Introduction
The famous cardiologist, Eugene Braunwald indicated
that atrial fibrillation (AF) has become an epidemic in the 21st
century. Therefore, to prevent thromboembolic events in patients
with AF antithrombotic therapy has been studied. A study determined
that the average rate of ischemic stroke among patients with AF is
∼5% per year (1). However, it has
been indicated that warfarin may significantly reduce the incidence
of a stroke in patients with AF (2). Clinical trials utilizing warfarin for
the prevention of cerebral embolism in patients with AF, have
demonstrated that an appropriate anticoagulation intensity directed
by international normalized ratio (INR), may be an effective and
safe method for the use of warfarin. In addition, the Boston area
anticoagulation trial (BATT) determined that low-dose warfarin
therapy (INR 2.0–3.0) is highly effective in preventing a stroke in
patients with non-rheumatic AF, without an increased risk of major
bleeding, and may be a safe treatment with careful monitoring
(3). Furthermore, a meta-analysis
of five randomized trials of oral anticoagulants (OACs) compared
with the control, for the primary prevention of a stroke in
patients with non-valvular AF [aspirin versus warfarin standard
dose (AFASAK I); aspirin versus warfarin standard dose, age >75
(SPAF II); warfarin versus no treatment (BAATAF); warfarin versus
placebo (SPINAF); and Canadian Atrial Fibrillation Anticoagulation
(CAFA)], identified that INR 2.0–2.6 provided the lowest risk of
bleeding and a stroke. However, if INR>3, the risk of bleeding
increased (4). The increase in
risk of a stroke was similar between chronic AF and paroxysmal AF
(PAF) (5). Studies that have
investigated AF anticoagulation strategies in China have been
established according to the European or American guidelines.
However, a large-scale clinical study has yet to be performed and
may confirm an appropriate anticoagulation concentration (INR
range) for Chinese elderly patients with AF. Moreover, it is a
controversial issue as to whether elderly patients with PAF who
have a middle- to high-risk of a stroke, should receive
antithrombotic therapy. Additionally, the optimal concentration of
warfarin is still unknown. A study has demonstrated that blood
coagulation in Asian patients is lower than that of European and
American patients; therefore, lower intensity anticoagulation
therapy is recommended (6). Thus,
large-scale clinical studies on anticoagulation of elderly Chinese
patients with PAF, is required to ensure effective and safe
treatment with warfarin.
Patients and methods
Patients
The present investigation was a prospective,
randomized, controlled, parallel and multicentered study. The
definition and classification in the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines/European Society of Cardiology Committee (ACC/AHA/ESC;
2006) guidelines for the management of patients with AF was
followed (7). Patients with PAF
were divided into three groups on the basis of stroke risk: low-,
middle- and high-risk. Middle-risk patients had one of the
following risk factors: age ≥75 years, heart failure, hypertension,
diabetes, and left ventricular ejection fraction (LVEF) ≤0.35.
High-risk patients had two of the risk factors listed, or one of
the following risk factors: previous history of a stroke, transient
ischemic attack or thromboembolism.
Population
Elderly patients aged ≥65 years with PAF were
randomly assigned to aspirin or varied concentrations of warfarin
for antithrombotic therapy. Patients who met all the inclusion
criteria and none of the exclusion criteria were recruited at each
center. This included patients aged ≥65 years; had indicated at
least two documented AF episodes in the previous six months with a
duration of <3 days [confirmed by electrocardiography (ECG) or
Holter]; patients that demonstrated palpitations, chest tightness,
dizziness and sweating; and patients that were either at a middle
or high-risk of a stroke. The exclusion criteria were recorded by
the clinicians and determined the eligibility of a patient. The
criteria included patients with non-artherosclerosis AF (rheumatic
heart disease, cardiomyopathy, hyperthyroidism and electrolyte
disturbances); AF due to reversible underlying disease (acute
myocardial infarction, acute myocarditis and untreated
hyperthyroidism); AF induced by electrophysiological examination,
coronary angiography or pacemaker implantation; patients with a
recent history of cardiothoracic surgery, gastrointestinal and
intracranial bleeding or other bleeding; severe liver or renal
dysfunction; cancer or blood disease; and acute inflammation of the
respiratory tract or urinary tract. This study had a total of 1,472
patients. The local ethic committee approved the study protocol and
written informed consent was obtained from all patients.
