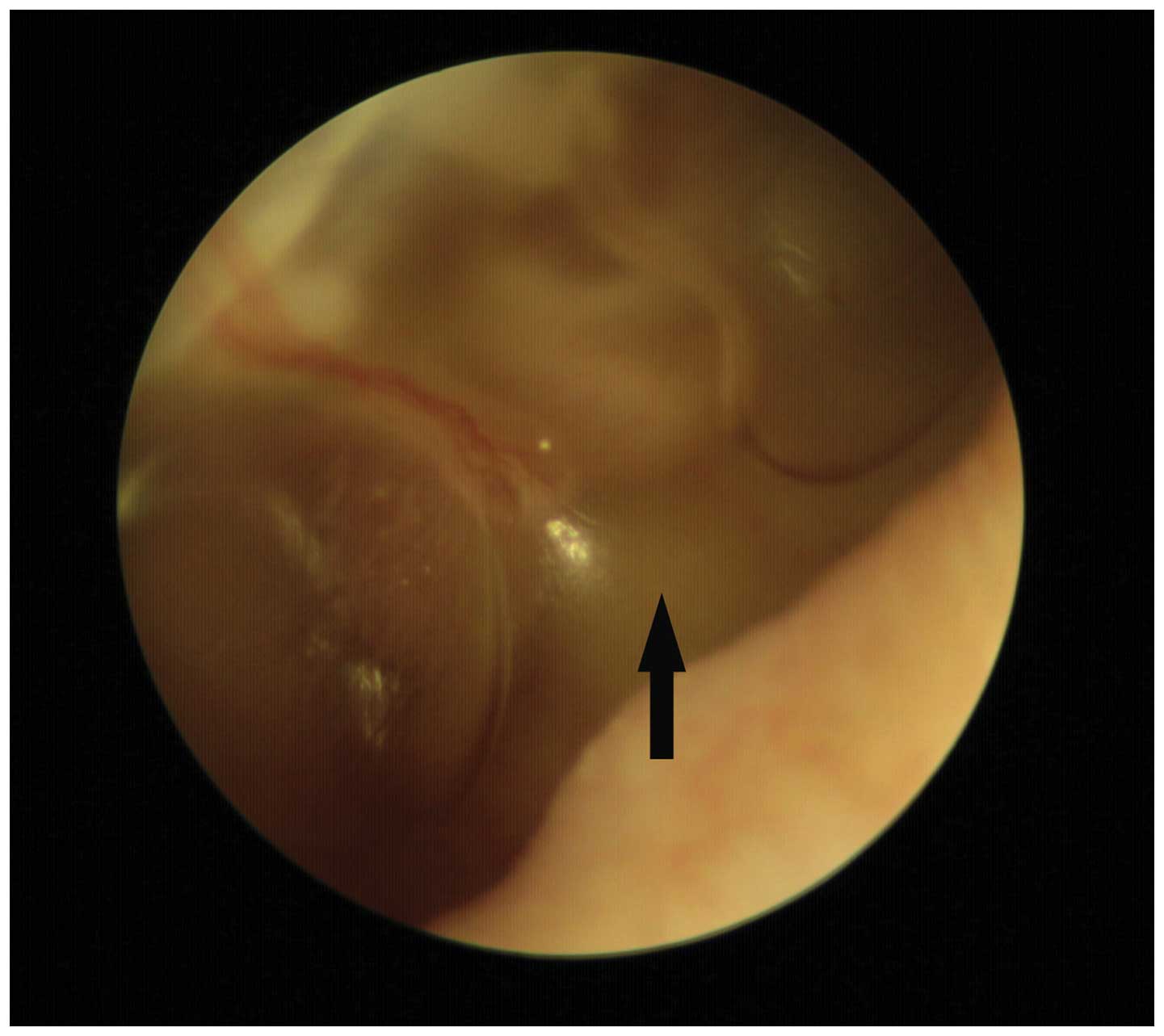
remaining animals, 22 exhibited effusion 3–7 days after surgery, as
indicated by visualization of an air-fluid interface or air bubbles
through the tympanic membrane (Fig.
