Introduction
Acute myeloid leukemia (AML) is a heterogeneous
malignancy characterized by the rapid growth of immature myeloid
cells that undergo a differentiation block, resulting in an
accumulation of leukemia cells in the bone marrow and the
inhibition of normal hematopoiesis (1). Although intensive chemotherapy
induces complete remission in the majority of patients with AML, a
number of patients eventually relapse. The optimum strategy at the
time of relapse or for patients with the refractory disease remains
uncertain. Therefore, novel therapeutic approaches are required to
improve the outcome of patients with AML. Microtubules are highly
dynamic structures that are important in the maintenance of cell
shape and organization of the mitotic spindle, which is necessary
for mitosis (2).
Microtubule-targeted agents (MTAs) are classified as destabilizing
and stabilizing agents according to their binding site on tubulin
or microtubules. MTAs exert a high cytotoxic efficiency, and also
cause anti-angiogenic and vascular-disruptive effects (2,3).
When utilized as anticancer agents, MTAs generally cause cell cycle
arrest and apoptosis, as a consequence of caspase activation
through the intrinsic apoptotic pathway. Similar effects have been
observed in leukemia cells; certain MTAs, including PBOX-15
(4), CA4P (5) and PBOX-6 (6), displayed cytotoxic efficiency in
various leukemia cell types through the inhibition of cellular
proliferation. In addition, it has been demonstrated that CA4P, a
novel tubulin-destabilizing agent, reduced the interaction of
leukemia cells with neovessels by downregulating the expression of
the adhesion molecule VCAM-1, thereby increasing leukemia cell
death (5). Therefore, disrupting
the microtubule function in leukemia cells is a potentially
promising method for overcoming drug resistance in AML cells.
CYT997, a structurally novel orally active MTA, has
been shown to inhibit tubulin polymerization and disrupt cellular
microtubules (7). In addition,
CYT997 has demonstrated potent cytotoxic activity against tumor
cells, including hematopoietic malignancies in vitro; its
effects are associated with the induction of apoptosis and cell
cycle arrest at the G2/M phase (8–10).
Notably, CYT997 also causes extensive ablation of the tumor
vasculature, and thus inhibits the enlargement of tumors (10). In phase I clinical trials, the
efficacy and safety of the drug has been investigated in patients
with solid malignant tumors that were refractory to standard
treatment (11,12). Eighteen patients (82%) that were
treated with CYT997 for >2 cycles demonstrated a stable disease
(11), indicating that CYT997 may
be an important agent for the treatment of patients with refractory
disease or may be used in combination with other anticancer
therapies. In the present study, the in vitro effect of
CYT997 on human AML cell lines was investigated. The cytotoxic
mechanisms of CYT997 in leukemia cells were also investigated, with
a particular focus on its effect on the cyclin-dependent kinase
(cdc2) pathway, which regulates the entry of cells into mitosis,
and the inhibition of PI3K/Akt/mTOR pathway proteins.
Methods and materials
Cell culture and reagents
Cell culture reagents, including RPMI-1640 and fetal
bovine serum (FBS), were purchased from Gibco (Grand Island, NY,
USA). z-IETD-FMK was obtained from BioVision (Palo Alto, CA, USA).
Anti-CD123 antibody was purchased from BioLegend (San Diego, CA,
USA) and CYT997 was obtained from Selleck (Houston, TX, USA).
Methylcellulose and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). All the antibodies
used in the western blot analysis were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). The human AML cell
lines, K562, HL-60, KG-1, THP-1, Kasumi-1 and HEL, were obtained
from the Institute of Hematology, Zhejiang University (Hangzhou,
China). All leukemia cell lines were maintained in RPMI-1640 medium
supplemented with 10% FBS at 37°C in a humidified atmosphere of 5%
CO2.
Cell viability assay
Cells were cultured at a density of 5×104
cells/well in a 96-well plate and treated with CYT997 at
concentrations of 12.5, 25, 50, 100 and 200 nM. Following 24 and 48
h of incubation, the medium was removed and fresh medium containing
MTT was added to each well. This was followed by the addition of
200 μl dimethyl sulfoxide (Amresco LLC, Solon, OH, USA), and
10 min of oscillation to dissolve the formazan crystals following 4
h of culture at 37°C. Subsequently, the mixture underwent
centrifugation at 600 × g for 5 min, and the supernatant was
discarded. The absorbance at 570 nm (A570) was measured using an
enzyme-linked immunosorbent assay plate reader (Bio-Rad, Hercules,
CA, USA).
Annexin V binding assay
Cells were seeded in a 6-well plate and treated with
CYT997 at concentrations of 0, 50, 100 and 200 nM. Following 24 h
of treatment at 37°C, the cells were trypsinized and washed.
