Introduction
Naturally occurring coumarins, a group of
plant-derived polyphenolic compounds, serve as antimitotic,
immunomodulating, antiviral, anticancer and cytotoxic agents in
humans (1,2). A coumarin derivative,
7,8-dihydroxycoumarin, an active plant lactone extracted from
Daphne Korean Nakai (3), is mainly
used as an analgesic, antibacterial and antiviral agent, as well as
to prevent and treat liver fibrosis in the clinic (1).
7,8-Dihydroxycoumarin and analogs have demonstrated
significant antitumor effects and promote tumor apoptosis (4–7) via
multiple signaling pathways. Elinos-Báez et al reported that
7-hydroxycoumarin inhibits anti-apoptotic Bcl-2 expression in lung
cancer cells and promotes the expression of pro-apoptotic
Bcl-2-associated X protein (Bax) (8). Other studies identified that coumarin
is able to induce cervical and colon cancer cell apoptosis by
activating the mitochondrial pathway and the caspase-3-dependent
apoptotic pathway, to downregulate the anti-apoptotic NF-κB, Bcl-2
and Bcl-xL, and upregulate caspase-3 to promote the release of
cytochrome (cyt) c(9,10).
The coumarin derivative psoralidin is also able to enhance the role
of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
in promoting the apoptosis and necrosis of HeLa cervical cancer
cells (11). Rasul et al
reported that the coumarin derivative xanthoxyletin induces S phase
arrest and apoptosis in SGC-7901 gastric cancer cells (12). Bhattacharyya et al
demonstrated that 7-hydroxy-6-methoxycoumarin induces the
downregulation of aryl hydrocarbon receptor (AhR), CYP1A1,
proliferating cell nuclear antigen (PCNA), Stat-3, survivin, matrix
metalloproteinase (MMP)-2, cyclin D1 and c-myc, and upregulation of
p53, caspase-3 and tissue inhibitor of metalloproteinases (TIMP)-2
(13). Singh et al reported
a coumarin derivative (RKS262) that inhibits the ovarian cancer
cell cycle and promotes apoptosis in cancer cells (14). Additionally, the authors identified
that the coumarin derivative upregulates pro-apoptotic proteins Bid
and Bok and inhibits anti-apoptotic Bcl-xL and Mcl-1, independently
of pro-apoptotic mitogen-activated protein kinase (MAPK) p38 and
stress-activated protein (SAP)/c-Jun N-terminal kinase (JNK)
activation. Bhattacharyya et al reported that coumarin
enhances pro-apoptotic p53, PCNA, Bad, Bax, apoptotic protease
activating factor (Apaf), cyt c, caspase-3 and caspase-9
expression in melanoma (skin cancer) cells, and inhibits the
anti-apoptotic factors Akt, Bcl-2, Bcl-xL and NF-κB (15). Thati et al also identified
that coumarin derivatives enhance the malignancy of pro-apoptotic
factors caspase-3 and -9 (16).
The main types of lung carcinoma include small-cell
lung cancer (SCLC) and non-small-cell lung cancer (NSCLC); lung
adenocarcinoma accounts for 40% of NSCLCs (17,18).
Lung adenocarcinoma cells overexpress multiple anti-apoptotic
signals. The Akt/NF-κB pathways are involved in a number of
anti-apoptotic and drug-resistant events that occur in lung
adenocarcinoma (17,18). Therefore, we hypothesize that
7,8-dihydroxycoumarin may also play an important role in promoting
the apoptosis of lung adenocarcinoma cells by suppressing the Akt
and NF-κB signaling pathways.
In the present study, 7,8-dihydroxycoumarin was
administered to lung adenocarcinoma cells to investigate its effect
on the apoptotic signaling pathways.