1). None of the control ears exhibited evidence of disease by
otomicroscopic examination.
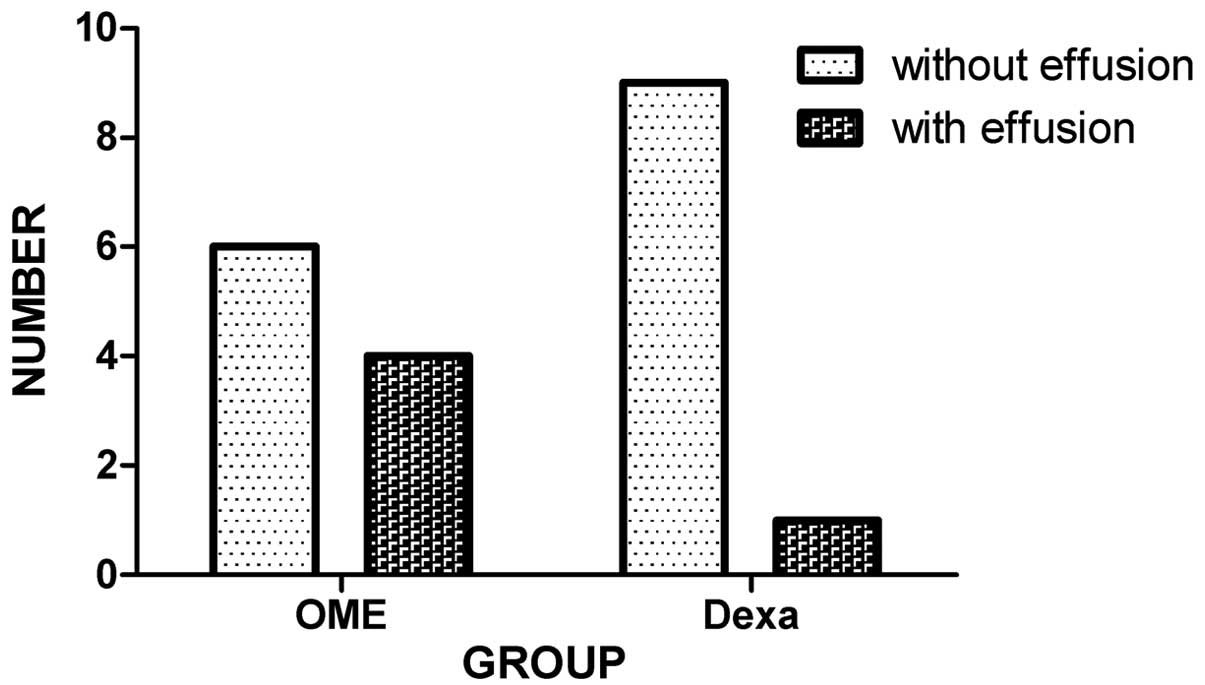
As shown in Fig. 2,
6 (60%) animals in the OME group and 9 (90%) in the dexa group
presented no sign of effusion within the ME cavity on postoperative
day 14. These observations were confirmed by microscopic
examination of the open bullae.