Aliquots of the cells were resuspended in binding buffer and
stained with 5 μl Annexin V and 5 μl propidium iodide
(PI; Biouniquer, Nanjing, China) according to the manufacturer’s
instructions. A fluorescence-activated cell-sorting (FACS) assay
was performed immediately following the staining. To measure
apoptosis in CD123+ cells, KG-1 cells were gated for
CD123 expression, then the CD123+ cell subset was
analyzed for positivity to Annexin V by flow cytometry following 24
or 48 h of treatment with CYT997 (100 nM).
Cell cycle analysis
Following trypsinization, the cells were washed with
phosphate-buffered saline (PBS) and subsequently fixed in 85%
ethanol. Following fixation, the cells were washed with PBS/1%
fetal calf serum (FCS), resuspended in PBS/1% FCS containing 10
μg/ml PI and 250 μg/ml RNase A, and incubated for 30
min at 37°C. Samples were tested using a FACSCalibur machine and
CellQuest software (Becton-Dickinson, Mountain View, CA, USA).
Leukemia colony-forming assay
The CYT997-treated cells were seeded in
methylcellulose medium and incubated at 37°C in a humidified
atmosphere with 5% CO2. Following 7 days of incubation,
the number of leukemia colony-forming units (CFU-Ls) that contained
>40 cells were scored manually under a light microscope
(Olympus, Tokyo, Japan).
Western blot analysis
Following treatment, cells were collected and lysed
using 10 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 10
mM KCl, and 0.3% Triton (pH 7.9). The concentration of the protein
samples was measured by the Bradford method. The protein samples
were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then electroblotted onto
Hybond-polyvinylidene fluoride (PVDF) membranes. The membranes were
subjected to western blot analysis with primary antibodies to
caspase-8, -9 and -3, poly ADP-ribose polymerase (PARP), Bid,
phosphoinositide 3-kinase (PI3K) class III, Akt, phospho (p)-Akt,
mechanistic target of rapamycin (mTOR), p-mTOR, p65, p-p65, cdc2,
p-cdc2, cdc25c, p-cdc25c and β-actin.
Statistical analysis
The experimental results are presented as the mean ±
standard deviation. Statistical analysis was performed by the
unpaired Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of CYT997 on the viability of
human AML cell lines
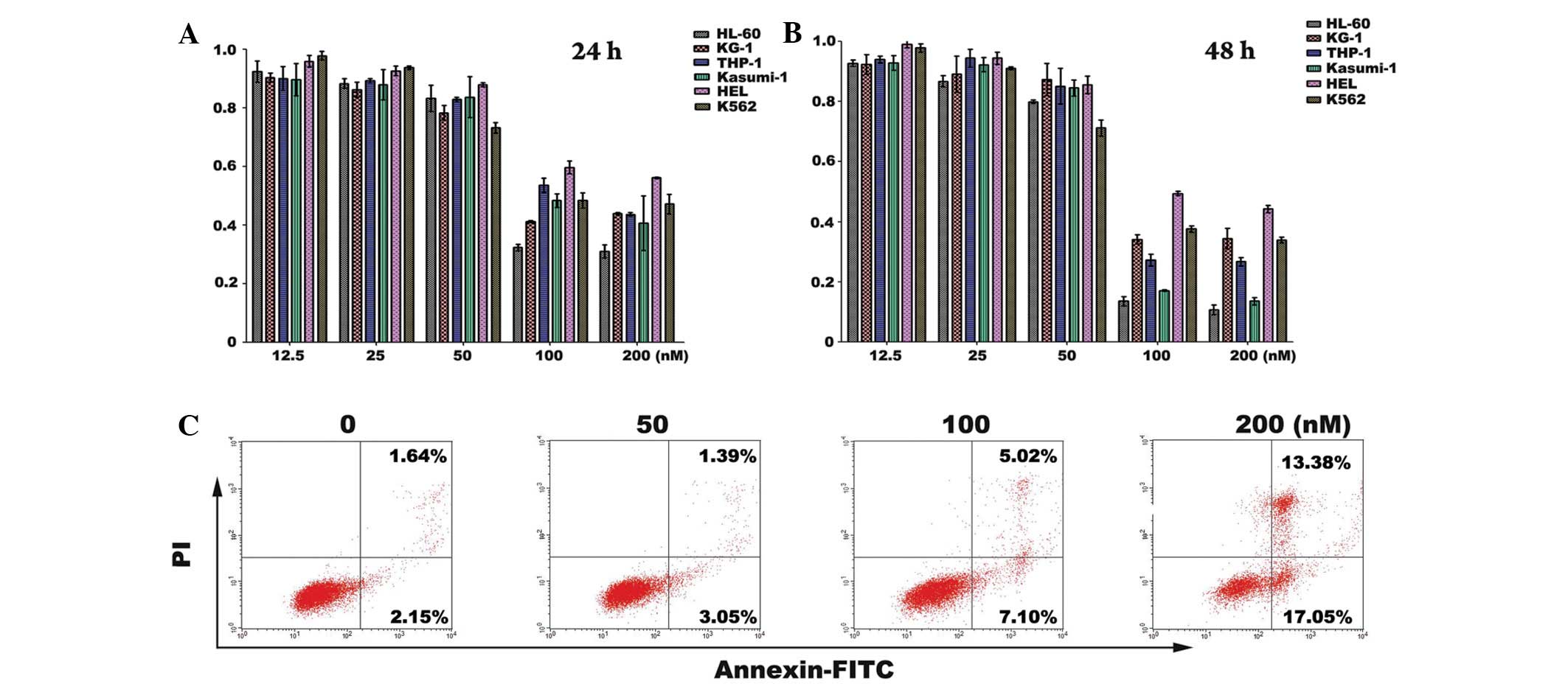
A panel of human AML cell lines and K562 cells was
tested for sensitivity to CYT997 in a cell proliferation assay
(Figs. 1A and B). Variability
between the cell lines in sensitivity to CYT997 was indicated,
regardless of the fact that cell growth inhibition occurred in a
concentration- and time-dependent manner in all tested cell lines.
