Introduction
Obesity is a pandemic medical and social problem
that is associated with several adverse health outcomes, including
type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia,
cardiovascular disease and cancer (1,2), all
of which result in increased mortality. Notably, 20–30% of the
adult obese population, referred to as metabolically healthy but
obese (MHO) individuals, have been identified who, despite having
excessive adiposity, are relatively insulin sensitive and have a
favorable cardiovascular risk profile (3,4).
Several studies have examined characteristics associated with the
protective profile of MHO individuals (5,6).
Brochu et al reported that an early age of obesity onset and
low amounts of visceral adipose tissue had protective effects on
MHO postmenopausal women (5).
Karelis et al reported that a lower inflammation state, as
attested by low C-reactive protein (CRP) levels, may play a role in
the protective profile of MHO postmenopausal women (6). Dai et al reported a positive
association between plasma epinephrine level and insulin
sensitivity in MHO individuals (7). In multiple regression analysis, CRP,
epinephrine, triglycerides and the lean body mass index were
identified as independent predictors of glucose disposal,
collectively explaining 39.4% of the variance in insulin
sensitivity.
Angiopoietin-like proteins (ANGPTLs), which are
structurally similar to angiopoietins, are characterized by a
coiled-coil domain in the N-terminus and a fibrinogen-like domain
in the C-terminus (8). ANGPTL2, a
member of the ANGPTL family, has been shown to be expressed
abundantly in adipose tissues and is reportedly a key mediator
linking obesity to adipose tissue inflammation and systemic insulin
resistance in mice and humans (9,10).
However, the association of serum ANGPTL2 levels with MHO phenotype
has not yet been investigated. In the present study, we explored
the association of serum ANGPTL2 levels with insulin sensitivity
and serum epinephrine levels in non-diabetic obese postmenopausal
women, and investigated the effects of epinephrine on ANGPTL2
expression in adipocytes in vitro.
Materials and methods
Subjects
A total of 100 non-diabetic obese postmenopausal
women aged between 50 and 76 years old were enrolled in this study
using the following criteria: i) body mass index (BMI) >27
kg/m2; ii) cessation of menstruation for >1 year and
a follicle-stimulating hormone level of ≥30 U/l; iii) sedentary
(<2 h of structured exercise per week); iv) nonsmoker; v) low to
moderate alcohol consumer (fewer than two drinks per day); vi) free
of known inflammatory disease; and vii) no use of hormone
replacement therapy. On physical examination or biological testing,
all participants had no history or evidence of the following: i)
cardiovascular disease, peripheral vascular disease or stroke; ii)
diabetes 2-h plasma glucose <11.0 mmol/l after a 75-g oral
glucose tolerance test; iii) orthopedic limitations; iv) body
weight fluctuation within 2 kg in the last 6 months; v) thyroid or
pituitary disease; vi) infection according to medical questionnaire
examination and complete blood count; and vii) medication that may
affect cardiovascular function and/or metabolism. Informed consent
was obtained from all subjects prior to the start of the study.
After a 4-week period of weight stabilization, patients underwent a
3-hour hyperinsulinemic-euglycemic (HE) clamp. A blood draw was
performed for determination of a fasting lipid profile and analyses
of insulin and glucose. Body composition was assessed by
dual-energy X-ray absorptiometry a few days after the HE clamp.
This study was approved by the Ethics Committee of Xiangya
Hospital, Changsha, China.
Identification of MHO and at-risk
subjects
As previously described by Karelis et
al(6), we identified MHO and
at-risk subjects by dividing the entire cohort of 100 patients into
quartiles based on glucose disposal rates (M values/FFM). Glucose
disposal (M(clamp)) was calculated as the mean rate of
glucose infusion measured during the last 30 minutes of the clamp
(steady-state) and is expressed as milligrams per minute per
kilogram body weight or as milligrams per minute per kilogram
fat-free mass (FFM). Women with M/FFM values in the upper quartile
(M≥12.84; n=25) were classified as having high insulin sensitivity
and placed in the MHO group, whereas women with M/FFM values in the
lower quartile (M≤9.05; n=25) were classified as low insulin
sensitivity and categorized as at-risk subjects. The at-risk group
was defined as a group that presents metabolic abnormalities (i.e.
insulin resistance and dyslipidemia), which may be associated with
an increased risk of T2DM and/or cardiovascular disease.
