Introduction
Atherosclerosis (AS) is a common disease that is
seriously detrimental to human health. Therefore, its mechanism and
related treatment methods are areas of interest in medical
research. Urotensin II (UII) is the most potent vosoactive cyclic
peptide (1,2). By binding to its receptor, G
protein-coupled receptor 14 (GPR14), it exhibits biological,
hemodynamic and non-hemodynamic effects, as well as apparently
pathophysiologic effects on the formation and development of a
number of diseases. A large number of fundamental and clinical
studies have indicated that UII is closely related to the formation
and development of AS (3–5). Thus, the UII receptor antagonist,
urantide, may have potential clinical value in the treatment of
AS.
Urantide is a peptide similar to human UII and is
one of the most potent UII receptor antagonists. Its antagonistic
effect is 50 times stronger than that of other chemical compounds
(6,7). However, the effect of urantide on AS
remains unknown. The present study used a rat model of AS to
examine the expression of UII and its receptor GPR14 in rat aorta
pectoralis, aiming to determine the effect of urantide on the
expression of UII and GPR14 in atherosclerotic rats, thus providing
an experimental basis for the clinical prevention of AS.
Materials and methods
Reagents
Urantide was synthesized by Shanghai Huadatianyuan
Biology Technology Ltd., Co. (Shanghai, China). Fluvastatin (40
mg/box) was purchased from Beijing Nuohua Pharmacy Ltd., Co.
(Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM) and UII
were purchased from Gibco (Carlsbad, CA, USA); fetal bovine serum
(FBS) was purchased from Tianjin Jingyang Corporation (China);
α-smooth muscle actin (SMA) was purchased from Beijing Beaosen
Biology Technology Ltd., Co. (China); the UII polyclonal and GPR14
polyclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA); biotin-labeled IgG was purchased from
Wuhan Boster Biological Engineering Co., Ltd. (China); and S-ABC
chemical reagent and 3,3′-diaminobenzidine (DAB) developer kits
were purchased from Fuzhou Maixin Biotechnology Development Co.,
Ltd. (China)
Animals and modeling
A total of 160 healthy male Wistar rats (180–200 g
body weight) were provided by the Lab Animal Center of Jilin
University (license number: SCXK[JI]-2009-0004). The rats were
randomized into two groups: i) the normal group: 20 rats fed on
common forage; and ii) the model group: 140 rats fed on a high fat
diet and injected with 70 U/kg vitamin D3 (VD3) for three
continuous days. The ingredients of the high fat diet included
common forage, 3.5% cholesterol, 10% hog fat, 0.2%
propylthiouracil, 0.5% sodium cholate and 5% refined sugar. Four
weeks later, a hematoxylin and eosin (H&E) staining assay was
peformed to observe the morphological changes in the aorta
pectoralis in the AS model rats.
Following successful modeling, AS rats were randomly
divided into three groups: the model group (20 rats, control), the
fluvastatin group (20 rats) and the urantide group (20 rats,
divided into three subgroups according to the duration of treatment
of 3, 7 and 14 days). The rats in the normal and model groups were
intravenously injected with 30 μg/kg normal saline every
day. The rats in the fluvastatin group were injected with 5
μg/kg fluvastatin every day for 14 continuous days. The rats
in the urantide group were injected with 30 μg/kg urantide
once daily and the duration of treatment was 3, 7 and 14 days,
respectively. All animal experiments were approved by the Ethics
Committee of Jilin University College of Pharmaceutical Sciences
(Changchun, China).
Blood fat and calcium (Ca2+)
levels of AS rats
Blood samples were collected at the beginning of the
experiment, before the injection and at the end of the experiment.
Blood collection procedures were as follows: all rats were fasted
overnight. Following anesthesia with 0.3% pentobarbital sodium (30
mg/kg), the aorta pectoralis was dissected and arterial blood was
collected with a 5-ml injector. Blood serum was separated via 1,500
× g centrifugation for 15 min, allocated to Eppendorf tubes and
stored at −20°C.
An automatic biochemistry analyzer was used to
examine the levels of triglycerides (TG) in blood serum, as well as
total cholesterol (TC), high-density lipoprotein (HDL), low-density
lipoprotein (LDL) and Ca2+ levels. A hydroxyproline
(HYP) kit was used to analyze the concentration of HYP in the blood
serum and urine.