Two cell lines, HL-60 and Kasumi-1, were particularly sensitive to
the effects of CYT997. The IC50 values of CYT997 in
HL-60, KG-1, THP-1, Kasumi-1 and HEL cell lines at 48 h were 60.75,
111.38, 96.06, 71.43 and 134.33 nM, respectively.
Treatment with CYT997 resulted in the
induction of apoptosis via activation of the caspase pathway
Annexin V versus PI staining was used to assess the
apoptotic status of HL-60 cells treated with increasing
concentrations of CYT997. Leukemia cells treated with the drug
exhibited a robust increase in apoptotic events, and the maximum
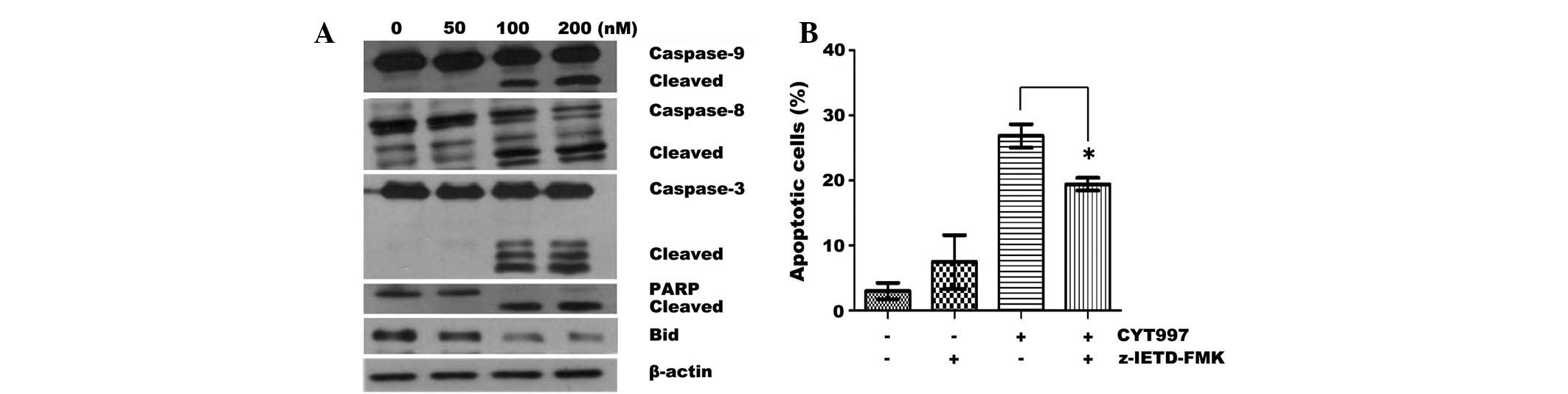
number of apoptotic events were observed at 200 nM CYT997 (Fig. 1C). Western blot analysis was
conducted to measure the activation of the caspase pathway. CYT997
triggered the concentration-dependent cleavage of caspase-8, -9 and
-3, followed by cleavage of PARP (Fig.
2A). Moreover, treatment with CYT997 resulted in a reduction in
the expression of the proapoptotic Bcl-2-related protein, Bid. This
suggested that the Bid protein was truncated and thus may have
resulted in the induction of the intrinsic pathway. As the
effectiveness of MTAs is largely considered to be a consequence of
caspase activation through the intrinsic apoptotic pathway
(3), this study investigated the
effect of the z-IETD-FMK caspase-8 inhibitor on CYT997-induced
apoptosis. z-IETD-FMK (8 μM) partially inhibited
CYT997-induced apoptosis (Fig.