Laboratory analysis
Prior to the HE clamp, blood samples were drawn from
subjects who had been resting quietly for 30 min in a recumbent
position following the insertion of a venous catheter. The subjects
refrained from eating, using tobacco, or drinking coffee or tea for
at least 4 h prior to veni-puncture. The procedure room was kept
quiet and comfortable at a temperature of 23–24° C. Serum
high-sensitivity CRP (hsCRP) and β-1 anti-trypsin were assessed
with ELISA kits from antibodies-online.com (Atlanta, GA, USA) and Bethyl
Laboratories, Inc. (Montgomery, TX, USA), respectively. Basal serum
epinephrine, norepinephrine and dopamine levels were assessed with
a 3-CAT RIA kit from Rocky Mountain Diagnostics, Inc. (Colorado
Springs, CO, USA).
Cell line and reagents
The 3T3-L1 cell line was purchased from the American
Type Culture Collection (Manassas, VA, USA). Anti-ANGPTL2
(sc-107143) antibody and anti-β-actin (sc-130656) antibody were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). All secondary antibodies were purchased from Jackson
ImmunoResearch Laboratories (West Grove, PA, USA). Epinephrine
bitartrate, phentolamine, propranolol, protein kinase A inhibitor
fragment 6–22 amide (PKAI), LY294002 and all chemicals of reagent
grade were purchased from Sigma (St. Louis, MO, USA).
Cell culture and treatment
3T3-L1 cells were cultured in DMEM with 10% FCS, 10
U/ml penicillin, 10 μg/ml streptomycin and 0.5 μg/ml
amphotericin B for 10 days in 5% CO2 at 37°C. The cells
were differentiated into mature adipocytes in medium supplemented
with insulin (1.7 M), IBMX (1 μM) and dexamethasone (25 pM)
for 2 days. After culture in medium with insulin (1.7 μM)
for an additional 6 days, the adipocytes were used for experiments
within 7–11 days. At the time of the experiments >90% of the
cells had accumulated lipid droplets. 3T3-L1 cells were treated
with epinephrine (10, 30, or 50 nM) in the presence or absence of
phentolamine (10 μM), propranolol (0.3 μM), LY294002
(50 μM) or PKAI (1 mM) for 24 h.
Western blot analysis
Protein was extracted with lysis buffer containing
150 mM NaCl, 2% Triton, 0.1% SDS, 50 mM Tris pH 8.0 and 10%
protease inhibitor cocktail (Sigma) and stored at −20° C. Equal
amounts of protein (25 μg) for each sample were loaded into
pre-cast 7.5% Mini Protean TGX gels (Bio-Rad, Hercules, CA, USA)
and separated by electrophoresis for 50 min at 200 V. The separated
proteins were transferred to a PVDF transfer membrane (Amersham
Biosciences/GE Healthcare, Piscataway, NJ, USA) for 55 min at 100
V. The membranes were incubated for 1 h with a 1/500 dilution of
anti-ANGPTL2 and 1/1000 dilution of anti-β-actin antibody, and then
washed and revealed using secondary antibodies with horseradish
peroxidase conjugate (1/5000, 1 h). Peroxidase was revealed with an
GE Healthcare ECL kit. Proteins were quantified before being loaded
onto the gel and equal loading of protein was verified by Ponceau
coloration.