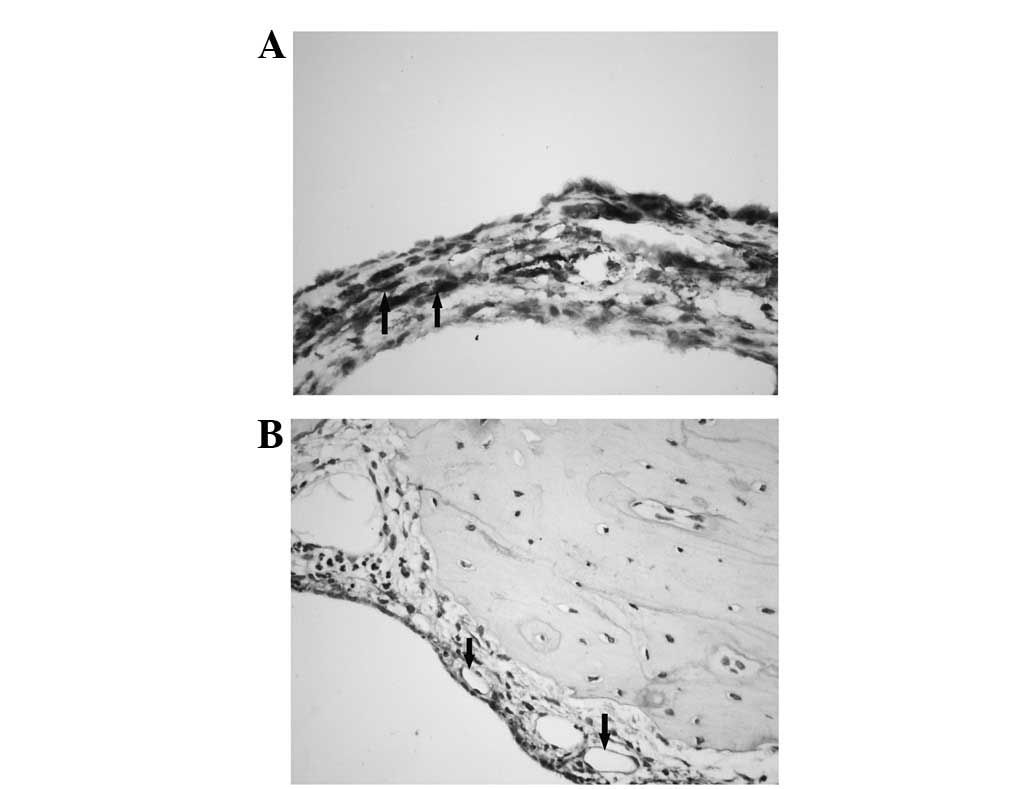
Immunohistochemistry
Immunohistochemistry was used to determine the
cellular distribution of AQP1 in the MEs of guinea pigs with OME.
AQP1 expression was observed in the subepithelial fibroblasts and
capillary endothelial cells; however, it was not identified in the
ciliated and secretory cells within the ME (Fig. 3).
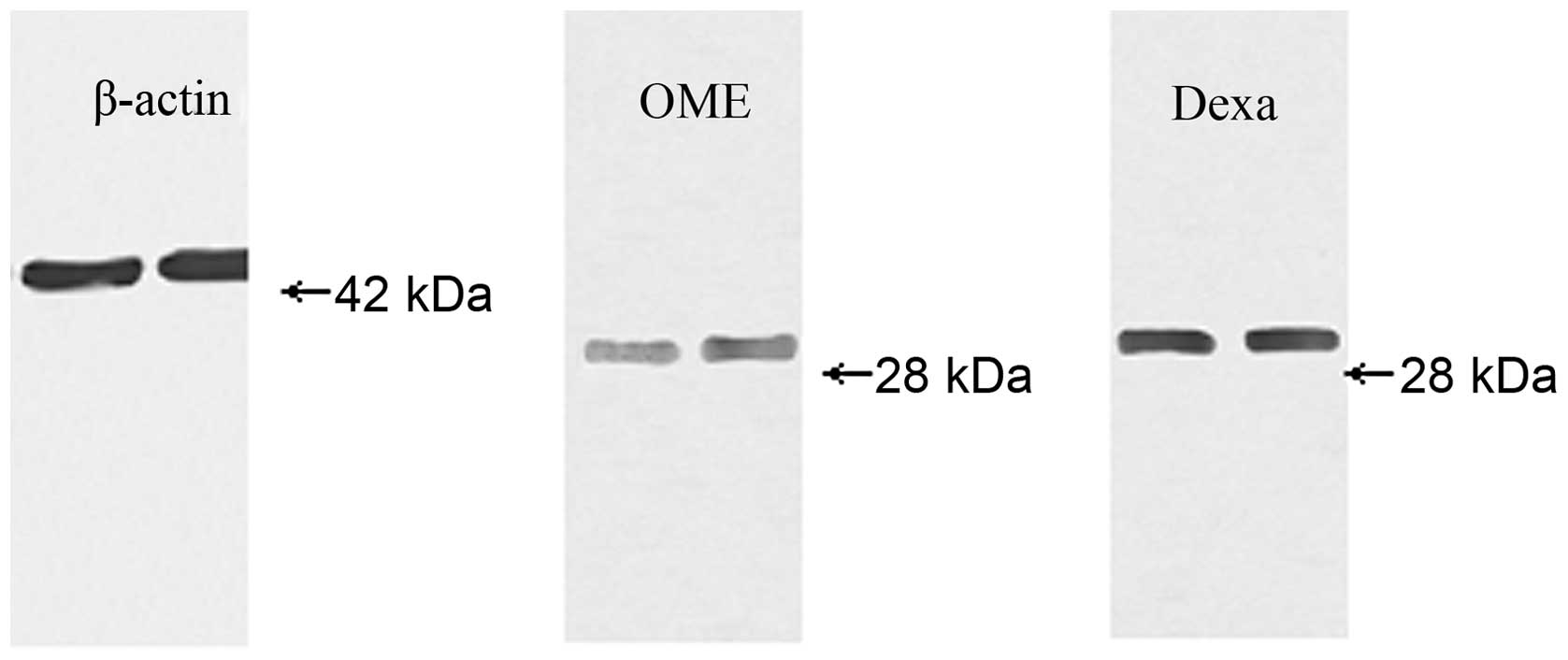
Western blot analysis
AQP1 was detected as a 28-kDa protein in the ME
mucosa of the animals from the OME and dexa groups (Fig. 4). The level of AQP1 protein was
markedly higher in the dexa group than in the OME group (t=2.733,
P<0.05; Table I).
 | Table IExpression of AQP1 protein in the two
groups. |
Table I
Expression of AQP1 protein in the two
groups.
| Group | N | Protein amount |
|---|
| OME | 10 | 0.2435±0.04422 |
| Dexa | 10 |
0.4145±0.04427a |
Discussion
The etiology of OME is multifactorial; however,
dysfunction of the ET is one of the factors essential to the
formation of ME effusion (14).
Moreover, inflammation of the ME is often self-limiting and ∼90% of
acute episodes (either symptomatic or asymptomatic) resolve within
3 months of presentation with or without medical treatment
(15). To date, multiple animal
models of OME induced via ET obstruction have been established and
used to explore the pathogenesis and treatment of this condition.
In the present study, we introduce a novel OME model with
reversible ET obstruction obtained via an improved surgical
procedure. We blocked the ET with polyvinyl acetal material rather
than argent nitrate solution, which produces permanent obstruction
(10). As a result, the effusion
formed in the ME may be gradually absorbed or pumped out within 2–3
weeks, which is similar to the spontaneous resolution of the
majority of cases of OME.
AQP1 is not only the first-identified subtype of the
AQP family, but also the most extensively distributed and
important. In the plasma membrane, AQP1 molecules form
homotetramers, with each 28-kD subunit containing an independent
water pore. The atomic-level structure of AQP1 explains its
selectivity for water and its ability to facilitate the rapid
transport of water across membranes (16). AQP1 is distributed mainly in the
endothelial layer of the vasculature throughout the body and helps
to increase vasopermeability by facilitating transcellular water
movement in the direction of the osmotic gradient (17).
