Introduction
Cancer is the second leading cause of mortality
worldwide. The mortality rate of cancer is only inferior to that of
cardiovascular disease (1). The
threat of cancer to human health has not previously been
effectively controlled. Chemotherapy is the main treatment for
cancer, but its therapeutic effect is severely limited by
side-effects and multidrug resistance (2). Traditional Chinese medicine has a
long history of use in the treatment of diseases. ‘Shen Nong’s
Herbal Classic’ and other herbal monographs have documented and
discussed multiple traditional Chinese medicine prescriptions for
treating severe ulcers and malignant tumors. Traditional Chinese
medicine has notable advantages of multi-component, multi-link and
multi-target for treating cancer (3). The extraction of active antitumor
ingredients from Chinese herbal medicines has gained an increasing
amount of attention.
In recent years, polysaccharides of plant origin
have emerged as an important class of bioactive natural products. A
wide range of polysaccharides have been reported to exhibit
properties, including antioxidant activity (4,5),
free radical-scavenging activity (6), the enhancement of immune capacity
(7,8), the ability to lower blood sugar and
blood lipids (9,10) and anti-viral activity (11). Furthermore, polysaccharides have
been shown to exert antitumor activity by enhancing the immune
capacity of the body and inducing tumor cell apoptosis (12–15).
The majority of methods for extracting polysaccharides involve hot
water extraction and low temperature alkali extraction (16,17).
Research has revealed that plant polysaccharides extracted with hot
water exhibit in vivo and in vitro antitumor
activities (18). The extraction
yields differ depending on the plants and extraction methods
used.
Fomes officinalis Ames (‘Kubaiti’ in
Chinese), is the dried fruiting body of the Fomes
officinalis fungus, and is commonly used as a medicine by
Uyghur doctors in China. It has many functions such as warming
lung, eliminating phlegm, relieving asthma, activating blood and
dispersing swelling, inducing diuresis, enhancing physical
strength, prolonging the anti-fatigue and hypoxia tolerance time
and improving the emergency response capacity of the body. It is
often used for treating chronic bronchitis, abdominal pain,
influenza, tuberculosis and cancer (19). As reported by Wu et al
(20), terpene and steroid
compounds have been identified to be the main active components of
Fomes officinalis Ames, and are related to the observed
efficacies. Polysaccharides from medicinal fungi and their
derivatives have become important in immune regulation and cancer
treatment. The Fomes officinalis Ames polysaccharides
(FOAPs) have also obtained increasing attention (21,22).
However, studies concerning the extraction of FOAPs and their
efficacy have been seldom reported. In the current study, the
extraction methods of FOAPs and the anti-tumor activities of the
extracts were investigated. The objective was to provide a
theoretical foundation and experimental basis for the study of
other drugs derived from fungi.
Materials and methods
Apparatus and reagents
The main apparatus and reagents were as follows:
1700 UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan), RE-3000
rotary evaporators (Shanghai Yarong Biochemical Instrument Factory,
Shanghai, China), TD-1500 low speed centrifuge (Hunan Kaida
Scientific Instruments Co., Ltd, Changsha, China), FA1104N
electronic balance (Shanghai Precision & Scientific Instrument
Co., Ltd, Shanghai, China), GSY-II thermostatic water bath (Beijing
Medical Equipment Factory, Beijing, China) and Fomes
officinalis Ames (Ningbo Dekang Biological Products Co., Ltd,
Ningbo, China). Other reagents were analytically pure, and
deionized water was used in all experiments.
Animals
Clean Kunming mice inoculated with S180 tumor cells
were provided by the Key Laboratory of Forest Plant Ecology of
Ministry of Education, Northeast Forestry University (Heilongjiang,
China). The study was approved by the ethics committee if The
Second Hispital Affiliated to The Third Military Medical University
of PLA (Chongqing, China).
Identification of Fomes officinalis
Ames
Fomes officinalis Ames was identified by the
following methods: a) 0.1 g Fomes officinalis Ames was
placed in a bottle and 2 ml ethyl acetate was added. Following
agitation and impregnation for 1 h, the mixture was filtered. The
fluorescence of the filtrate was observed with a spectrophotometer.
b) The filtrate (0.5 ml) was placed into a test tube, and 0.5 ml
sulfuric acid was added slowly along the tube wall. The color of
the mixture was observed, and then the fluorescence of the mixture
was observed with the spectrophotometer.