Real-time quantitative reverse
transcription (RT)-PCR
RNA was prepared from 3T3-L1 adipocytes using TRIzol
reagent followed by purification with TURBO DNA-free system
(Ambion, Austin, TX, USA). The cDNAs were synthesized using
SuperScript II reverse transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA). Real-time quantitative PCR was performed on the
LightCycler thermal cycler system (Roche Diagnostics, Indianapolis,
IN, USA) using a SYBR-Green I kit (Roche) according to the
manufacturer’s instructions. The results were normalized against
the level of the housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) in the same sample. The primers used were as
follows: for ANGPTL2, 5′-GGAGGTTGGACTGTCATCCAGAG-3′ (forward) and
5′-GCCTTGGTTCGTCAGCCAGTA-3′ (reverse); for GAPDH,
5′-ATTCAACGGCACAGTCAAGG-3′ (forward) and 5′-TGTTAGTGGGGTCTCGCTCC-3′
(reverse). Each experiment was repeated twice in triplicate.
Statistical analysis
All continuous variable values were expressed as
mean ± standard deviation. Comparisons of means between two groups
were performed using a Student’s t-test upon test of normality and
equality of variances. Comparisons of means among multiple groups
were performed with one-way ANOVA followed by post hoc pairwise
comparisons using the least significant difference method. Discrete
variables were compared with Chi-square tests. A stepwise
multi-linear regression model determined which variables explained
unique variance in glucose disposal values. Statistical analyses
were performed with SPSS for Windows 13.0 (SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results
By design [MHO, upper quartile of insulin
sensitivity (IS) vs. at-risk, lower quartile of IS], the two groups
were significantly different in absolute and relative levels of
glucose disposal rates and insulin sensitivity
(IS(clamp); P<0.01). As shown in Table I, the MHO and the at-risk groups of
obese postmenopausal women were comparable in age, BMI, fat mass
index and waist circumference. While there were no significant
group differences in total cholesterol, LDL-cholesterol and resting
systolic and diastolic pressure, the MHO group showed higher levels
of epinephrine and HDL-cholesterol and lower levels of
triglycerides than the at-risk group (P<0.01). In addition, the
MHO group showed significantly lower levels of ANGPTL2, hsCRP and
α-1 anti-trypsin than the at-risk group (P<0.01).
 | Table ICharacteristics of MHO and at-risk
subjects. |
Table I
Characteristics of MHO and at-risk
subjects.
| Characteristics | MHO (n=25) | At-risk (n=25) |
|---|
| Physical
characteristics | | |
| Age (years) | 59.5±7.3 | 60.8±6.1 |
| Age group (years), n
(%) | | |
| <50 | 4 (16) | 4 (16) |
| 50–59 | 7 (28) | 9 (36) |
| 60–69 | 10 (40) | 9 (36) |
| ≥70 | 4 (16) | 3 (12) |
| BMI
(kg/m2) | 33.1±4.5 | 35.2±3.9 |
| Fat mass index
(kg/m2) | 15.9±3.6 | 15.7±3.1 |
| Lean body mass index
(kg/m2) | 16.2±2.3a | 19.3±2.6 |
| Waist circumference
(cm) | 97.2±9.3 | 101.8±9.5 |
| Metabolic
characteristics | | |
| Total cholesterol
(mmol/l) | 5.9±1.1 | 5.7±1.4 |
| LDL-cholesterol
(mmol/l) | 3.8±0.9 | 3.9±0.7 |
| HDL-cholesterol
(mmol/l) | 1.9±0.3a | 1.4±0.4 |
| Triglycerides
(mmol/l) | 1.3±0.7a | 2.5±1.3 |
| Systolic blood
pressure (mmHg) | 121.3±18.5 | 120.0±16.5 |
| Diastolic blood
pressure (mmHg) | 79.7±9.2 | 80.2±8.9 |
| Insulin sensitivity
index | | |
| Fasting glucose
(mmol/l) | 4.6±1.3 | 5.4±0.9 |
| Fasting insulin
(μU/ml) | 10.9±4.1a | 19.8±6.1 |
| HOMA-IR | 2.4±1.2a | 4.4±1.7 |
| IS (clamp) | 304.3±75.9a | 166.5±47.0 |
| M (clamp)
(mg/min/kg) | 8.6±1.3a | 4.3±0.9 |
| M/FFM (clamp)
(mg/min/kg FFM) | 15.9±2.7a | 7.3±1.5 |
| Inflammation
markers | | |
| hsCRP (mg/l) | 2.2±2.3a | 5.4±4.7 |
| α-1 anti-trypsin
(g/l) | 1.5±0.2a | 1.9±0.3 |
| ANGPTL2
(ng/ml) | 2.9±0.8a | 4.2±1.3 |
| Serum
catecholamines | | |
| Epinephrine
(pg/ml) | 81±24a | 32±19 |
| Norepinephrine
(pg/ml) | 335±42 | 319±30 |
| Dopamine
(pg/ml) | 56±23 | 60±32 |
Statistical analyses were performed for the entire
cohort (n=100) of non-diabetic obese postmenopausal women for
stepwise multi-linear regression analysis and correlation analyses.