In the present study, we revealed that AQP1 is
localized to the cell surface of capillary endothelial cells and
fibroblasts in the ME of guinea pigs. Previous findings support the
hypothesis that AQP1 is involved in maintaining the ion gradient in
the subepithelial milieu of the guinea pig ME (10). Furthermore, we identified that dexa
treatment affects AQP1 in a guinea pig model of OME. We
administered dexa at a dose of 5 mg/kg since the guinea pig is
relatively resistant to glucocorticoids compared with other
species, including rats and mice (18). Immunoblotting revealed that dexa
treatment increases the expression of AQP1 in the OME-affected ME.
In addition, the outcome on postoperative day 14 tended to be
better for the dexa group than for the OME group.
Patients suffering from diseases involving edema,
including OME, have been successfully treated by the local and oral
administration of glucocorticoids. Increasing evidence indicates
that in addition to increasing Na+/K+-ATPase
activity (19), glucocorticoids
may also regulate water transport in various organs by altering the
expression of AQP1. Fukushima et al reported that
intratympanic injection of steroids upregulates AQP1 mRNA
expression in the rat cochlea in a dose-dependent manner (13). Stoenoiu et al demonstrated
that dexa injection induces the expression of AQP1 in the capillary
endothelium of the peritoneal membrane and that a glucocorticoid
receptor antagonist inhibits this effect (12). A previous study demonstrated that
dexa alleviates pulmonary edema in mice by upregulating the
expression of AQP1 in the lung (20). These studies are consistent with
our data and indicate that AQP1 is directly involved in these
diseases and relieves their symptoms by facilitating water
transport. The effect of glucocorticoids on AQP1 may be due to the
presence of the multiple glucocorticoid response elements that have
been identified in the promoters of mammalian AQP1 genes (21,22).
This regulatory mechanism warrants further investigation.
One limitation of the present study is that only a
single dose and duration of dexa treatment was used. Further
studies with a greater number of groups and analysis of the dose-
and time-dependence of the response should be performed to
elucidate the effect of dexa on AQP1 more fully.
In conclusion, corticosteroids induce the expression
of AQP1 protein in the mucosa of the ME. Our data emphasize the
significance of AQP1 in the pathophysiology of OME and suggest that
glucocorticoids regulate water homeostasis via an AQP1 pathway,
which may be a new target for drug therapy.
Acknowledgements
This work was supported by the Medical
Science Program of Nanjing Municipality (No: QYK09171), the
Foundation of Medical Key Talents of Jiangsu Provincial Health
Bureau (No: RC2007010) and Medical Youth Talent Cultivation Project
of Nanjing Municipality ([2011] No. 42).
References
|
1.
|
Morris LM, DeGagne JM, Kempton JB, Hausman
F and Trune DR: Mouse middle ear ion homeostasis channels and
intercellular junctions. PLoS One. 7:e390042012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Topcuoglu N, Keskin F, Ciftci S, Paltura
C, Kulekci M, Ustek D and Kulekci G: Relationship between oral
anaerobic bacteria and otitis media with effusion. Int J Med Sci.
9:256–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bluestone CD and Klein JO: Physiology,
pathophysiology and pathogenesis. Otitis Media in Infants and
Children. 4th Edition. BC Decker; pp. 41–42. 2007
|
|
4.
|
Post JC: Direct evidence of bacterial
biofilms in otitis media. Laryngoscope. 111:2083–2094. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Darrow DH, Dash N and Derkay CS: Otitis
media: concepts and controversies. Curr Opin Otolaryngol Head Neck
Surg. 11:416–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Takata K, Matsuzaki T and Tajika Y:
Aquaporins: water channel proteins of the cell membrane. Prog
Histochem Cytochem. 39:1–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Altuntas A, Yilmaz MD, Aktepe F, Kahveci
OK, Derekoy S, Dilek H and Serteser M: Expression and distribution
of aquaporin-1 in nasal polyps: does it have any significance in
edema formation? Am J Rhinol. 20:128–131. 2006.PubMed/NCBI
|
|
8.