Extraction of FOAPs
Raw Fomes officinalis Ames was pulverized and
the powder was dried at 80°C for 2 h. Dried powder (25 g) was
placed in a flask (500 ml), followed by 125 ml deionized water. The
mixture was refluxed for 2 h and subsequently filtered. The
extraction was repeated twice, and the three filtrates were mixed.
Chloroform was added to the mixed filtrate in order to remove
proteins and pigments. After discarding the chloroform layer, a 95%
ethanol solution was added to induce precipitation. Following
centrifugation (1227 × g, 8 min), washing with acetone and drying,
the FOAPs were obtained.
For the acid and alkali extraction methods, the
extractions were performed at room temperature, and hydrochloric
acid solution and sodium hydroxide solution were used as the
respective extraction solvents. Following extraction, the
extraction solution was immediately neutralized. Other procedures
were the same as those used in the hot water extraction method.
Determination of FOAP yield
A standard glucose solution (250 ml, 0.2 mg/ml) was
prepared and 2.5, 5, 7.5, 10, 12.5, 15 and 17.5 ml of the standard
solution were diluted to 50 ml with distilled water, respectively.
Subsequently, 2 ml diluted solution was added to a test tube. Then,
1 ml 6% phenol reagent was added and the mixture was fully blended,
and 5 ml concentrated sulfuric acid was added quickly. After
standing for 5 min, the mixture was incubated in a boiling water
bath for 15 min, and then cooled down to room temperature.
Concurrently, a reagent blank control experiment was performed. The
absorbance of the mixture was determined at 490 nm using a UV-Vis
spectrophotometer. The standard glucose curve was established by
plotting the absorbance value (y-axis) against the glucose
concentration (x-axis, mg/ml). The regression equation took the
form: y=16.314× − 0.0619, r=0.9992.
The yield of FOAPs was expressed as follows: Yield
(%) = (m/M) × 100 (1), where m is
the weight of FOAPs analyzed by UV-Vis analysis (g), and M is the
weight of Fomes officinalis Ames (g).
Weighing of the organs of the immune
system and determination of the tumor inhibition rate
A cell suspension (1×106/ml) of S180
mouse ascites tumor cells (seven days following vaccination) was
prepared with saline. Subsequently, 0.2 ml cell suspension was
subcutaneously inoculated into the right axillary region of the
mice. The mice were randomly divided 24 h later into the normal
group, the model group, the fluorouracil (5-Fu) group and three
FOAP groups, with 10 mice per group. The three FOAPs groups were
intragastricly administrated with FOAPs at doses of 50, 100 and 200
mg/(kg·d), respectively. Equal volumes of saline were administered
to the normal and model groups and the 5-Fu group was treated with
5-Fu (30 mg/kg·d). All irrigations were conducted for seven
consecutive days. On the eighth day, all mice were sacrificed. The
tumor, thymus and spleen were dissected and weighed. The tumor
inhibition rate, thymus index and spleen index were determined
according to previously described methods (23), as follows: Tumor inhibition rate
(%) = [1 - (mean tumor weight of treatment group/mean tumor weight
of model group)] × 100%. Thymus index (mg/g) = (thymus weight/body
weight) × 1,000. Spleen index (mg/g) = (spleen weight/body weight)
× 1,000.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analyses were performed using SPSS 13.0 statistical software (SPSS,
Inc., Chicago, IL, USA). A t-test was used to analyze the
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
Identification of Fomes officinalis
Ames
According to the identification method a), the
filtrate displayed light blue fluorescence under a UV light. In
method b), following the addition of sulfuric acid, the filtrate
exhibited green fluorescence and yellow fluorescence was observed
under UV light (365 nm). The Fomes officinalis Ames material
met the criteria specified in Standard of Medicine PRC-Uygur
Medicine Fascicule (24).