Gene and protein expression levels of UII
and GPR14 in the AS rat model
At the end of the experiment (16 weeks), the aorta
pectoralis (∼1 cm long) was sampled, placed into a sample bag and
immediately frozen in liquid nitrogen at −80°C for storage. Reverse
transcription-polymerase chain reaction (RT-PCR) and western
blotting were performed to determine the gene and protein
expression levels of UII and GPR14 in the AS rat model.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). Significant differences were examined using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Analysis of variance or
rank test were used as the statistical methods to analyze the
interclass difference and the least significant difference (LSD)
method was used for multiple comparisons of ad hoc test. P<0.05
was considered to indicate a statistically significant result.
Results
Textural structure of the aorta
pectoralis in the AS rat model
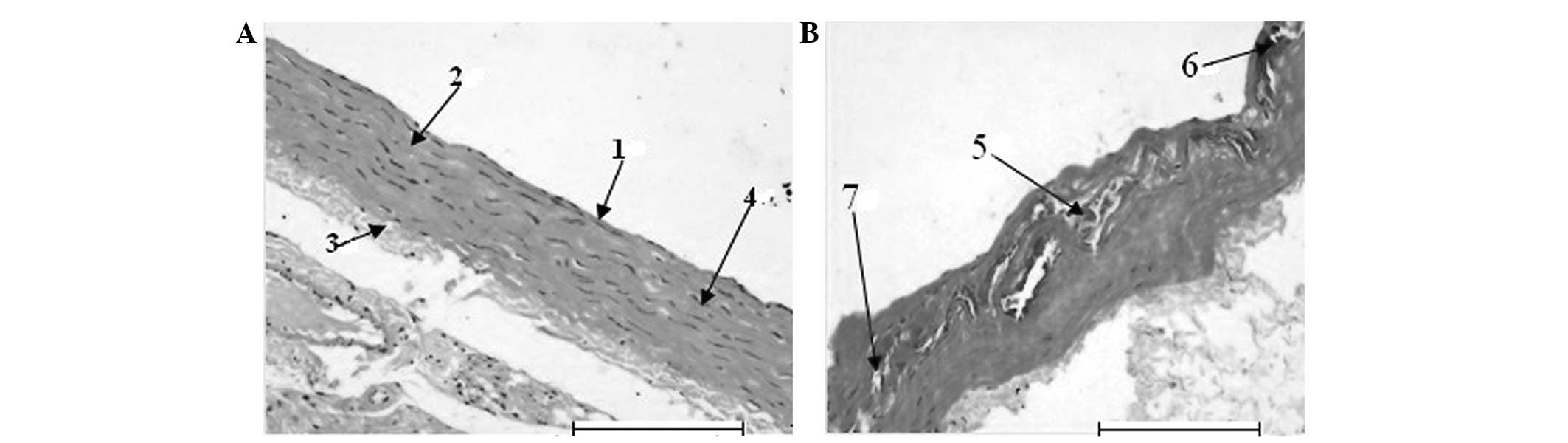
The H&E staining revealed that the blood vessel
endothelium in the normal group was integrated, the tunica media
had fusiform-shaped smooth muscle cells and the structure of
elastic fibers was clear and integrated (Fig. 1A). The tunica intima of the disease
region in the model group thickened significantly. The vascular
endothelium was not integrated. The tunica media presented apparent
hyperplasia, calcification and abundant foam-like accumulation of
smooth muscle cells, degeneration, breakage and disintegration of
elastic fibers and atrophia, which are typical pathological changes
of AS (Fig. 1B). All the results
demonstrated that the AS rat model was successfully created by
injection with VD3 and feeding with a high-fat diet.
Effect of urantide on blood fat and
Ca2+ levels in the AS rat model
At the end of the experiment (16 weeks), the animals
were sacrificed. Table I shows
that the Ca2+, TG, TC, HDL and LDL levels of blood serum
in the model group were increased significantly compared with those
in the normal group (P<0.01, respectively). However, the
Ca2+, TG, TC, HDL and LDL levels of blood serum in the
fluvastatin group were significantly reduced compared with those in
the model group (P<0.01, respectively). Indicators of blood
serum in the urantide groups decreased gradually in a
dose-dependent manner. Levels in the fluvastatin group were
significantly different compared with normal (P<0.01).