|
Takeda T and Taguchi D: Aquaporins as
potential drug targets for Meniere’s disease and its related
diseases. Handb Exp Pharmacol. 171–184. 2009.PubMed/NCBI
|
|
9.
|
Hasegawa H, Ma T, Skach W, Matthay MA and
Verkman AS: Molecular cloning of a mercurial-insensitive water
channel expressed in selected water-transporting tissues. J Biol
Chem. 269:5497–5500. 1994.PubMed/NCBI
|
|
10.
|
Zhang Q, Liu C, Gao X, Hu Y, Guo W, Sun J
and Li X: Expression pattern of aquaporin 1 in the middle ear of
the guinea pig with secretory otitis media. ORL J Otorhinolaryngol
Relat Spec. 71:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu H, Hooper SB, Armugam A, Dawson N,
Ferraro T, Jeyaseelan K, Thiel A, Koukoulas I and Wintour EM:
Aquaporin gene expression and regulation in the ovine fetal lung. J
Physiol. 551:503–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Stoenoiu MS, Ni J, Verkaeren C, Debaix H,
Jonas JC, Lameire N, Verbavatz JM and Devuyst O: Corticosteroids
induce expression of aquaporin-1 and increase transcellular water
transport in rat peritoneum. J Am Soc Nephrol. 14:555–565. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fukushima M, Kitahara T, Uno Y, Fuse Y,
Doi K and Kubo T: Effects of intratympanic injection of steroids on
changes in the rat inner ear aquaporin expression. Acta
Otolaryngol. 122:600–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Honjo I, Okazaki N, Nozoe T, Ushiro K and
Kumazawa T: Experimental study of the pumping function of the
Eustachian tube. Acta Otolaryngol (Stockh). 91:85–89. 1981.
View Article : Google Scholar
|
|
15.
|
Hebda PA, Piltcher OB, Swarts JD, Alper
CM, Zeevi A and Doyle WJ: Cytokine profiles in a rat model of
otitis media with effusion caused by eustachian tube obstruction
with and without Streptococcus pneumoniae infection.
Laryngoscope. 112:1657–1662. 2002. View Article : Google Scholar
|
|
16.
|
Kozono D, Yasui M, King LS and Agre P:
Aquaporin water channels: Atomic structure molecular dynamics meet
clinical medicine. J Clin Invest. 109:1395–1399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Verkman AS: Aquaporin water channels and
endothelial cell function. J Anat. 200:617–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nagata T, Nabe T, Fujii M, Mizutani N and
Kohno S: Effects of multiple dexamethasone treatments on
aggravation of allergic conjunctivitis associated with mast cell
hyperplasia. Biol Pharm Bull. 31:464–468. 2008. View Article : Google Scholar
|
|
19.
|
Kim CR, Sadowska GB, Newton SA, Merino M,
Petersson KH, Padbury JF and Stonestreet BS:
Na+,K+-ATPase activity and subunit protein
expression: ontogeny and effects of exogenous and endogenous
steroids on the cerebral cortex and renal cortex of sheep. Reprod
Sci. 18:359–373. 2011.
|
|
20.
|
Dong C, Wang G, Li B, Xiao K, Ma Z, Huang
H, Wang X and Bai C: Anti-asthmatic agents alleviate pulmonary
edema by upregulating AQP1 and AQP5 expression in the lungs of mice
with OVA-induced asthma. Respir Physiol Neurobiol. 181:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
de Arteaga J, Ledesma F, Garay G,
Chiurchiu C, de la Fuente J, Douthat W, Massari P, Terryn S and
Devuyst O: High-dose steroid treatment increases free water
transport in peritoneal dialysis patients. Nephrol Dial Transplant.
26:4142–4145. 2011.PubMed/NCBI
|
|
22.
|
Moon C, King LS and Agre P: Aqp1
expression in erythroleukemia cells: genetic regulation of
glucocorticoid and chemical induction. Am J Physiol.
273:C1562–C1570. 1997.PubMed/NCBI
|