2B). These results suggest that treatment with CYT997 activates
the cascades to the caspase-8 and -9 pathways in AML cells.
CYT997 induced cell death in AML stem and
progenitor cells
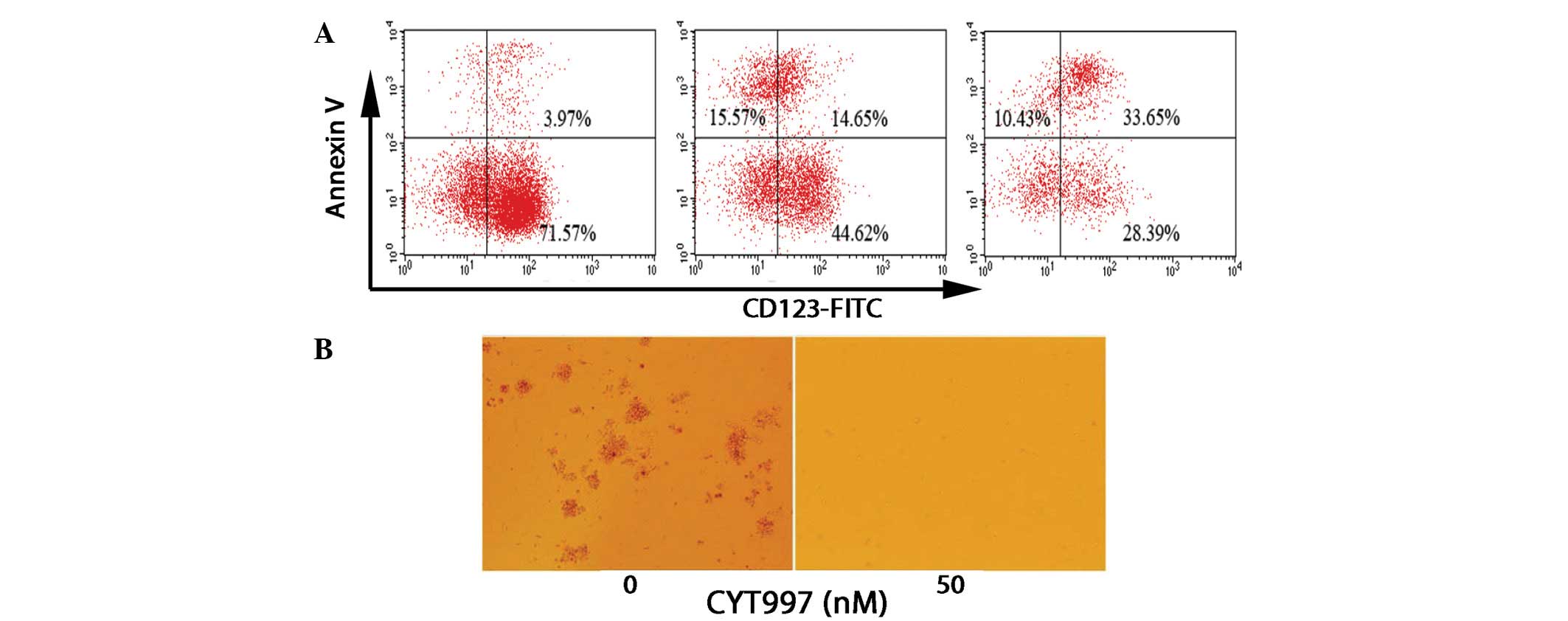
The interleukin-3 receptor α chain (CD123) has been
demonstrated to be strongly expressed in AML stem cells, but not in
human normal hematopoietic stem cells (13). The present study investigated
whether CYT997 may induce apoptosis in leukemia progenitor cells.
Following electronic gating on the CD123+ subpopulation,
the cells were analyzed for positivity to Annexin V staining. KG-1
cells treated with CYT997 for 24 and 48 h demonstrated a
time-dependent increase in the frequency of Annexin V+
and CD123+ cells, while the percentage of Annexin
V− and CD123+ cells significantly decreased
(Fig. 3A). In the HL60-derived
leukemia progenitor colony formation assays, CYT997 significantly
reduced the colony formation ability (Fig. 3B). Collectively, the results
suggest that CYT997 has cytotoxic activity against leukemia stem
and progenitor cells.