 | Table IEffect of urantide on lipid and
calcium concentrations of AS rats at the end of the 16-week
experiment (mmol/l). |
Table I
Effect of urantide on lipid and
calcium concentrations of AS rats at the end of the 16-week
experiment (mmol/l).
| Group | n | Ca2+ | TG | TC | HDL | LDL |
|---|
| Normal | 10 | 2.62±0.06 | 0.03±0.01 | 1.20±0.02 | 0.57±0.04 | 0.16±0.01 |
| Model | 10 | 3.82±0.01a | 1.74±0.08a | 17.61±0.08a | 2.19±0.05a | 16.05±0.15a |
| Fluvastatin | 10 |
3.12±0.01a,c |
0.74±0.05a,c |
12.45±0.02a,c |
1.27±0.00a,c |
11.95±0.06a,c |
| Urantide | | | | | | |
| 3 days | 10 |
3.57±0.00a,c |
1.04±0.00a,c |
17.03±0.01a,c |
1.98±0.00a,c |
15.16±0.01a,c |
| 7 days | 10 |
3.48±0.01a,c |
0.72±0.14a,b |
15.87±0.00a,c |
1.81±0.02a,c |
14.20±0.01a,c |
| 14 days | 10 |
3.43±0.39a,c |
0.43±0.04a,c |
12.83±0.06a,c |
1.48±0.06a,c |
10.16±0.05a,c |
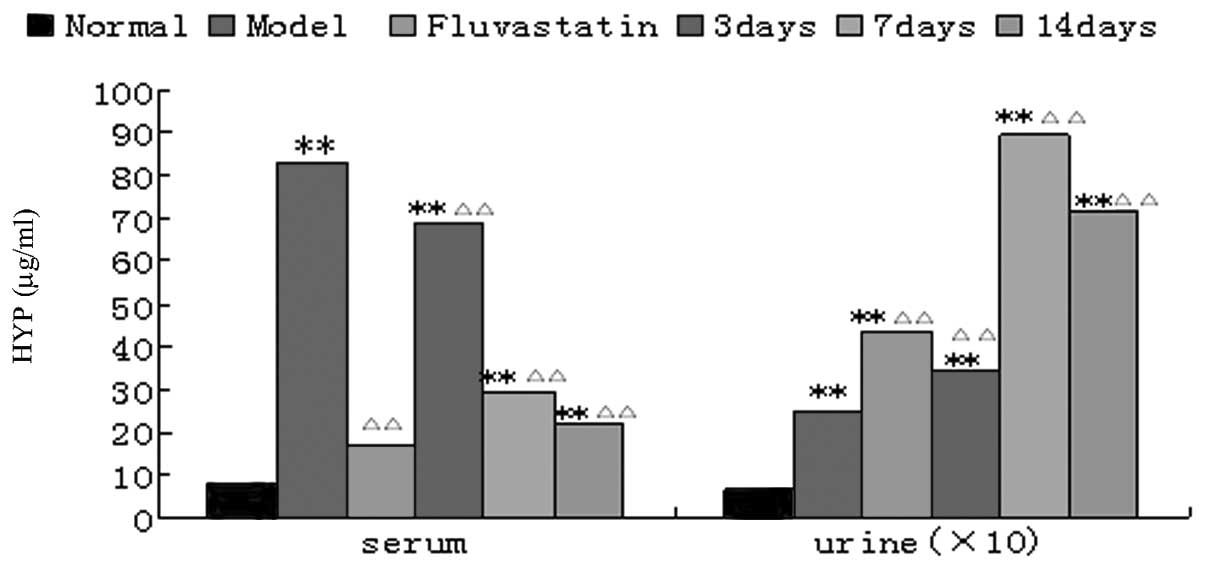
Effect of urantide on HYP levels in the
AS rat model
The concentrations of HYP in blood serum and urine
in the model group were significantly increased compared with those
in the normal group (P<0.01). The HYP level in blood serum in
the fluvastatin group was significantly reduced compared with that
in the model group (P<0.01). The HYP level in blood serum was
significantly lower than that in the model group after injecting
urantide for three days (P<0.01), was markedly lower after seven
days and even lower after 14 days, and approximate to the level in
the fluvastatin group. The urinary HYP levels in the urantide
groups and the fluvastatin group were significantly increased
(P<0.01) compared with the HYP level in the model group; in the
urantide group, the level peaked after 7 days of drug
administration (Fig. 2).