Materials and methods
Materials
7,8-Dihydroxycoumarin (purity, 99.6%; Tauto Biotech
Ltd. Co., Shanghai, China) was dissolved in 0.9% NaCl solution,
followed by filtration with a 0.02-mm filter (Millipore, Billerica,
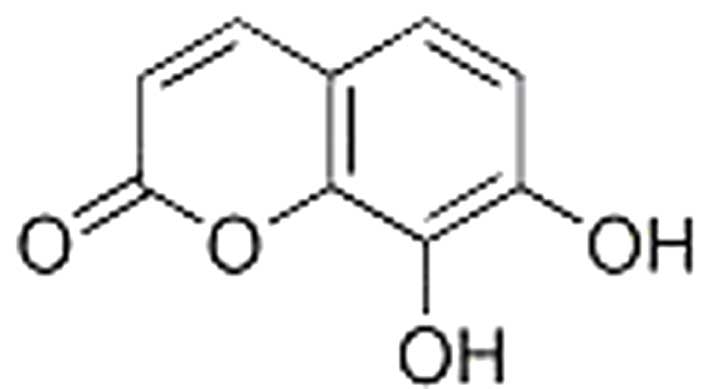
MA, USA). The structure of 7,8-dihydroxycoumarin is shown in
Fig. 1. A total protein extraction
kit and a TRIzol total RNA extraction kit were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The
anti-phospho-IκBα (phospho S32/S36; sc-8404), anti-NF-κBp65
(sc-8008), anti-Bcl-2 (sc-509) and anti-caspase-3 (sc-7272)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The anti-phospho-Akt1 (phospho T308;
ab105731) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
ab8245) antibodies were purchased from Abcam (Beijing, China);
these antibodies were mouse monoclonal. The horse-radish peroxidase
(HRP)-labeled goat anti-mouse secondary antibody was purchased from
Abcam. 3-(4,5-Dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide
(MTT) was purchased from Sigma (St. Louis, MO, USA). The Moloney
murine leukemia virus reverse transcriptase (M-MLV RTase) kit was
purchased from Promega Corporation (Shanghai, China). The 2X SYBR
real-time polymerase chain reaction (PCR) kit was purchased from
Roche Diagnostics (Shanghai, China). The bicinchoninic acid (BCA)
protein detection kit and enhanced chemiluminescence (ECL)
detection kit were purchased from Pierce Chemicals, Thermo Fisher
Scientific Inc. (Rockford, IL, USA).
Cell line
The A549 human lung adenocarcinoma cell line was
purchased from American Type Culture Collection (ATCC no. CCL-185;
Manassas, VA, USA). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% fetal bovine serum (Gibco,
Invitrogen Life Technologies) in a 5% CO2 incubator and
passaged with 0.25% trypsin (Sigma, Ronkonoma, NY, USA) and 0.03%
ethylenediamine tetraacetic acid (EDTA) solution.
Treatment
The A549 cells were digested, suspended and seeded
into each well of six-well plates with a density of
1.0×106 cells/ml in 2 ml complete culture medium. The
cells were cultured for 24 h and then exposed to
7,8-dihydroxycou-marin for 48 h. 7,8-Dihydroxycoumarin was
dissolved in 0.9% NaCl solution and added to cells, forming a final
concentration of 25, 50 and 100 μmol/l. Equivalent 0.9% NaCl
solution was added to cells as the control.
Quantitative PCR (qPCR)
The A549 cells were harvested and total RNA was
extracted with the total RNA extraction kit using the TRIzol
method. The first strand cDNA was synthesized using M-MLV RTase
according to the manufacturer’s instructions. Real-time PCR was
performed using the cDNA template according to the manufacturer’s
instructions. Amplification of GAPDH was used as an inner control
in each reaction system. The reaction conditions were as follows:
40 cycles of 95°C for 30 sec, 58°C for 60 sec and 72°C for 60 sec.
The primers were designed based on the Genbank sequence using
Beacon Designer 7 (PREMIER Biosoft, Palo Alto, CA, USA). Primer
synthesis and DNA sequencing were performed by Shanghai Sangon
(China). The primer sequences were as follows: NF-κBp65, sense:
5′-GCAAAGGAAACGCCAGAAGC-3′ and antisense: 5′-
CACTACCGAACATGCCTCCAC-3′; Bcl-2, sense: 5′-ATGACTTCTCTCGTCGCTACT-3′
and antisense: 5′-CCCATCCCTGAAGAGTTCCGA-3′; caspase-3, sense:
5′-CATGGCCTGTCAGAAAATAC-3′ and antisense:
5′-TAACCCGAGTAAGAATGTGC-3′; GAPDH (housekeeper gene), sense:
5′-AATGTGTCCGTCGTGGATCTG-3′ and antisense
5′-CAACCTGGTCCTCAGTGTAGC-3′.