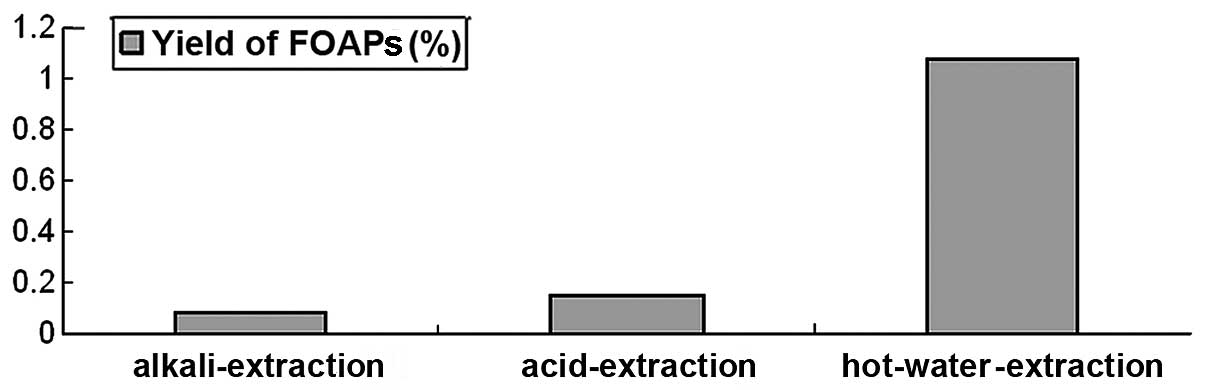
Effects of various extraction methods on
the yield of FOAPs
The effects of various extraction methods on the
yield of FOAPs are shown in Fig.
1. The yield of FOAPs obtained by the hot water extraction
method was higher than that by the acid and alkali extraction
methods. Water is a safe and economic extraction solvent. It
effectively penetrates plant tissue, resulting in a high extraction
yield. In the acid or alkali extraction method, the acid or alkali
may cause glycoside bonds in the polysaccharide to rupture,
resulting in a low yield. Therefore, following acid or alkali
extraction, the extraction solution should be neutralized
immediately, concentrated and precipitated in order to maximize the
yield of polysaccharides. In this study, the hot water extraction
method was suitable for extracting FOAPs. There are many methods
for the extraction and separation of polysaccharides. The optimum
extraction method and technology should be determined according to
the characteristics of the polysaccharide, its physical and
chemical properties and its experimental results.
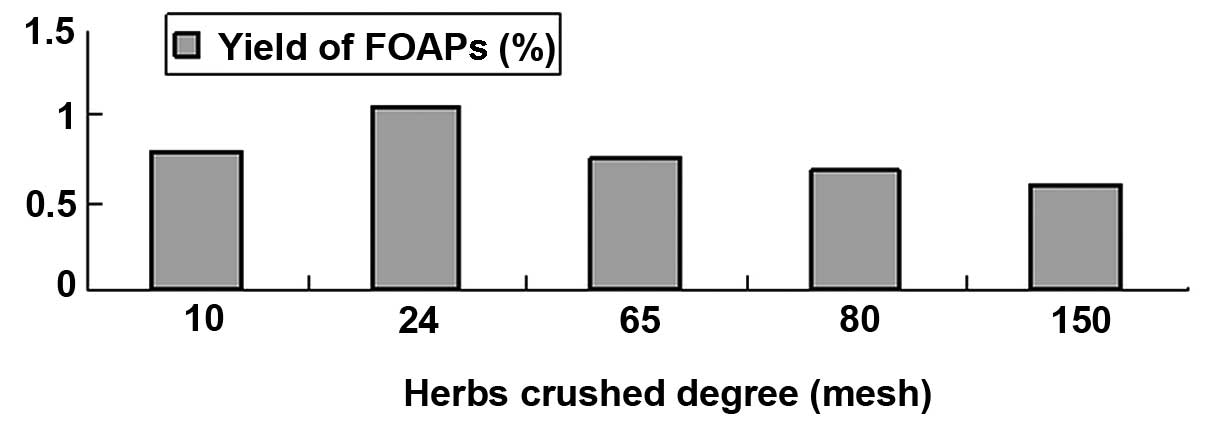
Effects of raw material particle size on
the yield of FOAPs
The effect of the particle size distribution of the
raw material (10, 24, 65, 80 and 150 mesh) on the FOAP yield was
investigated using an extraction time of 2 h and material-liquid
ratio of 1 g:10 ml. The results are shown in Fig. 2. In general, as the particle size
increased, the FOAP yield decreased. The yield was at its highest
(1.05%), however, when the particle size was 24 mesh rather than 10
mesh. The reason for this may be that too small a particle size
enhances the interaction between the material particles, resulting
in a low yield. Furthermore, a too small particle size may increase
the difficulty of follow-up filtration. Therefore, 24 mesh was
selected as an optimum raw material particle size for subsequent
experiments.