As shown in Table II, Pearsons’
correlation analyses showed that the serum ANGPTL level was
negatively correlated with the glucose disposal rate
[M(clamp) and M/FFM(clamp)], insulin
sensitivity [IS(clamp)], and the serum epinephrine
level. No statistically significant correlation was noted between
the serum ANGPTL2 level and the serum hsCRP or α-1 anti-trypsin
level.
 | Table IICorrelation of plasma epinephrine
level with glucose disposal rate and blood lipid and inflammation
marker levels. |
Table II
Correlation of plasma epinephrine
level with glucose disposal rate and blood lipid and inflammation
marker levels.
| Variable | M(clamp)
(mg/min/kg) |
M/FFM(clamp) (mg/min/kg
FFM) |
IS(clamp) | HDL cholesterol
(mmol/l) | Triglycerides
(mmol/l) | hsCRP (mg/l) | α-1 Anti-trypsin
(g/l) | Epinephrine
(pg/ml) |
|---|
| ANGPTL2
(ng/ml) | r=−0.25 | r=−0.27 | r=−0.23 | r=−0.21 | r=0.22 | r=0.18 | r=0.17 | r=−0.62 |
| P=0.015a | P=0.008a | P=0.021a | P=0.028a | P=0.024a | P=0.077 | P=0.095 | P<0.001a |
Multivariate regression analysis showed that among
all variables listed in Table I,
with the exception of the serum epinephrine level, hsCRP, ANGPTL2,
triglycerides and lean body mass index were independent predictors
of glucose disposal, collectively explaining 41.2% of the variance
(P<0.05; Table IIIA). However,
following additional adjustment for the serum epinephrine level,
ANGPTL2 was no longer an independent predictor of glucose disposal
(Table IIIB), suggesting that the
serum epinephrine level accounted for the variances caused by the
serum ANGPTL2 level in glucose disposal.
 | Table IIIMultivariate regression analysis of
independent predictors of glucose disposal in obese postmenopausal
women. |
Table III
Multivariate regression analysis of