Western blotting
Western blotting was used to detect the protein
expression of phospho-Akt1 (pAkt1), phospho-IκBα (pIκBα), NF-κBp65,
Bcl-2 and caspase-3. The A549 cells were harvested and cell lysis
was performed using the eukaryotic cell lysis buffer followed by
extraction of total protein, according to the manufacturer’s
instructions (Beyotime Inst. Biotech., Nanjing, Jiangsu, China).
Protein quantity was determined by a BCA method. Using 30 μg
for each sample, proteins were separated by 12% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were
blotted with a wet transfer device (Bio-Rad, Shanghai, China) to a
nitrocellulose membrane. The membrane was then immersed in blocking
solution containing 10% skimmed milk in phosphate-buffered saline
(PBS) Tween-20 (PBST), followed by agitation for 1 h. After washing
three times with Tris-buffered saline Tween-20 (TBST) for 5 min
each, the membrane was immersed in primary antibody diluted to
1:1,000 in the blocking solution at room temperature and then
agitated for 1 h. After washing again, the membrane was incubated
in HRP-labeled secondary antibody diluted to 1:10,000 in blocking
solution at room temperature and then agitated for 1 h. After
another rinse, the membrane underwent color development by an ECL
method, followed by X-film photography. GAPDH protein was used as
an inner control. The gray scale values (total raw density) of
blots were measured with the VisionWorksLS analysis software
available in the UVP EC3 (600) Imaging System (UVP, LLC, Upland,
CA, USA).
MTT assay
After 48 h, the medium was refreshed to discard the
7,8-dihydroxycoumarin. Cells were supplemented with 200 μl