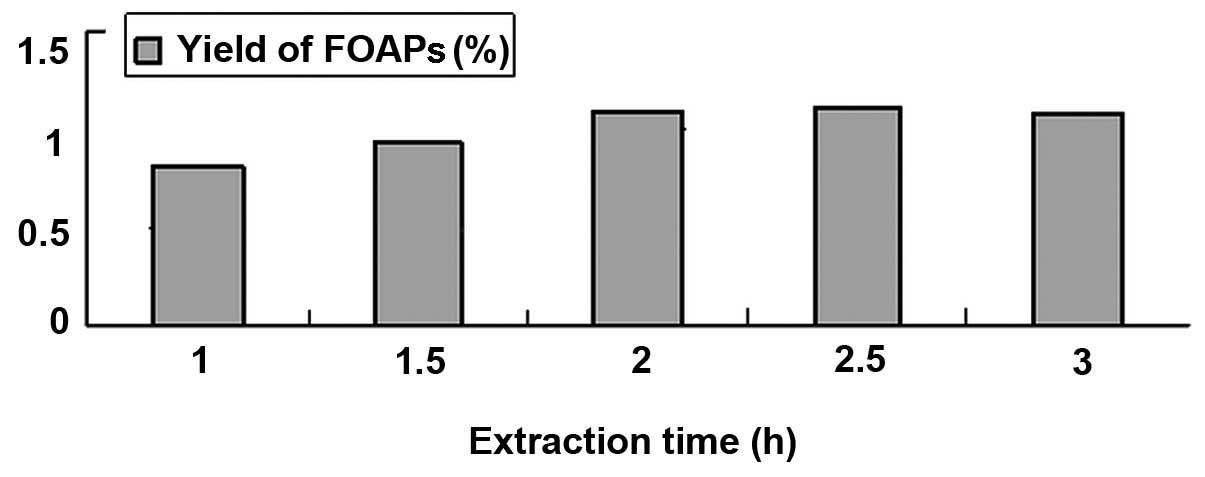
Effects of various extraction times on
the yield of FOAPs
Extraction times ranging from 1 to 3 h were
investigated, with a 24 mesh raw material particle size and a
material-liquid ratio of 1 g:10 ml. The results are shown in
Fig. 3. The yield of FOAPs
increased with increasing extraction time, but began to reduce when
the extraction time was >2.5 h. This indicates that when the
extraction time is too long, greater amounts of other components
are extracted, resulting in a lower FOAP content. Furthermore, too
long an extraction time may increase energy consumption.
Considering the feasibility in a practical application, 2.5 h was
selected as a suitable extraction time.
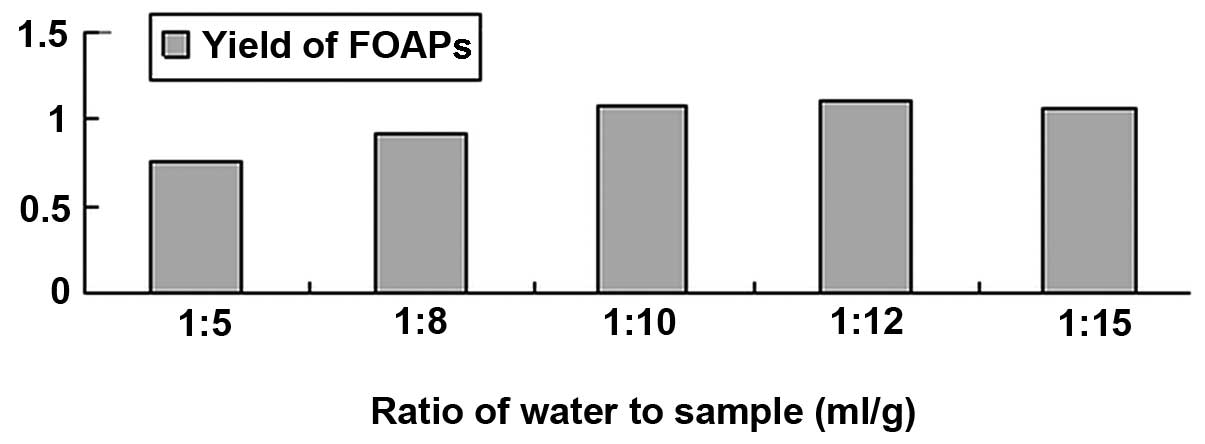
Effects of various material-liquid ratios
on the yield of FOAPs
The effect of various material-liquid ratios (g:ml;
1:5, 1:8, 1:10, 1:12 and 1:15, respectively) on the yield of FOAPs
was investigated (extraction time was 2 h and raw material particle
size was 24 mesh). As shown in Fig.