Baseline evaluation and grouping
Prior to the randomization, baseline characters of
the patients were evaluated. This included their medical history, a
physical examination, 12-lead ECG, 24-h Holter, assay of D-dimer
and prothrombin times, vascular ultrasound of the carotid and leg
arteries and an echocardiogram. In addition, all patients were
computer randomized using SPSS software, version 13.0 (SPSS, Inc.
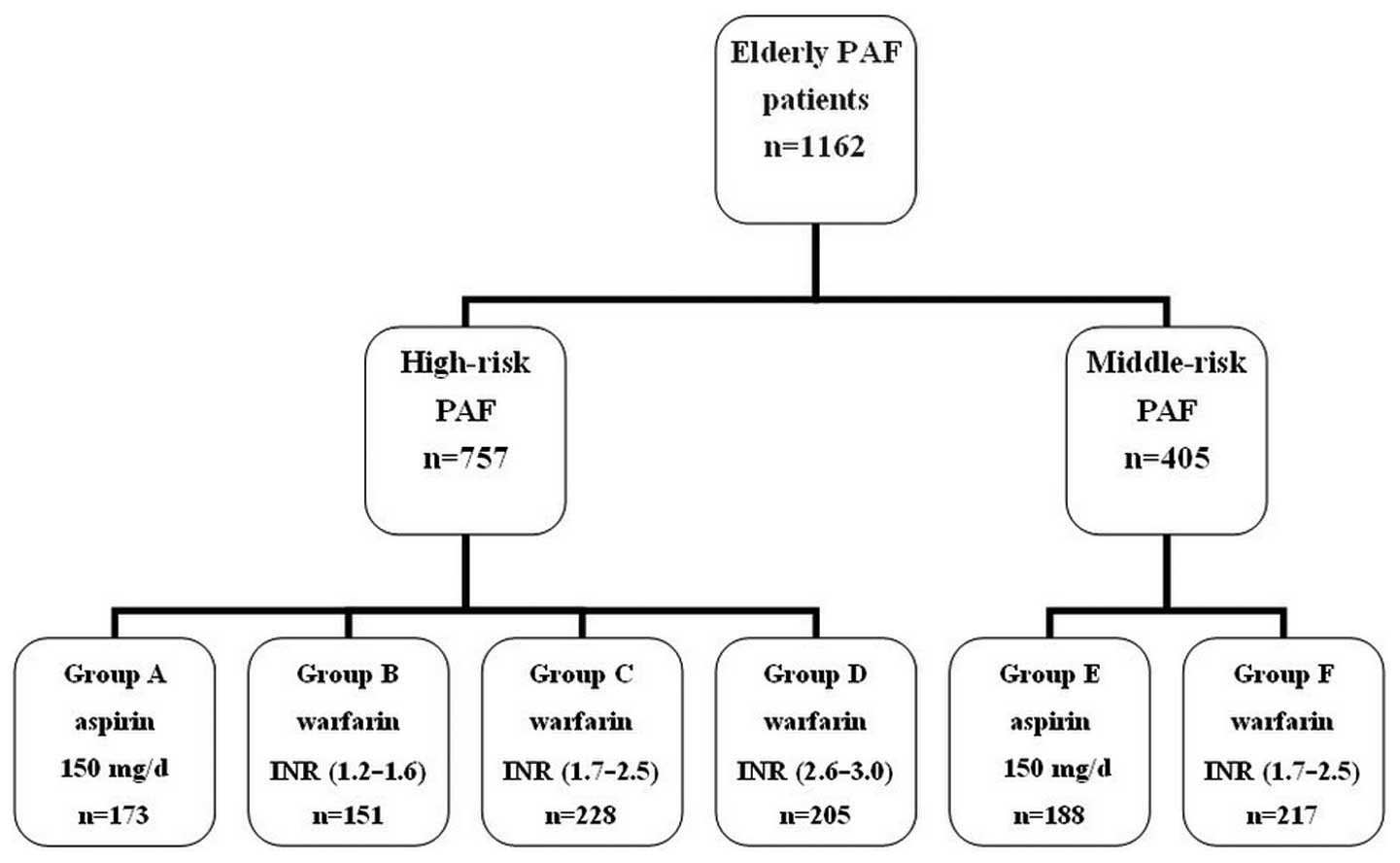
Chicago, Il, USA) into four high-risk groups and two middle-risk
groups; Group A: high-risk, administered 150 mg/day aspirin; Group
B: high-risk, administered 1.875 mg/day warfarin, then the dose was
adjusted to maintain a target INR range of 1.2–1.6; Group C:
high-risk, administered 2.5 mg/day warfarin, then the dose was
adjusted to a target INR range of 1.7–2.5; Group D: high-risk,
administered 2.5 mg/day warfarin, then dose was adjusted to a
target INR range of 2.6–3.0. The two middle-risk groups were Group
E: administered 150 mg/day aspirin; and Group F: administered 2.5
mg/day warfarin, then dose was adjusted to a target INR range of
1.7–2.5 (Fig. 1). Prior to
enrollment in this study, the target INR range was achieved in the
groups administered with warfarin (Groups B, C, D and F).