MTT solution (5 mg/ml), followed by incubation in a CO2
incubator for another 4 h. The supernatant was discarded and each
well was supplemented with 500 μl dimethylsulfoxide (DMSO;
Sigma). When the purple crystals at the bottom of the well were
completely dissolved, the absorbance value was measured with a
Thermo Multiskan MK3 microplate reader (Thermo Fisher Scientific
Inc., Waltham, MA, USA) at a wavelength of 490 nm. Cell viability
(%) = experimental absorbance/normal absorbance ×100.
Statistical analysis
Data are presented as means ± standard deviation
(SD). The statistical software SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Paired comparisons were
performed by the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
mRNA levels detected by qPCR
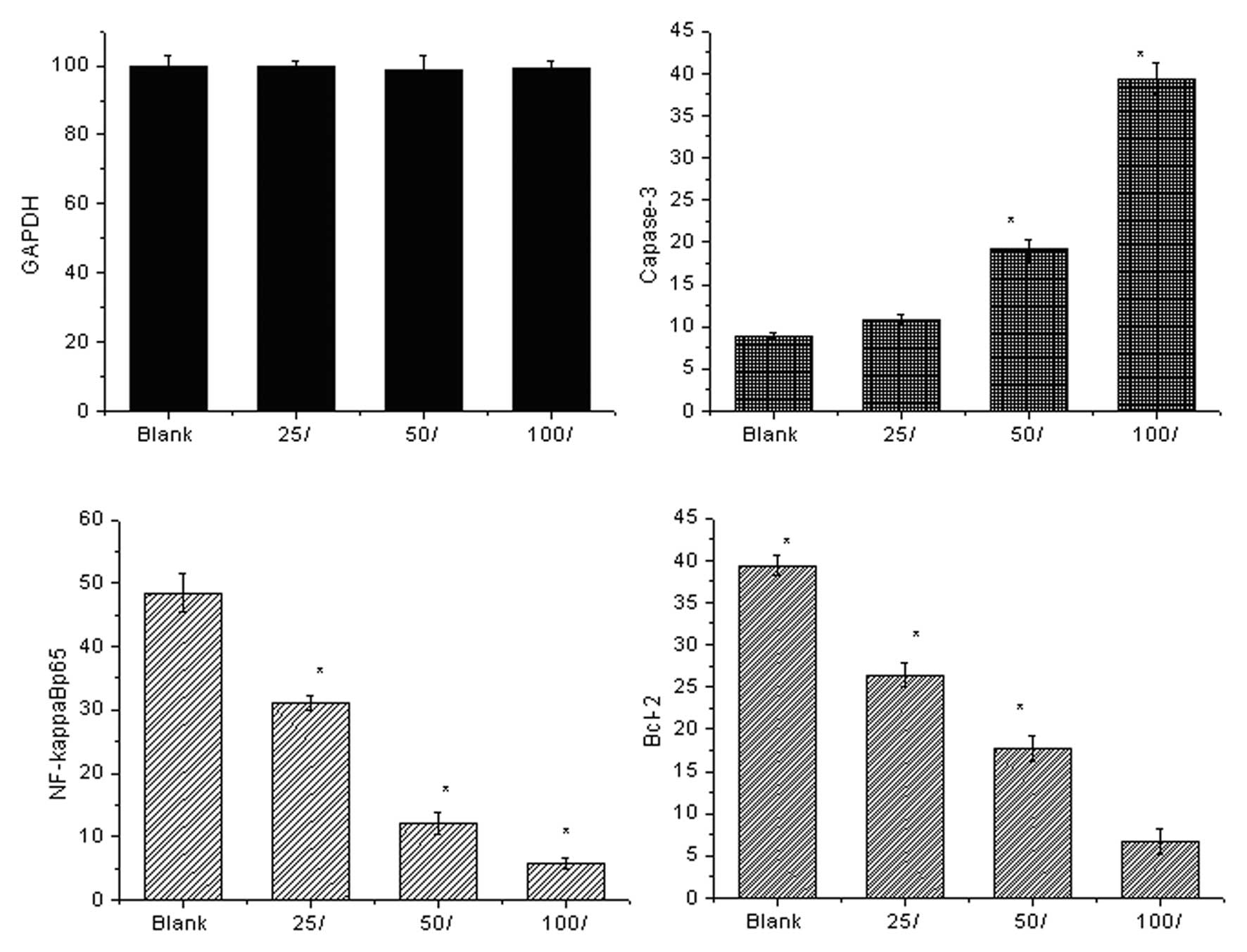
Fig. 2 shows the
expression levels of the cell signaling molecules detected by qPCR.
Prior to 7,8-dihydroxycoumarin treatment, the control cells
expressed high levels of anti-apoptotic NF-κBp65 and Bcl-2 mRNAs
and a low level of pro-apoptotic caspase-3. As
7,8-dihydroxycoumarin was used in a series of dilutions (25, 50 and
100 μmol/l), the anti-apoptotic signaling was inhibited and
the pro-apoptotic signaling was activated. The anti-apoptotic
NF-κBp65 mRNA expression levels decreased 0.64 (31.04/48.5)-, 0.25
(12.1/48.5)- and 0.12 (5.82/48.5)-fold, respectively; and the
levels of Bcl-2 mRNA decreased 0.67 (26.4/39.4)-, 0.45 (17.7/39.4)-
and 0.17 (6.7/39.4)-fold, respectively. The pro-apoptotic caspase-3
increased 1.23 (10.9/8.9)-, 2.14 (19.1/8.9)-, and 4.43
(39.4/8.9)-fold, respectively. The pro-apoptotic induction effect
of 7,8-dihydroxycoumarin is concentration-dependent.