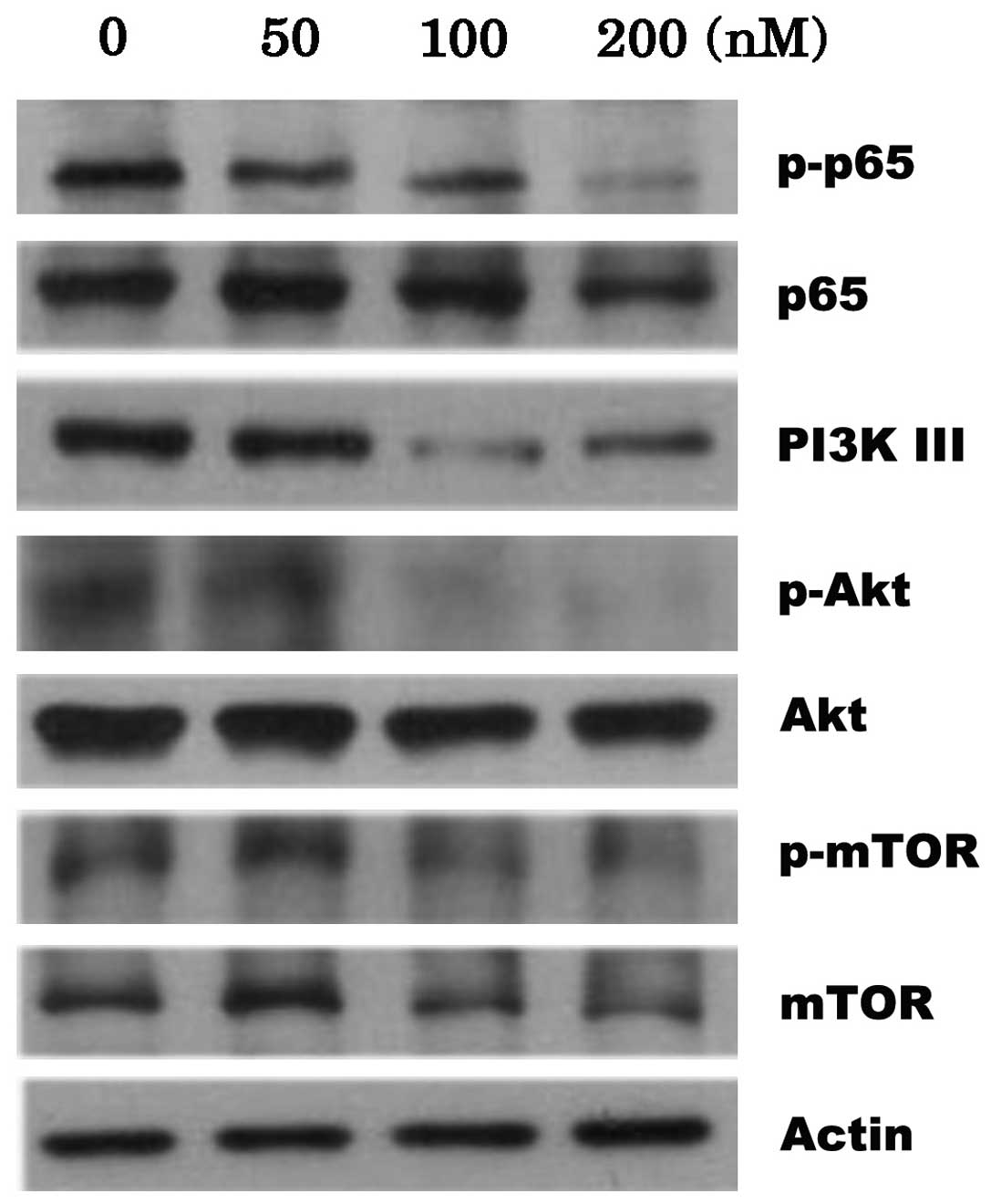
CYT997 inhibited PI3K/Akt/mTOR
signaling
The activation of the nuclear factor κB (NF-κB)
pathway and the PI3K/AKT/mTOR axis has been shown to promote AML
cell proliferation and contribute to drug resistance (14). Therefore, the present study
investigated the mechanisms of CYT997, by studying possible
alterations in the levels of proteins in the PI3K/Akt/mTOR pathway.
The results indicated that CYT997 concentration-dependently reduced
the phosphorylation of p65 and p-mTOR, accompanied by slight
degradation of the proteins. Furthermore, treatment with CYT997
resulted in the downregulation of PI3K class III protein
expression. In addition, the phosphorylation of Akt in the HL-60
cells was completely inhibited by treatment with 100 and 200 nM
CYT997 (Fig. 4).