Patient follow-up
Patients were seen in the institutional outpatient
clinics one, two, three and six months following treatment, then
follow-up every 3–6 months and then as required. Routine tests
including prothrombin time and INR were performed. In addition,
various events were recorded including; primary endpoint events
(death, stroke, pulmonary embolism) and secondary endpoint events
[acute myocardial infarction, lacunar infarction, transient
ischemic attack (TIA), asymptomatic stroke and peripheral arterial
embolism], major bleeding events (cerebral hemorrhage,
gastrointestinal bleeding) and minor bleeding events (skin, mucous
membrane and gums bleeding, hematuria). In addition, the total
primary events (primary endpoint events and major bleeding events),
total secondary events (secondary endpoint events and minor
bleeding events) and total events (total primary events and total
secondary events) were calculated. All the events were evaluated
synergistically by a cardiologist and neurologist.
Statistical analysis
Statistical analysis of the data was performed on
SPSS software, version 13.0 (SPSS Inc, Chicago, Il, USA). All means
were expressed with SD for continuous variables when normally
distributed and comparisons were evalutated using the Student’s
t-test or Mann-Whitney U-test, as appropriate. Categorical
variables were expressed as numbers (with %), differences between
groups were analysed using Pearson’s χ2 test or Fisher’s
exact test. P<0.05 is considered to indicate a statistically
significant difference.
Results
Demographic and clinical
characteristics
The demographic and clinical characteristics of the
patients in this study were identical in the high- or middle-risk
groups (Table I). The total
patients at the end of the study was 1,472. There were 310 invalid
cases as they had met one of the exclusion criteria including;
<6 months treatment duration; patients were unable to follow the
instructions, refused to continue the drugs, or did not monitor the
INR regularly; a new onset of other diseases was indicated during
the follow-up period, including rheumatic heart disease, idiopathic
cardiomyopathy, encephalitis, hyperthyroidism, cancer, blood
diseases or the patient needed surgery; and complication of severe
liver or renal dysfunction during the follow-up period. The total
1,162 cases (females; 458) were regarded as valid cases, the mean
(SD) age of these patients was 72.5±4.4 years (range; 65–78 years).
Between the four high-risk groups (A, B, C and D), or between the
two middle-risk groups (E and F), age, sex distribution and smoking
status were approximately identical. There were no significant
differences in the history of hypertension, diabetes,
hyperlipidemia, stroke, TIA, myocardial infarction, peripheral
vascular thrombosis, pulmonary embolism and other diseases. The use
of ACEI/ARB and β-blockers were similar between Groups A, B, C and
D, or Groups E and F. The incidence of LVEF<35%, follow-up time
and INR value prior to treatment also indicated no significant
differences (P>0.05).