Protein levels detected by western
blotting
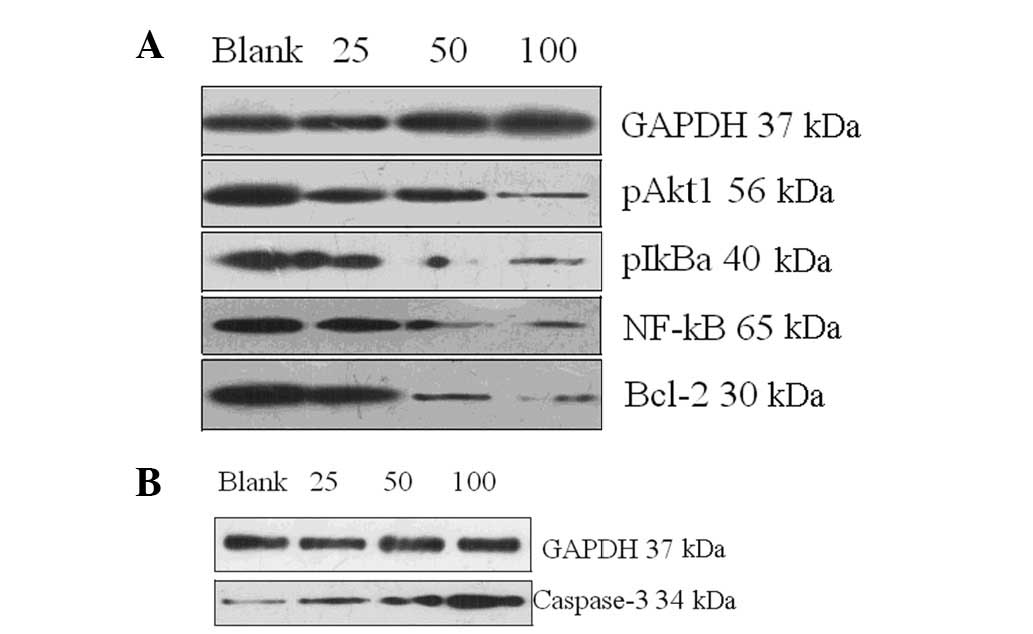
Fig. 3 shows the
expression of the cell signaling molecules detected by western
blotting. Prior to treatment with 7,8-dihydroxycoumarin, the
control cells expressed high levels of anti-apoptotic pAkt1, pIκBα,
NF-κBp65 and Bcl-2 proteins and a low-level of pro-apoptotic
caspase-3 protein. As 7,8-dihydroxycoumarin was used in a series of
dilutions (25, 50 and 100 μmol/l), the anti-apoptotic
signaling was inhibited (Fig. 3A)
and the pro-apoptotic signaling was upregulated (Fig. 3B).
Table I presents
the complete gray scales of the blots shown in Fig. 3 to represent the total levels of
the detected proteins. The anti-apoptotic pAkt1 protein blot
grayscales were 36.5, 18.1 and 7.3 vs. 52.4 (each
7,8-dihydroxycoumarin dose vs. control), respectively; the pIκBα
blot grayscales were 13.7, 7.6 and 4.3 vs. 42.2, respectively; the
pNF-κBp65 blot grayscales were 23.3, 12.6 and 5.08 vs. 44.5; the
Bcl-2 blot grayscales were 23.6, 17.9 and 5.92 vs. 38.5; and the
pro-apoptotic caspase-3 blot grayscales were 7.61, 16.1 and 27.8
vs. 5.8, respectively. The pro-apoptotic induction effect of
7,8-dihydroxycoumarin is concentration-dependent.
 | Table IGray scales of the western blots (48
h, %/GAPDH). |
Table I
Gray scales of the western blots (48
h, %/GAPDH).