independent predictors of glucose disposal in obese postmenopausal
women.
| A, Without
adjustment for plasma epinephrine |
|
| Variable | Partial
r2 | Total
r2 | β Coefficient | P-Value |
|
| Glucose disposal
(mg/min/kg) | | | | |
| hsCRP | 0.185 | 0.185 | −0.267 | 0.011 |
| ANGPTL2 | 0.149 | 0.334 | −0.243 | 0.015 |
|
Triglycerides | 0.048 | 0.382 | −0.216 | 0.016 |
| Lean body mass
index | 0.030 | 0.412 | −0.202 | 0.040 |
|
| B, With adjustment
for plasma epinephrine |
|
| Variable | Partial
r2 | Total
r2 | β Coefficient | P-Value |
|
| Glucose disposal
(mg/min/kg) | | | | |
| HsCRP | 0.176 | 0.176 | −0.262 | 0.017 |
| Epinephrine | 0.172 | 0.348 | 0.259 | 0.010 |
|
Triglycerides | 0.037 | 0.385 | −0.183 | 0.021 |
| Lean body mass
index | 0.020 | 0.405 | −0.174 | 0.046 |
Based on the in vivo data, we hypothesized
that there was a causal relationship between serum epinephrine and
ANGPTL2 levels. As ANGPTL2 is primarily secreted by adipose tissue
(9), we further explored the
effect of epinephrine on ANGPTL2 expression in 3T3-L1 adipocyte
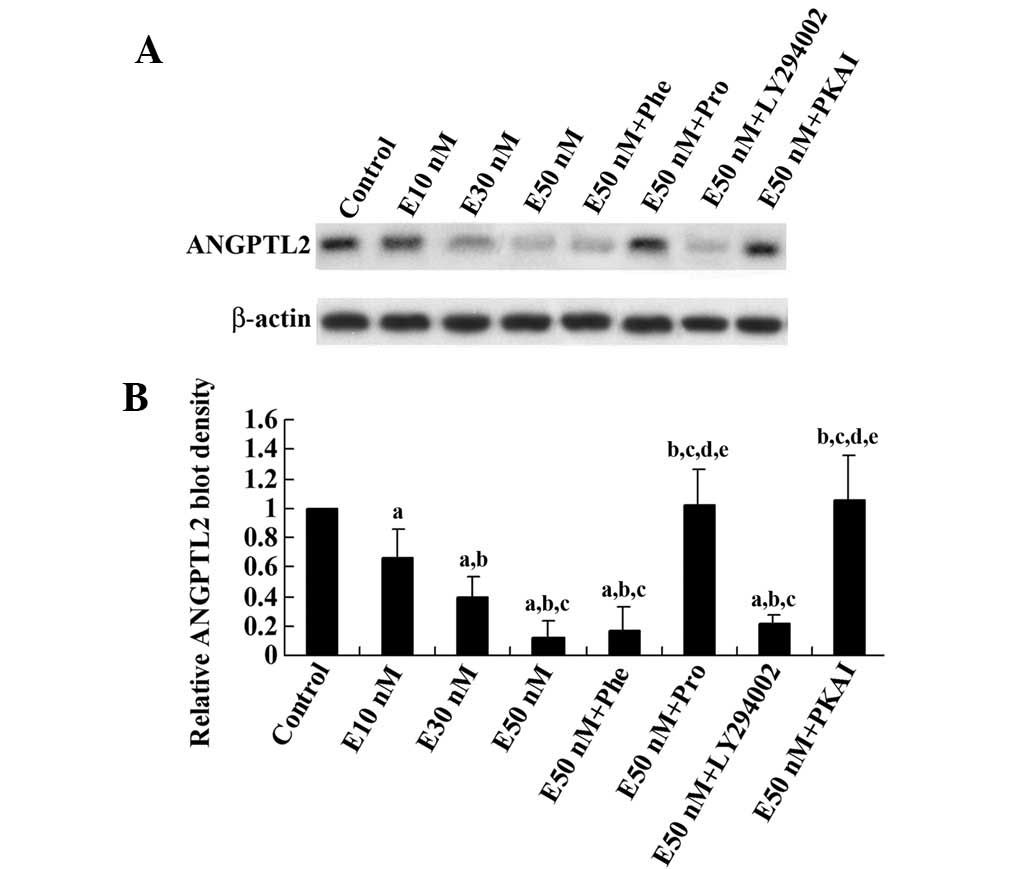
cells in vitro. As shown in Fig. 1, epinephrine reduced the ANGPTL2
protein level in differentiated 3T3-L1 adipocytes in a
concentration-dependent manner. This effect was eliminated by the
β-adrenoceptor blocker propranolol and PKAI, but not by the
β-adrenoceptor blocker phentolamine or the phosphatidylinositol
3-kinase (PI3K) inhibitor LY294002. Real-time RT-PCR showed that
the ANGPTL2 mRNA level was decreased by epinephrine in a
concentration-dependent manner, which was eliminated by propranolol
or PKAI, but not by phentolamine or LY294002 (Fig. 2). These results suggest that
epinephrine reduces ANGPTL expression at both the mRNA and protein
levels through β-adrenoceptors and the PKA signaling pathway.