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
| Variable (n) | Group A (n=173) | Group B (n=151) | Group C (n=228) | Group D (n=205) | Group E (n=188) | Group F (n=217) |
|---|
| Age (years) | 72.4±4.9 | 73.1±4.7 | 72.8±4.5 | 72.2±4.9 | 72.8±4.5 | 71.9±4.3 |
| Male (%) | 108 (62.4) | 92 (60.9) | 141 (61.8) | 123 (60.0) | 114 (60. 6) | 126 (58.1) |
| Smoking (%) | 65 (37.6) | 53 (35.1) | 85 (37.3) | 73 (35.6) | 67 (35.6) | 80 (36.8) |
| Hypertension (%) | 72 (41.6) | 61 (40.4) | 91 (39) | 82 (40.0) | 75 (39.9) | 86 (39.6) |
| Diabetes (%) | 65 (37.6) | 57 (37.7) | 83 (36.4) | 75 (36.6) | 69 (36.7) | 83 (38.2) |
| Hyperlipidemia
(%) | 51 (29.5) | 45 (29.8) | 66 (28.9) | 60 (29.3) | 54 (28.7) | 63 (29.0) |
| Prior stroke (%) | 38 (21.9) | 34 (22.5) | 49 (21.5) | 44 (21.5) | 0 | 0 |
| Prior TIA (%) | 25 (14.5) | 21 (13.9) | 32 (14.0) | 29 (14.1) | 0 | 0 |
| Prior AMI (%) | 16 (9.2) | 12 (8.0) | 20 (8.8) | 18 (8.8) | 10 (5.3) | 12 (5.5) |
| LVEF<35% (%) | 18 (10.4) | 14 (9.3) | 23 (10.1) | 20 (9.8) | 0 | 0 |
| Prior peripheral
vascular thrombosis | 6 (3.5) | 5 (3.3) | 8 (3.5) | 7 (3.4) | 0 | 0 |
| Prior pulmonary
embolism (%) | 10 (5.8) | 9 (5.9) | 13 (5.7) | 11 (5.4) | 0 | 0 |
| ACEI/ARB (%) | 102 (59.0) | 90 (59.6) | 134 (58.8) | 124 (60.5) | 110 (58.5) | 126 (56.7) |
| β-blockers (%) | 68 (39.3) | 60 (39.7) | 88 (38.6) | 80 (39.0) | 74 (39.4) | 85 (39.2) |
| Follow-up
(months) | 50.7±13.8 | 51.3±12.8 | 51.6±13.8 | 51.3±12.9 | 50.7±11.6 | 51.4±12.2 |
| INR prior to
treatment | 0.86±0.12 | 0.88±0.15 | 0.84±0.17 | 0.87±0.12 | 0.81±0.11 | 0.79±0.10 |
Comparison of events in high-risk
patients
In high-risk patients, the primary and secondary
endpoint events in Groups C and D were significantly lower
(P<0.05) compared with those in Groups A and B (Table II). However, there were no
significant differences between Groups A and B or between Groups C
and D (P>0.05). The major and minor bleeding events among the
four groups (A, B, C and D) indicated no significant differences
(P>0.05). However, Groups C and D indicated a higher number of
major bleeding events compared with those in Groups A and B. In
addition, the total primary events in Group C were significantly
lower (P<0.05) compared with those in Groups A and B
(P<0.05). However, there were no significant differences between
Groups A, B, D or between Groups C and D (P>0.05). Total
secondary events in Group C were significantly lower than those in
Group B (P<0.05); however, there were no significant differences
between Groups A, B and D or between Groups C, A and D (P>0.05).
The total events in Group C were significantly lower than those in
Groups A and B (P<0.05); however, there were no significant
differences between Groups A, B and D or between Groups C and D
(P>0.05).
 | Table II.Comparison of events in high-risk
patients. |
Table II.
Comparison of events in high-risk
patients.
| Variable (n) | Group A (n=173) | Group B (n=151) | Group C (n=228) | Group D (n=205) |
|---|
| Primary endpoint
events | 19 | 14 | 9a,b | 8a,b |
| Death | 6 | 5 | 3 | 4 |
| Ischemic
stroke | 10 | 8 | 4 | 2 |
| Pulmonary
embolism | 3 | 3 | 2 | 1 |
| Secondary endpoint
events | 30 | 25 | 19a,b | 16a,b |
| Acute myocardial
infarction | 3 | 4 | 4 | 4 |
| Lacunar
infarction | 8 | 6 | 5 | 3 |
| TIA | 7 | 6 | 4 | 3 |
| Peripheral arterial
embolism | 6 | 4 | 2 | 3 |
| Asymptomatic
stroke | 6 | 5 | 4 | 3 |
| Major bleeding
events | 5 | 5 | 7 | 13 |
| Cerebral
hemorrhage | 2 | 3 | 4 | 9 |
| Gastrointestinal
bleeding | 3 | 3 | 3 | 4 |
| Minor bleeding
events | 11 | 14 | 20 | 24 |
| Hematuria | 5 | 6 | 9 | 9 |
| Skin, mucous
membrane and gums bleeding | 6 | 8 | 11 | 15 |
| Total primary
events | 24 | 20 | 16a,b | 21 |
| Total secondary
events | 41 | 39 | 39b | 40 |
| Total events | 65 | 56 | 55a,b | 61 |
Comparison of aspirin with warfarin in
high-risk or middle-risk patients
In high-risk patients with PAF, warfarin
significantly reduced the primary and secondary endpoint events,
total primary events and total secondary events (P<0.05)
compared with aspirin, whereas major and minor bleeding events were
not significantly different (P>0.05; Table III). In middle-risk patients with
PAF, there were no significant differences in any events between
the aspirin or warfarin groups (Table
IV).