| Protein blot | Cell control | 7,8-Dihydroxycoumarin
concentration (μmol/l)
|
|---|
| 25 | 50 | 100 |
|---|
| GAPDH (37 kDa) | 100.30 | 101.40 | 99.50 | 102.20 |
| pAkt1 (56 kDa) | 52.40 | 36.50 | 18.10 | 7.30 |
| pIκBα (40 kDa) | 42.20 | 13.70 | 7.60 | 4.30 |
| NF-κBp65 (65
kDa) | 44.50 | 23.30 | 12.60 | 5.08 |
| Bcl-2 (30 kDa) | 38.50 | 23.60 | 17.90 | 5.92 |
| Caspase-3 (34
kDa) | 5.80 | 7.61 | 16.10 | 27.80 |
Cell proliferation
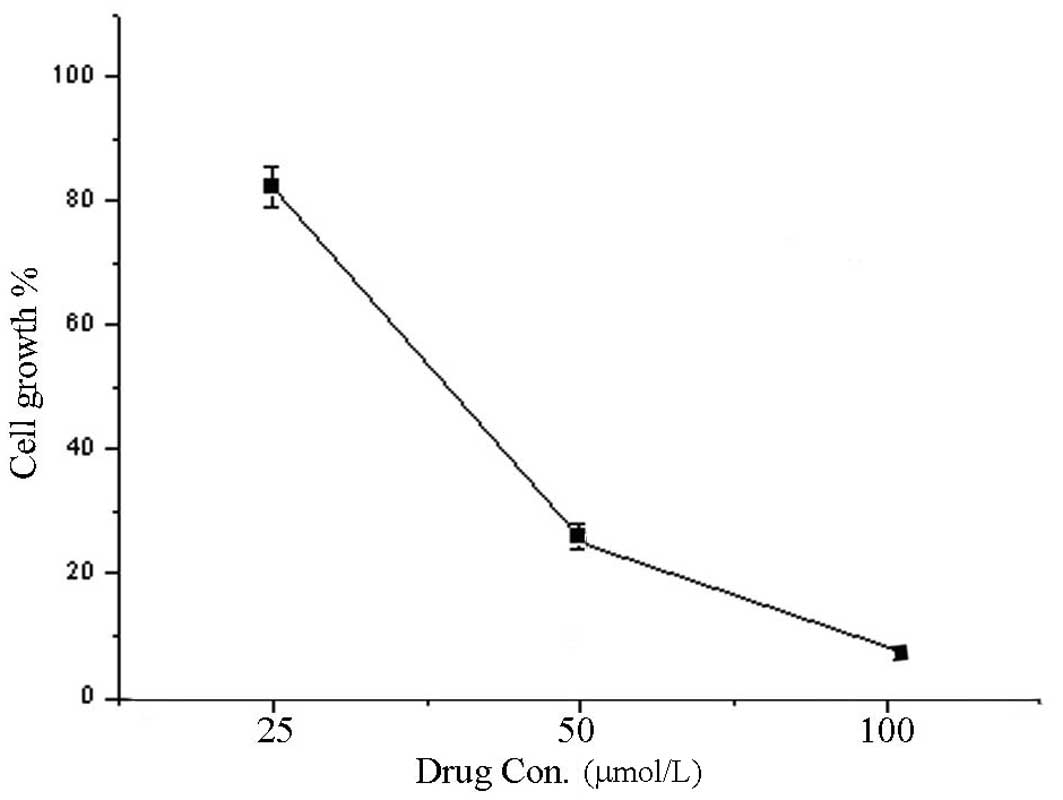
Fig. 4 illustrates
cell viability at 48 h. The proliferative activity of A549 cells
treated with 7,8-dihydroxycoumarin decreased and was significantly
lower compared with that of the control cells (83.7, 27.2 and 9.5
vs. 100%, respectively; P<0.05 for each). 7,8-Dihydroxycoumarin
inhibited tumor cell proliferation in a concentration-dependent
manner.
Discussion
Inhibition of the Akt/NF-κB pathways results in the
upregulation of pro-apoptotic Fas/APO-1, FasL, Bax (17), caspase-8, caspase-3 and cyt
c, with simultaneous downregulation of NF-κBα, Akt, Bcl-2
and Bcl-xL (18). In the present
study, we used 7,8-dihydroxycoumarin to treat A549 lung
adenocarcinoma cells and then performed qPCR and western blotting
to detect the ability of 7,8-dihydroxycoumarin to change the levels
of anti-apoptotic pAkt, pIκBα, pNF-κB p65 and Bcl-2, as well as
pro-apoptotic caspase-3.
Prior to treatment with 7,8-dihydroxycoumarin, there
is an overexpression of Akt1 phosphorylated in control cells
(19). Hyperactivated pAkt1 has
serine-threonine protein kinase activity and triggers the cascaded
enzymes, resulting in an increased phosphorylation of IκBα at
serines 32 and 36. pIκBα was disassociated from the IκBα/NF-κB
complex, resulting in a release of pNF-κB causing an increase of
NF-κBp65 at the mRNA and protein levels. The hyperactivated pAkt1
also causes anti-apoptotic Bcl-2 to be maintained at high mRNA and
protein levels, resulting in the sustained proliferation of the
A549 control cells.