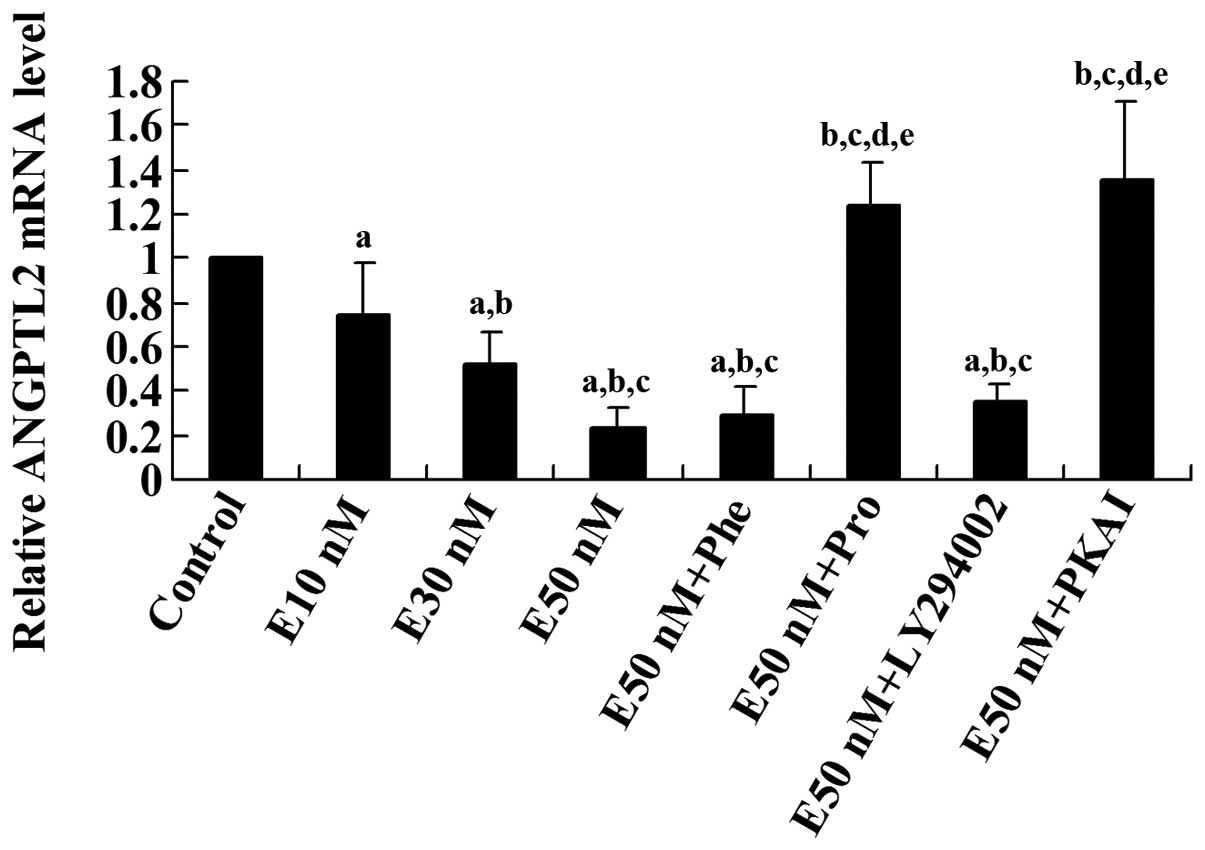 | Figure 2Real-time reverse transcription
(RT)-PCR analysis of the effect of epinephrine on the mRNA level of
angiopoietin-like protein 2 (ANGPTL2) in differentiated 3T3-L1
adipocytes. Differentiated 3T3-L1 cells were treated with
epinephrine in different concentrations (E10, 30, or 50 nM) for 24
h in the presence or absence of phentolamine (Phe, 10 μM),
propranolol (Pro, 0.3 μM), LY294002 (50 μM) or
protein kinase A inhibitor fragment 6–22 amide (PKAI, 1 mM) for 24
h. The ANGPTl2 mRNA level of treated cells was shown as fold change
to that of the untreated control cells (designated as 1).
aP<0.05, compared with untreated control cells;
bP<0.05, compared with E10 nM; cP<0.05,
compared with E30 nM; dP<0.05, compared with E50;
eP<0.05, compared with E50 nM+Phe (10 μM). |
Discussion
MHO individuals are insulin sensitive, normotensive
and have normal lipid profiles, despite having excessive adiposity
(11). Several studies have
reported the association of metabolic and inflammatory
characteristics with the protective profile of the MHO individual
(5,9,12).