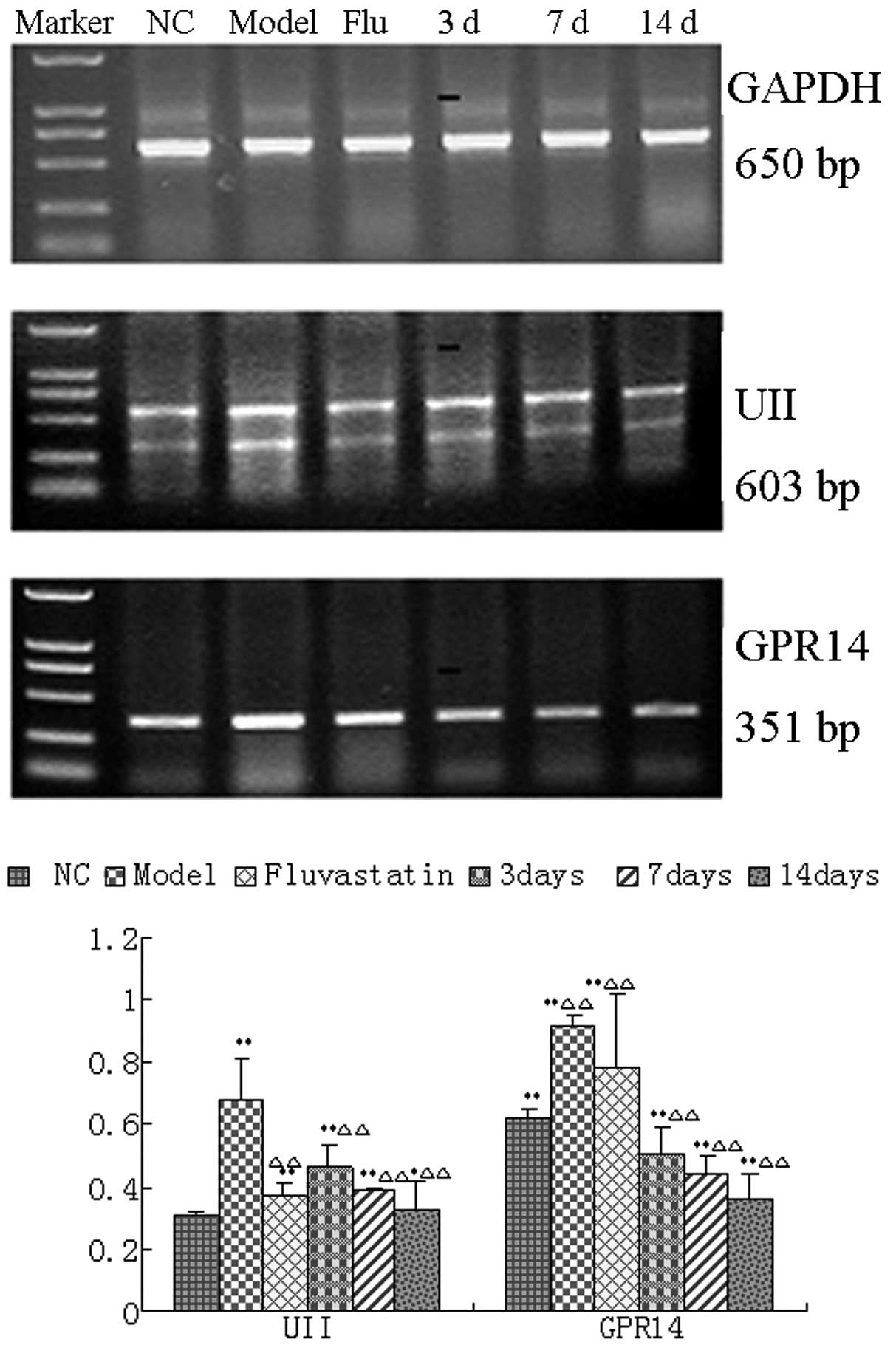
Effect of urantide on mRNA and protein
expression of UII and GPR14 in the AS rat model
The RT-PCR results revealed that UII and GPR14 mRNA
expression levels in the aorta pectoralis in the model group were
significantly increased compared with those in the normal group
(P<0.01). However, the mRNA expression levels in the aorta
pectoralis in the urantide groups and the fluvastatin group were
significantly reduced compared with those in the model group
(P<0.01). The mRNA expression levels in the urantide groups
decreased gradually in a dose-dependent manner, reaching the lowest
level after 14 days of injection of urantide (Fig. 3).