4, the yield of FOAPs was at its highest when the
material-liquid ratio was 1 g:12 ml. There should be sufficient
liquid to fully dissolve the polysaccharide, but the amount of
liquid should be as small as possible, in order to reduce the time
taken and the energy consumption during concentration. Based on
these results, 1 g:12 ml was selected as a suitable material-liquid
ratio.
Verification experiment
Triplicate experiments were performed under the
optimum hot water extraction conditions: a 24-mesh raw material
particle size, a 2.5-h extraction time and a material-liquid ratio
of 1 g:12 ml. The average yield of FOAPs was 1.13%.
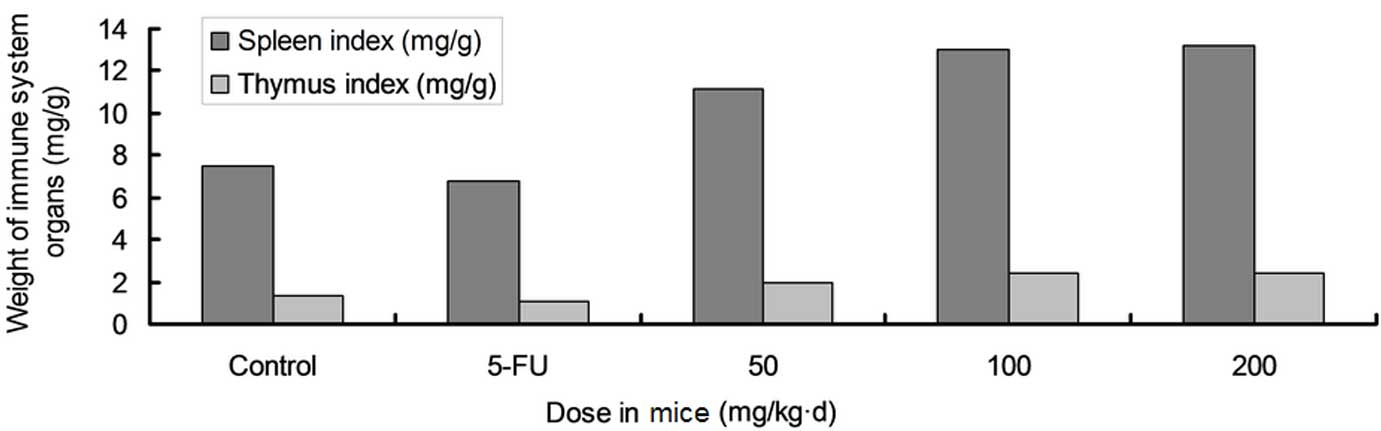
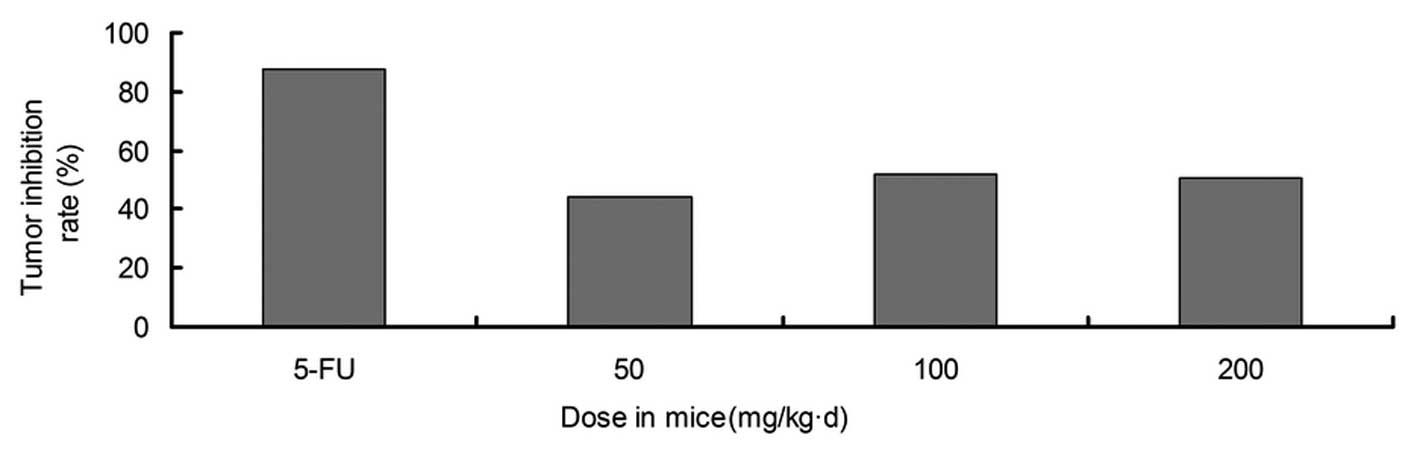
Effects of FOAPs on immune function and
their antitumor activity in mice
Effects of various doses of FOAPs on the weight of
the organs of the immune system and the tumor inhibition rate in
S180 tumor-bearing mice are shown in Figs. 5 and 6, respectively. FOAPs did not affect the
survival and body weight of the S180 tumor-bearing mice, but
inhibited tumor growth. There was a significant difference in the
tumor weight between each FOAP group and the model group
(P<0.05). Treatment with FOAPs increased the thymus and spleen
weights of the S180 tumor-bearing mice. Compared with the 5-Fu
group, the tumor inhibition rate in the FOAPs groups was lower, but
there was a more marked stimulation of the organs of the immune
system. Though 5-Fu effectively inhibited tumor growth, it markedly
suppressed the immune system. The combination of FOAPs and 5-Fu may
result in enhanced antitumor effects and simultaneously reduce the
poisonous side-effects of 5-Fu.
In conclusion, the hot water extraction method is
suitable for extracting FOAPs. The optimum extraction conditions
were as follows: a 24-mesh raw material particle size, a 2.5-h
extraction time and a 1 g:12 ml material-liquid ratio. Chloroform
removed the proteins and pigments from the extraction solution.