 | Table III.Comparison of aspirin (Group A) with
warfarin (Group C) in high-risk patients. |
Table III.
Comparison of aspirin (Group A) with
warfarin (Group C) in high-risk patients.
| Variable (n) | Group A
(n=173) | Group C
(n=228) | χ2 | P-value |
|---|
| Primary endpoint
event | 19 | 9 | 7.496 | 0.006a |
| Death | 6 | 3 |
| Ischemic
stroke | 10 | 4 |
| Pulmonary
embolism | 3 | 2 |
| Secondary end
points | 30 | 19 | 7.441 | 0.006a |
| Acute myocardial
infarction | 3 | 4 |
| Lacunar
infarction | 8 | 5 |
| TIA | 7 | 4 |
| Peripheral
arterial embolism | 6 | 2 |
| Asymptomatic
stroke | 6 | 4 |
| Major bleeding
event | 5 | 7 | 0.011 | 0.917 |
| Cerebral
hemorrhage | 2 | 4 |
| Gastrointestinal
tract bleeding | 3 | 3 |
| Minor bleeding | 11 | 20 | 0.803 | 0.370 |
| Hematuria | 5 | 9 |
| Skin and mucous
membrane bleeding gums | 6 | 11 |
| Total primary
events | 24 | 16 | 5.148 | 0.023a |
| Total secondary
events | 41 | 39 | 2.678 | 0.102 |
| Total events | 65 | 55 | 8.485 | 0.004a |
 | Table IV.Comparison of aspirin (Group E) with
warfarin (Group F) in middle-risk patients. |
Table IV.
Comparison of aspirin (Group E) with
warfarin (Group F) in middle-risk patients.
| Variable (n) | Group E (188) | Group F (217) | χ2 | P-value |
|---|
| Primary endpoint
event | 14 | 7 | 3.651 | 0.056 |
| Death | 4 | 3 |
| Ischemic
stroke | 7 | 3 |
| Pulmonary
embolism | 3 | 1 |
| Secondary end
points | 19 | 11 | 3.727 | 0.054 |
| Acute myocardial
infarction | 3 | 3 |
| Lacunar
infarction | 7 | 3 |
| TIA | 4 | 2 |
| Peripheral
arterial embolism | 1 | 1 |
| Asymptomatic
stroke | 4 | 2 |
| Major bleeding
event | 3 | 5 | 0.261 | 0.069 |
| Cerebral
hemorrhage | 1 | 3 |
| Gastrointestinal
tract bleeding | 2 | 2 |
| Minor bleeding | 8 | 12 | 0.349 | 0.555 |
| Hematuria | 4 | 6 |
| Skin and mucous
membrane bleeding gums | 4 | 6 |
| Total primary
events | 17 | 12 | 1.87 | 0.172 |
| Total secondary
events | 27 | 23 | 1.318 | 0.251 |
| Total events | 44 | 35 | 3.396 | 0.065 |
Discussion
AF is frequently divided into three categories: PAF,
persistent AF and chronic AF (8).
Chronic AF indicates the long-term presence of symptoms (seven
days-years), and is also known as permanent AF. PAF is a recurrent
AF and commonly lasts <7 days and may be inhibited without
treatment for the majority of cases. If AF continues >48 h (∼7
days), known as persistent AF, the patient is unlikely to revert
back to normal without treatment, and may regain a normal rhythm
with cardioversion (9).