The use of 7,8-dihydroxycoumarin to treat A549 cells
resulted in a marked downregulation of pAkt1 and pIκBα, as well as
NF-κBp65 at the mRNA and protein levels. The downregulation of
pAkt1 indicates that the serine-threonine protein kinase activity
of Akt was reduced. Subsequently, the phosphorylation of IκBα was
reduced. Thus the IκBα/NF-κB complex inhibited the release of
NF-κB, resulting in a reduction in NF-κBp65 levels. As the
serine-threonine protein kinase activity of Akt was reduced,
anti-apoptotic Bcl-2 was simultaneously downregulated, so that the
suppression of the apoptosis of A549 cells was reduced; therefore,
apoptosis was facilitated.
The downregulation of pAkt1 and NF-κBp65
demonstrated that the signal amplification and transduction
pathways were efficiently suppressed. Accordingly, the
pro-apoptotic caspase-3 expression was increased. As reported in
previous studies, upregulated caspase-3 inhibits IKK2 (20,21)
in necrotized or apoptotic cancer cells, resulting in a further
reduction in the phosphorylation of IκBα, causing the NF-κBp65
level to be further reduced. The upregulated caspase-3 also
directly inhibits the NF-κBp65 protein (22), causing a secondary downregulation
of NF-κBp65 in apoptotic cancer cells. Therefore, the NF-κBp65
signaling was markedly suppressed in A549 cells in the present
study. The apoptotic A549 cells were observed to undergo reduced
proliferation. The MTT assay results also demonstrated that the
proliferation of A549 cells was significantly inhibited by
7,8-dihydroxycoumarin. In addition, the pro-apoptotic induction
effect of 7,8-dihydroxycoumarin was concentration-dependent.
In conclusion, 7,8-dihydroxycoumarin inhibits the
proliferation of A549 human lung adenocarcinoma cells and induces
their apoptosis via Akt/NF-κB signaling suppression in a
concentration-dependent manner. Akt and NF-κB may be targets for
the treatment of lung adenocarcinoma. 7,8-Dihydroxycoumarin may be
a candidate naturally occurring drug for the treatment and
prevention of lung adenocarcinoma.
7,8-Dihydroxycoumarin, as an extract of naturally
occurring plants, is safe and has a high efficacy. Therefore, it
may be used in the clinic to treat lung carcinoma.
References
|
1.
|
Kontogiorgis C, Detsi A and
Hadjipavlou-Litina D: Coumarin-based drugs: a patent review (2008 -
present). Expert Opin Ther Pat. 22:437–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Anand P, Singh B and Singh N: A review on
coumarins as acetylcholinesterase inhibitors for Alzheimer’s
disease. Bioorg Med Chem. 20:1175–1180. 2012.
|
|
3.
|
Chakraborty AK, Funasaka Y, Pawelek JM,
Nagahama M, Ito A and Ichihashi M: Enhanced expression of
melanocortin-1 receptor (MC1-R) in normal human keratinocytes
during differentiation: evidence for increased expression of POMC
peptides near suprabasal layer of epidermis. J Invest Dermatol.
112:853–860. 1999. View Article : Google Scholar
|
|
4.
|
Yang EB, Zhao YN, Zhang K and Mack P:
Daphnetin, one of coumarin derivatives, is a protein kinase
inhibitor. Biochem Biophys Res Commun. 260:682–685. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Finn GJ, Creaven BS and Egan DA: Daphnetin
induced differentiation of human renal carcinoma cells and its
mediation by p38 mitogen-activated protein kinase. Biochem
Pharmacol. 67:1779–1788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Finn GJ, Kenealy E, Creaven BS and Egan
DA: In vitro cytotoxic potential and mechanism of action of
selected coumarins using human renal cell lines. Cancer Lett.
183:61–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Riveiro ME, Moglioni A, Vazquez R, Gomez
N, Facorro G, Piehl L, de Celis ER, Shayo C and Davio C: Structural
insights into hydroxycoumarin-induced apoptosis in U-937 cells.
Bioorg Med Chem. 16:2665–2675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Elinos-Báez CM, León F and Santos E:
Effects of coumarin and 7OH-coumarin on bcl-2 and Bax expression in
two human lung cancer cell lines in vitro. Cell Biol Int.