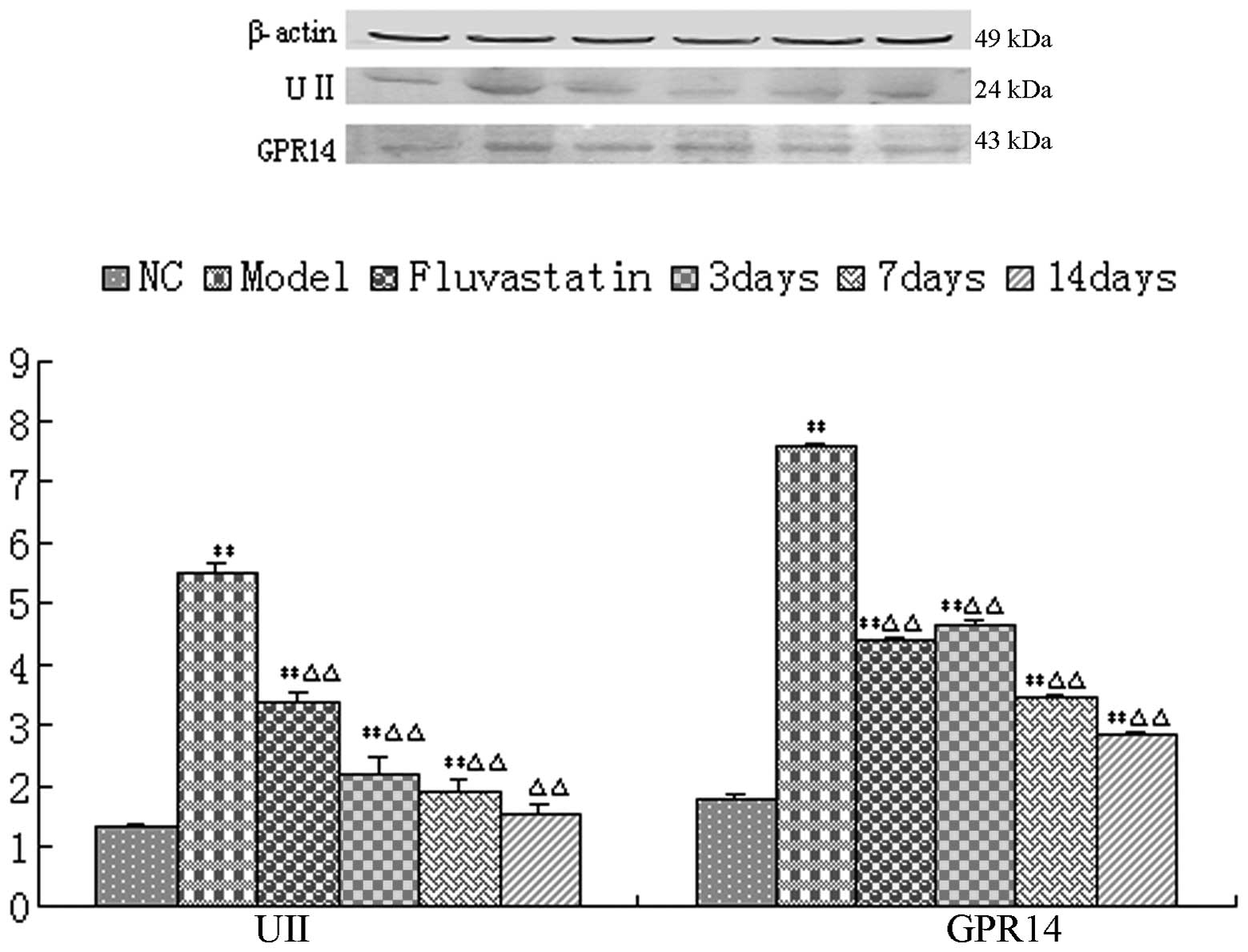
The western blotting results revealed that the UII
and GPR14 protein expression levels in the aorta pectoralis in the
model group were significantly increased compared with those in the
normal group (P<0.01). However, the protein expression levels in
the urantide groups and the fluvastatin group were significantly
reduced compared with those in the model group (P<0.01). The
protein expression levels in the urantide groups decreased
gradually as the injection of urantide continued, reaching the
lowest levels after 14 days of injection of urantide (Fig. 4).