At present, it has not been concluded whether
patients with PAF have a similar risk factor for developing
ischemic stroke as patients with chronic AF. The Framingham Heart
Study (10) demonstrated a
5.6-fold increased risk for the development of embolism in patients
with non-rheumatic AF when compared with controls. Non-rheumatic AF
is thought to be responsible for a large percentage of strokes, as
it is present in ∼15–20% of cerebrovascular accidents of ischemic
origin. In addition, the risk of thromboembolic events in patients
with non-rheumatic AF was ∼5% per year. Previously, it has been
determined that PAF and persistent AF may have a similar risk for
developing ischemic stroke. A study has indicated that chronic AF
carries a risk of 6% per year for the development of thromboembolic
events, this is higher than the 2–3% risk per year in paroxysmal AF
(11). A meta-analysis of five
randomized controlled trials demonstrated that type (paroxysmal or
chronic) and duration of AF has no significant effect on the
incidence of a stroke. The risk of embolism is higher immediately
following the onset of AF, during the first year of chronic AF or
following the early conversion to a sinus rhythm (1). Elderly patients with AF that were
more likely to suffer cerebrovascular accidents, represented 6.7%
of the total number in the 50-to-59-year-old group and 36.2% in the
80-to-89-year-old group (12).
It is important to study antithrombotic therapy in
elderly patients due to the diversity of individuals. According to
ACC/AHA/ESC 2006 Guidelines for the Management of Patients with AF
(7), the dose of oral warfarin may
be adjusted to maintain the target INR 2.0–3.0; however, the
guidelines are based on the American and European population. In
comparison, the Asian populations tend to have lower coagulation
activation, and it has been suggested they require a lower
intensity of anticoagulation therapy (6).
The present study lasted for >5 years and it was
a single-blind, randomized and multicentered study. Elderly
patients (n=1,472) with PAF who demonstrated a middle-high risk of
having a stroke, were used in this study. From the 1,472 cases, 310
patients were invalid due to various reasons, leaving 1,162 cases
which were analyzed. In this study, the total primary events
(primary endpoint event and major bleeding events) was the major
indicator for optimal antithrombotic therapy (aspirin or warfarin).
In Group C (high-risk patients, 2.5 mg/day warfarin then the dosage
was adjusted to a target INR 1.7–2.5) the primary and secondary
endpoint events, total primary events and total events were all
significantly lower compared with Group A (high-risk, administered
150 mg/day aspirin) and Group B (high-risk, administered 1.875
mg/day warfarin then the dose was adjusted to a target INR 1.2–1.6;
P<0.05). In addition, the primary and secondary endpoint events
were significantly lower in Group D (high-risk, administered 2.5
mg/day warfarin then the dose was adjusted to a target INR 2.6–3.0)
compared with Groups A and B (P<0.05). In Group C, the total
primary events (the major indicator for the optimal antithrombotic
therapy) was significantly less than those in Groups A and B.
However, no significant difference was identified in Group D,
possibly due to a high number of major bleeding events (13 cases).
Therefore, the increased anticoagulation (INR, 2.6–3.0) in Group D
did not benefit the patients. Furthermore, there were no
significant differences between Groups A and B in any event, which
indicated that low intensity anticoagulation (warfarin, INR
1.2–1.6) had no increased benefit compared with aspirin. This study
concluded that the optimal INR range is 1.7–2.5 for Chinese elderly
patients with PAF.
Hylek et al confirmed that anticoagulant
prophylaxis of ischemic stroke is effective at INRs of ≥2.0, in
patients with AF (13). The risk
of a stroke increased steeply at INRs of <2.0. At a decreasing
INR, the adjusted odds ratio determining the risk factor of a
stroke increased; INR of 1.7, ratio of 2.0; INR of 1.5, ratio of
3.3 and INR of 1.3, ratio of 6.0. This is in accordance with the
results of the present study. A prospective multicentered,
randomized, Japanese non-valvular AF study (6) showed that a high concentration of
warfarin anticoagulation treatment (INR 2.2–3.5) significantly
increased bleeding events compared with a lower intensity of
warfarin (INR 1.5–2.1). This indicated that blood coagulation in
Asian individuals is lower compared with European and American
individuals. A further study (14)
in China also demonstrated that in elderly patients with PAF, the
anticoagulation intensity of INR 1.7–2.5 is safe and effective, a
finding that is supported by the results from the present
study.