ANGPTL2 is a key adipocyte-derived inflammatory mediator linking
obesity to systemic insulin resistance (9). Doi et al reported that
elevated serum ANGPTL2 levels were positively associated with the
development of T2DM in a general population, independent of other
risk factors including hsCRP levels (8). In agreement with the previous
reports, our study showed that the serum ANGPTL2 level was
negatively correlated with the glucose disposal rate and insulin
sensitivity, but not with the serum hsCRP and α-1 anti-trypsin
levels in the study subjects, with the MHO subjects displaying
significantly lower serum ANGPTL2 levels than the at-risk subjects
(P<0.05).
The serum epinephrine and ANGPTL2 levels showed a
strong negative correlation and multivariate regression analysis
suggested a causal relationship between the serum epinephrine and
ANGPTL2 levels. As ANGPTL2 is primarily secreted by adipose tissue
in the human body, in subsequent in vitro experiments, we
employed differentiated 3T3-L1 adipocytes as a cell model, which
has been used in a previous adipocyte study (9). To examine the effects of epinephrine
on ANGPTL2 expression, we treated 3T3-L1 adipocytes with low
concentrations of epinephrine in small increments (10, 30 and 50
nM) for 24 h. This was due to: i) the difference in serum
epinephrine levels between the MHO group and the at-risk group was
∼3-fold; and ii) the effects of circulating epinephrine on insulin
sensitivity was expected to be chronic in the human body.
Despite a similar potency for α- and
β-adrenoceptors, epinephrine stimulates β-receptors (particularly
β2-receptors) to a greater extent than norepinephrine (13). In agreement with a previous study,
our in vitro experiments in the present study showed that a
β-, but not α-receptor blocker, completely eliminated the
inhibitory effects of epinephrine on ANGPTL2 expression in
adipocytes, suggesting that epinephrine increased ANGPTL2
expression via the β-receptors (13). Since serum ANGPTL2 leves links
obesity with systemic insulin resistance and is positively
associated with the development of T2DM (8), our results suggest that β-receptor
activation helps to maintain the metabolic profile of MHO and
prevent T2DM by decreasing serum ANGPTL2 levels. Although the acute
effect of pharmacological doses of epinephrine is to increase blood
glucose and diminish insulin sensitivity (13), the long-term effect of endogenous
epinephrine is reportedly protection against hyperglycemia and
insulin insensitivity in a hig-fat-diet-induced obesity mouse model
(14). Epinephrine is able to
stimulate intracellular AMP-activated protein kinase (AMPK) through
β-receptors, which may lead to improved insulin sensitivity
(15). However, the chronic use of
β-blockers decreases insulin sensitivity in humans (16). Thus, chronic stimulation of
β-receptors by an elevated serum level of epinephrine may increase
insulin sensitivity. Our study suggests that decreasing ANGTPL2
expression through the β-receptors is one of the mechanisms
underlying the protective effects of epinephrine on MHO subjects.
Further studies are required: i) to define which β-receptor
subtypes are involved in the effects of epinephrine on ANGPTL2
expression in adipocytes; and ii) to elaborate the underlying
molecular mechanisms.
There are several limitations to our in vivo
study. Firstly, our cohort consisted only of non-diabetic sedentary
obese postmenopausal women. Therefore, our findings are limited to
this population. Secondly, we used a cross-sectional approach,
which does not allow us to draw any causal connection among serum
ANGPTL2 and epinephrine levels and insulin sensitivity in MHO
subjects. However, our in vitro study provided evidence for
a causal relationship between serum ANGPTL2 and epinephrine levels,
which compensated for the deficiencies in the in vivo
study.