Discussion
The experiments of the present study were designed
with referrence to previous reports (8,9), in
order to establish a new method for generating the AS model with
more serious pathological changes in a short experimental period.
We used traditional high-fat diet supplemented with
propylthiouracil, sodium cholate and white sugar in order to
inhibit thyroid function and stimulate the absorption of
cholesterin, as well as overcome the bitter taste of
propylthiouracil and increase the blood sugar of rats. Furthermore,
we triggered the overload of calcium in the artery via the
intraperitoneal injection of 70 U/kg VD3 as the revulsant of
calcium ions.
The results of the present study indicated that the
supplemented high-fat forage increases the levels of
Ca2+, TG, TC, HDL and LDL in blood serum in the model
group four weeks after feeding. The aortic tunica intima of rats
presented typical pathological changes, including the apparent
calcification, hyperplasia and foam-like accumulation of vascular
smooth muscle cells, the degeneration, breakage and disintegration
of elastic fibers and the atrophia of the tunica media. These
results demonstrated that the intraperitoneal injection of 70 U/kg
VD3 combined with supplemented high-fat forage for four weeks
successfully produces a rat model of AS, with a 100% success rate
in two separate modeling experiments. Additionally, the survival
rate of rats in the AS model group reached 78%. Therefore, the AS
models we reproduced in the present study had the advantages of
short cycle time, high survival rate and stability compared with
other modeling methods (6,9–11).
UII is a vasoactive cyclic peptide originally
isolated from the caudal neurosecretory organ of a fish. UII and
its receptor GPR14 combine together to form a UII/GPR14 system,
thus initiating a series of biological effects, including
vasoconstriction (6,10). UII and GPR14 are mainly distributed
in the tunica externa of the chest aorta in healthy rats and a
small amount is distributed in the tunica intima and tunica media.
A certain amount of UII is present in coronal AS plaques, smooth
muscle cells with lipidosis and rich areas of macrophages. The
vasoconstriction observed in the present study indicated that
abundant particles positive for UII and GPR14 expression were
present in the aortic tunica intima and tunica media plaque. The
RT-PCR and western blotting results further indicated that the gene
and protein expression levels of UII and GPR14 were increased in
the chest aorta, consistent with previous reports (6,10).
Thus, we consider that UII may be directly or indirectly involved
in the formation and development of AS.
UII is strongly associated with vasoconstriction;
however, the underlying mechanism is complicated. The
vasoconstrictive action may be mediated by protein kinase C (PKC),
Ca2+, phosphatide C, protein kinase and protein tyrosine
kinase (PTK) and blocked by PKC inhibitors, Ca2+ tunnel
antagonists, phosphatidase C inhibitors, PTK inhibitors and kinase
inhibitors (7,12,13).
Urantide is one of the most potent UII receptor antagonists
(3). A previous study demonstrated
that urantide competitively antagonizes the pro-contraction effect
of UII in the chest aorta of rats and markedly inhibits the
increase of UII activity in human monocyte-derived macrophages,
thereby blocking the development and accumulation of foam cells
(3).
In the present study, the urantide groups were
divided into three groups according to the duration of treatment
(3, 7 and 14 days) with 30 μg/kg urantide, to observe
whether urantide protects against AS and blocks the development of
AS. We identified that after three days, urantide resulted in
reductions in the concentrations of TG, TC, HDL and LDL in blood
serum and reductions in the immunohistochemical intensity and range
in the plaques of the aortic tunica intima and tunica media
compared with the model group. Urantide also resulted in reductions
in the gene and protein expression levels of UII and GPR14.
Furthermore, as the injections of urantide continued, the
therapeutic effect became more evident. This finding demonstrated
that urantide protected the rats from AS, possibly by antagonizing
the UII receptor, GPR14.
In conclusion, UII accelerated the initiation and
development of AS; this action may be a direct result of the
binding of UII to its receptor, GPR14. The gene and protein
expression levels of UII and GPR14 in the chest aorta of the AS
rats was reduced due to inhibition by treatment with the
GPR14-specific antagonist urantide. Urantide inhibits AS and
relieves its symptoms, thus providing a theoretical basis for
clinical treatment.