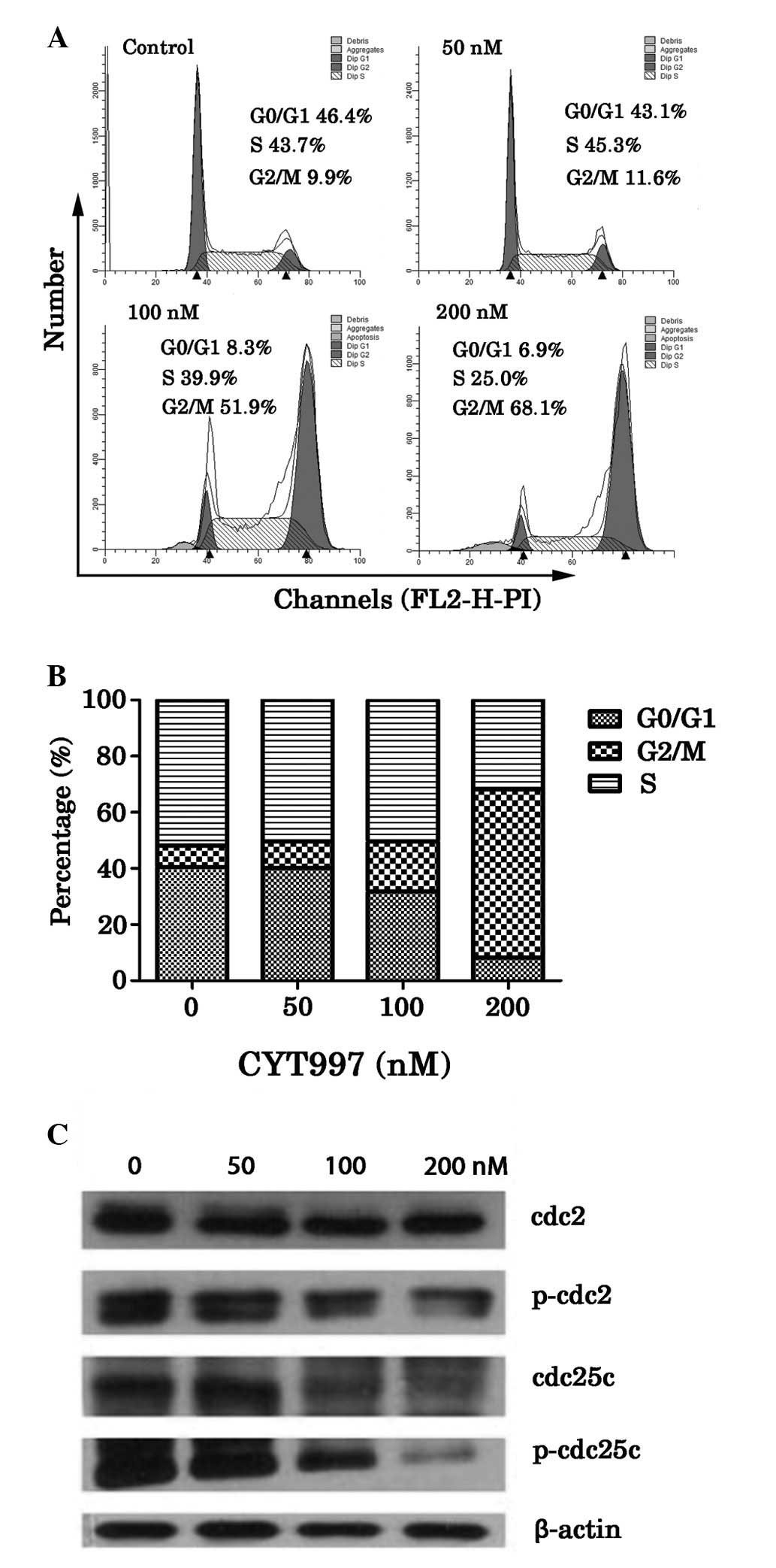
CYT997 induced G2/M arrest in HL-60 cells
via regulation of the expression of cell cycle regulatory
proteins
The present study determined the changes in the cell
cycle following the treatment of HL-60 cells with CYT997 for 24 h.
The percentage of cells in the G2/M phase increased following
treatment with CYT997 in a concentration-dependent manner; the
percentage increased from 9.9% in the absence of CT997 to 11.6,
51.9 and 68.1% in the presence of 50, 100 and 200 nM CT997,
respectively (Fig. 5). These
results suggest that CYT997 induced G2/M arrest in the AML cells,
which is consistent with previous results obtained using other MTAs
including PBOX-6, paclitaxel and vincristine (15). In the present study, the western
blot analysis identified that CYT997 downregulated the
phosphorylated form of cdc2, while the expression of cdc2 did not
change. CYT997 also mediated inhibition of the cell cycle protein
involved in the regulation of G2 to M phase transition, namely
cdc25C (Fig. 5C).