29:703–708. 2005.PubMed/NCBI
|
|
9.
|
Chuang JY, Huang YF, Lu HF, Ho HC, Yang
JS, Li TM, Chang NW and Chung JG: Coumarin induces cell cycle
arrest and apoptosis in human cervical cancer HeLa cells through a
mitochondria- and caspase-3 dependent mechanism and NF-kappaB
down-regulation. In Vivo. 21:1003–1009. 2007.PubMed/NCBI
|
|
10.
|
Saidu NE, Valente S, Bana E, Kirsch G,
Bagrel D and Montenarh M: Coumarin polysulfides inhibit cell growth
and induce apoptosis in HCT116 colon cancer cells. Bioorg Med Chem.
20:1584–1593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bronikowska J, Szliszka E, Jaworska D,
Czuba ZP and Krol W: The coumarin psoralidin enhances anticancer
effect of tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL). Molecules. 17:6449–6464. 2012. View Article : Google Scholar
|
|
12.
|
Rasul A, Khan M, Yu B, Ma T and Yang H:
Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in
human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer
Prev. 12:1219–1223. 2011.PubMed/NCBI
|
|
13.
|
Bhattacharyya SS, Paul S, Dutta S,
Boujedaini N and Khuda-Bukhsh AR: Anti-oncogenic potentials of a
plant coumarin (7-hydroxy-6-methoxy coumarin) against
7,12-dimethylbenz [a] anthracene-induced skin papilloma in mice:
the possible role of several key signal proteins. Zhong Xi Yi Jie
He Xue Bao. 8:645–654. 2010.PubMed/NCBI
|
|
14.
|
Singh RK, Lange TS, Kim KK and Brard L: A
coumarin derivative (RKS262) inhibits cell-cycle progression,
causes pro-apoptotic signaling and cytotoxicity in ovarian cancer
cells. Invest New Drugs. 29:63–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bhattacharyya SS, Paul S, Mandal SK,
Banerjee A, Boujedaini N and Khuda-Bukhsh AR: A synthetic coumarin
(4-methyl-7 hydroxy coumarin) has anti-cancer potentials against
DMBA-induced skin cancer in mice. Eur J Pharmacol. 614:128–136.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Thati B, Noble A, Creaven BS, Walsh M,
McCann M, Devereux M, Kavanagh K and Egan DA: Role of cell cycle
events and apoptosis in mediating the anti-cancer activity of a
silver(I) complex of 4-hydroxy-3-nitro-coumarin-bis(phenanthroline)
in human malignant cancer cells. Eur J Pharmacol. 602:203–214.
2009. View Article : Google Scholar
|
|
17.
|
Hsu YL, Kuo PL and Lin CC: Proliferative
inhibition, cell-cycle dysregulation, and induction of apoptosis by
7,8-dihydroxycoumarin in human non-small cell lung cancer A549
cells. Life Sci. 75:2303–2316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li Y, Xing D, Chen Q and Chen WR:
Enhancement of chemotherapeutic agent-induced apoptosis by
inhibition of NF-kappaB using 7,8-dihydroxycoumarin. Int J Cancer.
127:462–473. 2010.PubMed/NCBI
|
|
19.
|
Jang BC: The fruit juice of Morinda
citrifolia (noni) down-regulates HIF-1α protein expression
through inhibition of PKB, ERK-1/2, JNK-1 and S6 in
manganese-stimulated A549 human lung cancer cells. Int J Mol Med.
29:499–504. 2012.
|
|
20.
|
Barkett M and Gilmore TD: Control of
apoptosis by Rel/NF-κB transcription factors. Oncogene.
18:6910–6924. 1999.
|
|
21.
|
Tang G, Yang J, Minemoto Y and Lin A:
Blocking caspase-3 mediated proteolysis of IKKβ suppresses
TNF-α-induced apoptosis. Mol Cell. 8:1005–1016. 2001.PubMed/NCBI
|
|
22.
|
Ravi R, Mookerjee B, van Hensbergen Y,
Bedi GC, Giordano A, El-Deiry WS, Fuchs EJ and Bedi A: p53-mediated
repression of nuclear factor-kappaB RelA via the transcriptional
integrator p300. Cancer Res. 58:4531–4536. 1998.PubMed/NCBI
|