Acknowledgements
This study was supported by the
National Science Foundation of China (grant no. 81141045), the
Chengde Medical College Dr Foundation of China (grant no. 201102)
and the National Clinical Medicine Special Foundation of China
(grant no. L2011315).
References
|
1.
|
Ban Y, Watanabe T, Suguro T, Matsuyama TA,
Iso Y, Sakai T, Sato R, Idei T, Nakano Y, Ota H, Miyazaki A, Kato
N, Hirano T, Ban Y and Kobayashi Y: Increased plasma urotensin-II
and carotid atherosclerosis are associated with vascular dementia.
J Atheroscler Thromb. 16:179–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cheriyan J, Burton TJ, Bradley TJ, Wallace
SM, Mäki-Petäjä KM, Mackenzie IS, McEniery CM, Brown J and
Wilkinson IB: The effects of urotensin II and urantide on forearm
blood flow and systemic haemodynamics in humans. Br J Clin
Pharmacol. 68:518–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hassan GS, Douglas SA, Ohlstein EH and
Giaid EH: Expression of urotensin-II in human coronary
atherosclerosis. Peptides. 26:2464–2472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Babińska M, Holecki M, Prochaczek F,
Owczarek A, Kokocińska D, Chudek J and Więcek A: Is plasma
urotensin II concentration an indicator of myocardial damage in
patients with acute coronary syndrome? Arch Med Sci. 8:449–454.
2012.PubMed/NCBI
|
|
5.
|
You Z, Genest J Jr, Barrette PO, Hafiane
A, Behm DJ, D’Orleans-Juste P and Schwertani AG: Genetic and
pharmacological manipulation of urotensin II ameliorate the
metabolic and atherosclerosis sequalae in mice. Arterioscler Thromb
Vasc Biol. 32:1809–1816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Camarda V, Spagnol M, Song W, Vergura R,
Roth AL, Thompson JP, Rowbotham DJ, Guerrini R, Marzola E,
Salvadori S, Cavanni P, Regoli D, Douglas SA, Lambert DG and Calò
G: In vitro and in vivo pharmacological characterization of the
novel UT receptor ligand [Pen5,DTrp7,Dab8]urotensin II(4–11)
(UFP-803). Br J Pharmacol. 147:92–100. 2006.PubMed/NCBI
|
|
7.
|
Camarda V, Song W, Marzola E, Spagnol M,
Guerrini R, Salvadori S, Regoli D, Thompson JP, Rowbotham DJ, Behm
DJ, Douglas SA, Calo’ G and Lambert DG: Urantide mimics
urotensin-II induced calcium release in cells expressing
recombinant UT receptors. Eur J Pharmacol. 498:83–86. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sauzeau V, Le Mellionnec E, Bertoglio J,
Scalbert E, Pacaud P and Loirand G: Human urotensin II-induced
contraction and arterial smooth muscle cell proliferation are
mediated by RhoA and Rho-Kinase. Circ Res. 88:1102–1104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gao S, Oh YB, Shah A, Park WH, Chung MJ,
Lee YH and Kim SH: Urotensin II receptor antagonist attenuates
monocrotaline-induced cardiac hypertrophy in rats. Am J Physiol
Heart Circ Physiol. 299:H1782–H1789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Johns DG, Ao Z, Naselsky D, Herold CL,
Maniscalco K, Sarov-Blat L, Steplewski K, Aiyar N and Douglas SA:
Urotensin II-mediated cardiomyocyte hypertrophy: effect of receptor
antagonism and role of inflammatory mediators. Naunyn Schmiedebergs
Arch Pharmacol. 370:238–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Xu S, Wen H and Jiang H: Urotensin II
promotes the proliferation of endothelial progenitor cells through
p38 and p44/42 MAPK activation. Mol Med Report. 6:197–200.
2012.PubMed/NCBI
|
|
12.
|
Matsusaka S and Wakabayashi I: Enhancement
of vascular smooth muscle cell migration by urotensin II. Naunyn
Schmiedebergs Arch Pharmacol. 373:381–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shi L, Ding W, Li D, Wang Z, Jiang H,
Zhang J and Tang C: Proliferation and antiapoptotic effects of
human urotensin II on human endothelial cells. Atherosclerosis.
188:260–264. 2006. View Article : Google Scholar : PubMed/NCBI
|