The present study demonstrated that in high-risk
patients with PAF, warfarin reduced a larger number of primary and
secondary endpoint events, total primary events and total events
when compared with aspirin. In middle-risk patients with PAF, Group
F (warfarin) demonstrated a higher number of secondary endpoint
events when compared with Group E (aspirin); however, these were
not the major indicators. Therefore, in clinical practice, it is
necessary to analyze elderly patients with PAF based on their risk
of a stroke. This study concluded that warfarin may be used in
high-risk elderly patients with a target INR of 1.7–2.5. However,
in patients at middle-risk of a stroke, aspirin is the preferred
treatment of PAF and the use of warfarin is limited.
References
|
1.
|
Albers GW, Dalen JE, Laupacis A, Manning
WJ, Petersen P and Singer DE: Antithrombotic therapy in atrial
fibrillation. Chest. 119:S194–S206. 2001. View Article : Google Scholar
|
|
2.
|
No authors listed:. Risk factors for
stroke and efficacy of anti-thrombotic therapy in atrial
fibrillation. Analysis of pooled data from five randomized
controlled trials. Arch Intern Med. 154:1449–1457. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
No authors listed:. The effect of low-dose
warfarin on the risk of stroke in patients with nonrheumatic atrial
fibrillation. The Boston Area Anticoagulation Trial for Atrial
Fibrillation Investigators. N Engl J Med. 323:1505–1511. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Aguilar MI and Hart R: Oral anticoagulants
for preventing stroke in patients with non-valvular atrial
fibrillation and no previous history of stroke or transient
ischemic attacks. Cochrane Database Syst Rev. 20:CD0019272005.
|
|
5.
|
Connolly SJ, Laupacis A, Gent M, Roberts
RS, Cairns JA and Joyner C: Canadian Atrial Fibrillation
Anticoagulation (CAFA) study. J Am Coll Cardiol. 18:349–355. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yamaguchi T: Optimal intensity of warfarin
therapy for secondary prevention of stroke in patients with
nonvalvular atrial fibrillation: a multicenter, prospective,
randomized trial. Japanese Nonvalvular Atrial Fibrillation-Embolism
Secondary Prevention Cooperative Study Group. Stroke. 31:817–821.
2000.
|
|
7.
|
Fuster V, Rydén LE, Cannom DS, et al:
ACC/AHA/ESC 2006 Guidelines for the Management of Patients with
Atrial Fibrillation: a report of the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines and the European Society of Cardiology Committee for
Practice Guidelines (Writing Committee to Revise the 2001
Guidelines for the Management of Patients With Atrial
Fibrillation): developed in collaboration with the European Heart
Rhythm Association and the Heart Rhythm Society. Circulation.
114:e257–e354. 2006.
|
|
8.
|
Lévy S, Breithardt G, Campbell RW, et al:
Atrial fibrillation: current knowledge and recommendations for
management. Working Group on Arrhythmias of the European Society of
Cardiology. Eur Heart J. 19:1294–1320. 1998.PubMed/NCBI
|
|
9.
|
Bajorek BV, Ogle SJ, Duguid MJ, Shenfield
GM and Krass I: Management of warfarin in atrial fibrillation:
views of health professionals, older patients and their carers. Med
J Aust. 186:175–180. 2007.PubMed/NCBI
|
|
10.
|
Kannel WB, Wolf PA, Benjamin EJ and Levy
D: Prevalence, incidence, prognosis, and predisposing conditions
for atrial fibrillation: population-based estimates. Am J Cardiol.
82:2N–9N. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Petersen P and Godtferdsen J: Embolic
complications in paroxysmal atrial fibrillation. Stroke.
17:622–626. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wolf PA, Abbott RD and Kannel WB: Atrial
fibrillation: a major contributor to stroke in the elderly. The
Framingham Study. Arch Interm Med. 147:1561–1564. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hylek EM, Skates SJ, Sheehan MA and Singer
DE: An analysis of the lowest effective intensity of prophylactic
anticoagulation for patients with nonrheumatic atrial fibrillation.
N Engl J Med. 335:540–546. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chen XJ, Zheng RL, Zhang H, et al:
Clinical study of prevention thrombus on paroxysmal atrial
fibrillation patients with medium and high stroke risk. Chin J
Gerontology. 31:1126–1128. 2011.(In Chinese).
|