Discussion
Conventional antimicrotubule compounds have been
clinically used to treat patients with cancer; however, their
neurotoxicity and the emergence of acquired resistance limit the
success of therapy (16,17). There has been significant focus on
the discovery and development of novel, small, molecular
microtubule-targeted agents with a higher antitumor efficacy and
lower toxicity (8). It has been
demonstrated that PBOX compounds, a novel series of
microtubule-depolymerizing agents, are capable of inducing
apoptosis in drug-resistant HL60 cells expressing P-glycoprotein or
breast cancer resistance protein (BCRP) (15). Furthermore, CA4P, a
microtubule-destabilizing agent, not only inhibits leukemia cell
proliferation, but also causes a reduction in the interaction of
leukemia cells with neovessels, thereby augmenting leukemia cell
death in vivo (5). The
present study demonstrated that CYT997 induced the apoptosis and
inhibited the growth of AML cell lines including HL-60, KG-1,
THP-1, Kasumi-1 and HEL, with IC50 values of
60.75–134.33 nM at 48 h. These results are comparable with those
from a previous study where the IC50 values for various
solid tumor cell lines at 72 h were 9–101 nM (8). Notably, the present study showed that
treatment with 100 nM CYT997 for 24 and 48 h resulted in marked
levels of apoptosis in CD123+ KG-1 cells. Similar to
these results, the colony formation assay indicated a complete
cessation of leukemia colony formation at 50 nM in the HL-60 cells.
Therefore, the results demonstrated that CYT997 effectively induced
cytotoxicity against AML cells, and leukemia stem and progenitor
cells.
CYT997-induced apoptosis in the AML cell lines was
associated with the significant activation of caspase-3, -8 and -9.
Moreover, pretreatment with Z-IETD-FMK, a caspase-8 inhibitor,
partially inhibited the CYT997-induced apoptosis. These results
indicate that CYT997 induced apoptosis through activating the
caspase pathway, including the extrinsic and intrinsic apoptotic
pathways. The activation of Bid represents an important mechanism
accounting for cross-talk between the extrinsic and intrinsic
apoptotic pathways. In the current study, CYT997 significantly
downregulated Bid expression, suggesting that cleavage of Bid was
induced by the agent. Furthermore, it has been demonstrated that
the activation of the PI3K/Akt/mTOR axis is a common feature in
patients with AML, and inhibition of mTOR blocks the
phosphorylation of this kinase and results in cell death in
leukemia progenitor cells (14,18–20).
The present study demonstrated that CYT997 inhibited PI3K class III
protein expression and decreased the levels of p-65. CYT997 also
induced the downregulation of the expression levels of p-mTOR and
mTOR, and completely eliminated p-Akt. Previous studies have
identified that inhibition of the PI3K/Akt pathway by a specific
inhibitor failed to induce the apoptosis of AML cells, but
co-inhibition of the PI3K and mTOR pathways significantly induced
apoptosis (21,22). Therefore CYT997, which kills AML
cells by activation of the caspase pathway and dual inhibition of
the PI3K/Akt and mTOR pathways, may be useful for overcoming drug
resistance.
Due to their involvement in mitosis, disruption of
the function of microtubules results in the arrest of the cell
cycle at G2 phase, in which the cyclin-dependent kinase
(CDK1)/cyclin B complex is important for progression from the G2 to
the M phase (23). The activation
of the CDK1/cyclin B complex is maintained by cdc25 phosphatase.
Dephosphorylation of CDK1 is catalyzed by cdc25C (24). In the present study,
phosphorylation of CDK1, also known as cdc2, was remarkably
inhibited by CYT997. The results demonstrated that the levels of
cdc25C and p-cdc25C were reduced in cells treated with CYT997.
Therefore, CYT997 treatment induced a typical G2/M cell cycle
arrest in the AML cells by regulating CDK1 phosphorylation and
cdc25C expression.
In conclusion, the present study demonstrated that
CYT997 inhibited the proliferation of AML cells and induced
apoptosis through the activation of the extrinsic and intrinsic
apoptotic pathways. CYT997 induced cell death in CD123+
leukemia cells and reduced leukemia colony formation. Furthermore,
the drug exerted dual effects on the expression of PI3K/Akt and
mTOR signaling pathway proteins. These results suggested that
CTY997, used alone or in combination with chemotherapy, may
represent a promising approach for the treatment of AML.
Acknowledgements
This study was supported by funds from
the Hangzhou Science and Technology Bureau (grant no. 20120633B15),
the National Natural Science Foundation of China (grant no.
81200384), and the Funds of the Science and Technology Department
of Zhejiang Province (grant nos. 2012C13021-2 and 2012C37103).