In conclusion, our in vivo findings show that
the serum ANGPTL2 level is negatively associated with insulin
sensitivity and the serum epinephrine level in non-diabetic obese
postmenopausal women, with MHO subjects displaying significantly
lower serum ANGPTL2 and higher serum epinephrine levels than
at-risk subjects. Our in vitro findings indicate that
epinephrine decreases ANGPTL expression at the mRNA and protein
levels via β-adrenoceptors and the PKA signaling pathway. This
study suggests that β-receptor activation helps to maintain the
metabolic profile of MHO and prevent T2DM by decreasing serum
ANGPTL2 levels.
References
|
1.
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mokdad AH, Ford ES, Bowman BA, Dietz WH,
Vinicor F, Bales VS and Marks JS: Prevalence of obesity, diabetes,
and obesity-related health risk factors, 2001. JAMA. 289:76–79.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ruderman NB, Schneider SH and Berchtold P:
The ‘metabolically-obese,’ normal-weight individual. Am J Clin
Nutr. 34:1617–1621. 1981.
|
|
4.
|
Succurro E, Marini MA, Frontoni S, et al:
Insulin secretion in metabolically obese, but normal weight, and in
metabolically healthy but obese individuals. Obesity (Silver
Spring). 16:1881–1886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Brochu M, Tchernof A, Dionne IJ, Sites CK,
Eltabbakh GH, Sims EA and Poehlman ET: What are the physical
characteristics associated with a normal metabolic profile despite
a high level of obesity in postmenopausal women? J Clin Endocrinol
Metab. 86:1020–1025. 2001.
|
|
6.
|
Karelis AD, Faraj M, Bastard JP, St-Pierre
DH, Brochu M, Prud’homme D and Rabasa-Lhoret R: The metabolically
healthy but obese individual presents a favorable inflammation
profile. J Clin Endocrinol Metab. 90:4145–4150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dai XP, Liu ZQ, Xu LY, Gong ZC, Huang Q,
Dong M and Huang X: Association of plasma epinephrine level with
insulin sensitivity in metabolically healthy but obese individuals.
Auton Neurosci. 167:66–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Doi Y, Ninomiya T, Hirakawa Y, et al:
Angiopoietin-like protein 2 and risk of type 2 diabetes in a
general Japanese population: the Hisayama study. Diabetes Care.
36:98–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tabata M, Kadomatsu T, Fukuhara S, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Okada T, Tsukano H, Endo M, et al:
Synoviocyte-derived angiopoietin-like protein 2 contributes to
synovial chronic inflammation in rheumatoid arthritis. Am J Pathol.
176:2309–2319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wildman RP, Muntner P, Reynolds K, McGinn
AP, Rajpathak S, Wylie-Rosett J and Sowers MR: The obese without
cardio-metabolic risk factor clustering and the normal weight with
cardiometabolic risk factor clustering: prevalence and correlates
of 2 phenotypes among the US population (NHANES 1999–2004). Arch
Intern Med. 168:1617–1624. 2008.PubMed/NCBI
|
|
12.
|
Romano M, Guagnano MT, Pacini G, et al:
Association of inflammation markers with impaired insulin
sensitivity and coagulative activation in obese healthy women. J
Clin Endocrinol Metab. 88:5321–5326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Westfall TC and Westfall DP:
Neurotransmission: The autonomic and somatic motor nervous systems.
Goodman and Gilman’s The Pharmacological Basis of Therapeutics.
Brunton LL: 11th edition. McGraw-Hill; London, UK: pp. 137–182.
2006
|
|
14.
|
Ziegler MG, Milic M, Sun P, et al:
Endogenous epinephrine protects against obesity induced insulin
resistance. Auton Neurosci. 162:32–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Steinberg GR and Jørgensen SB: The
AMP-activated protein kinase: role in regulation of skeletal muscle
metabolism and insulin sensitivity. Mini Rev Med Chem. 7:519–526.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sharma AM, Pischon T, Hardt S, Kunz I and
Luft FC: Hypothesis: Beta-adrenergic receptor blockers and weight
gain: A systematic analysis. Hypertension. 37:250–254. 2001.
View Article : Google Scholar : PubMed/NCBI
|