References
|
1.
|
Steffen B, Müller-Tidow C, Schwäble J,
Berdel WE and Serve H: The molecular pathogenesis of acute myeloid
leukemia. Crit Rev Oncol Hematol. 56:195–221. 2005.PubMed/NCBI
|
|
2.
|
Kuppens IE: Current state of the art of
new tubulin inhibitors in the clinic. Curr Clin Pharmacol. 1:57–70.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rovini A, Savry A, Braguer D and Carré M:
Microtubule-targeted agents: when mitochondria become essential to
chemotherapy. Biochim Biophys Acta. 1807:679–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lysaght J, Verma NK, Maginn EN, et al: The
microtubule targeting agent PBOX-15 inhibits integrin-mediated cell
adhesion and induces apoptosis in acute lymphoblastic leukaemia
cells. Int J Oncol. 42:239–246. 2013.PubMed/NCBI
|
|
5.
|
Petit I, Karajannis MA, Vincent L, et al:
The microtubule-targeting agent CA4P regresses leukemic xenografts
by disrupting interaction with vascular cells and
mitochondrial-dependent cell death. Blood. 111:1951–1961. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Greene LM, Kelly L, Onnis V, et al:
STI-571 (imatinib mesylate) enhances the apoptotic efficacy of
pyrrolo-1,5-benzoxazepine-6, a novel microtubule-targeting agent,
in both STI-571-sensitive and -resistant Bcr-Abl-positive human
chronic myeloid leukemia cells. J Pharmacol Exp Ther. 321:288–297.
2007. View Article : Google Scholar
|
|
7.
|
Burns CJ, Harte MF, Bu X, et al: Discovery
of CYT997: a structurally novel orally active microtubule targeting
agent. Bioorg Med Chem Lett. 19:4639–4642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Burns CJ, Fantino E, Phillips ID, et al:
CYT997: a novel orally active tubulin polymerization inhibitor with
potent cytotoxic and vascular disrupting activity in vitro and in
vivo. Mol Cancer Ther. 8:3036–3045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Monaghan K, Khong T, Smith G and Spencer
A: CYT997 causes apoptosis in human multiple myeloma. Invest New
Drugs. 29:232–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Burns CJ, Fantino E, Powell AK, et al: The
microtubule depolymerizing agent CYT997 causes extensive ablation
of tumor vasculature in vivo. J Pharmacol Exp Ther. 339:799–806.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lickliter JD, Francesconi AB, Smith G, et
al: Phase I trial of CYT997, a novel cytotoxic and
vascular-disrupting agent. Br J Cancer. 103:597–606. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Burge M, Francesconi AB, Kotasek D, et al:
Phase I, pharmacokinetic and pharmacodynamic evaluation of CYT997,
an orally-bioavailable cytotoxic and vascular-disrupting agent.
Invest New Drugs. 31:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jordan CT, Upchurch D, Szilvassy SJ, et
al: The interleukin-3 receptor alpha chain is a unique marker for
human acute myelogenous leukemia stem cells. Leukemia.
14:1777–1784. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Xu Q, Simpson SE, Scialla TJ, Bagg A and
Carroll M: Survival of acute myeloid leukemia cells requires PI3
kinase activation. Blood. 102:972–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nathwani SM, Butler S, Fayne D, et al:
Novel microtubule-targeting agents, pyrrolo-1, 5-benzoxazepines,
induce apoptosis in multi-drug-resistant cancer cells. Cancer
Chemother Pharmacol. 66:585–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fojo AT, Ueda K, Slamon DJ, Poplack DG,
Gottesman MM and Pastan I: Expression of a multidrug-resistance
gene in human tumors and tissues. Proc Natl Acad Sci USA.
84:265–269. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Calligaris D, Verdier-Pinard P, Devred F,
Villard C, Braguer D and Lafitte D: Microtubule targeting agents:
from biophysics to proteomics. Cell Mol Life Sci. 67:1089–1104.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Martelli AM, Evangelisti C, Chiarini F and
McCubrey JA: The phosphatidylinositol 3-kinase/Akt/mTOR signaling
network as a therapeutic target in acute myelogenous leukemia
patients. Oncotarget. 1:89–103. 2010.PubMed/NCBI
|
|
19.
|
Meng H, Jin Y, Liu H, et al: SNS-032
inhibits mTORC1/mTORC2 activity in acute myeloid leukemia cells and
has synergistic activity with perifosine against Akt. J Hematol
Oncol. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Min YH, Eom JI, Cheong JW, et al:
Constitutive phosphorylation of Akt/PKB protein in acute myeloid
leukemia: its significance as a prognostic variable. Leukemia.
17:995–997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Récher C, Beyne-Rauzy O, Demur C, et al:
Antileukemic activity of rapamycin in acute myeloid leukemia.
Blood. 105:2527–2534. 2005.PubMed/NCBI
|
|
22.
|
Chapuis N, Tamburini J, Green AS, et al:
Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a
new therapeutic strategy for acute myeloid leukemia. Clin Cancer
Res. 16:5424–5435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gavet O and Pines J: Progressive
activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell.
18:533–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lu LX, Domingo-Sananes MR, Huzarska M,
Novak B and Gould KL: Multisite phosphoregulation of Cdc25 activity
refines the mitotic entrance and exit switches. Proc Natl Acad Sci
USA. 109:9899–8904. 2012. View Article : Google Scholar : PubMed/NCBI
|