Under these conditions, the yield of FOAP was 1.13%. FOAPs inhibit
tumor growth and enhance the immune function in mice.
Acknowledgements
This study was supported by grant of
National Natural Science Foundation of China (No. 30801366).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Cheng XQ, Li H, Yue XL, et al: Macrophage
immunomodulatory activity of the polysaccharides from the roots of
Bupleurum smithii var. parvifolium. J Ethnopharmacol.
130:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Eiznhamer DA and Xu ZQ: Betulinic acid: a
promising anti-cancer candidate. IDrugs. 7:359–73. 2004.
|
|
4.
|
Wang C, Chen Y, Hu M, Ding J, Xu C and
Wang R: In vitro antioxidant activities of the polysaccharides from
Tricholoma lobayense. Int J Biol Macromol. 50:534–539. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kong F, Zhang M, Liao S, Yu S, Chi J and
Wei Z: Antioxidant activity of polysaccharide-enriched fractions
extracted from pulp tissue of Litchi Chinensis sonn.
Molecules. 15:2152–2165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu ZF, Dong F, Ji YB, Miao J and Jin LN:
A study on process of pharmacology of selenium polysaccharide. J
Beijing Union Univ (Nat Sci). 25:36–40. 2011.(In Chinese).
|
|
7.
|
Tincer G, Yerlikaya S, Yagci FC, et al:
Immunostimulatory activity of polysaccharide-poly (I:C)
nanoparticles. Biomaterials. 32:4275–4282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yi Y, Liao ST, Zhang MW, et al:
Physicochemical characteristics and immunomodulatory activities of
three polysaccharide-protein complexes of longan pulp. Molecules.
16:6148–6164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gao T, Bi H, Ma S and Lu J: The antitumor
and immunostimulating activities of water soluble polysaccharides
from Radix Aconiti, Radix Aconiti Lateralis and
Radix Aconiti Kusnezoffii. Nat Prod Commun. 5:447–455.
2010.PubMed/NCBI
|
|
10.
|
Robitzer M and Quignard F: Marine
polysaccharides and their conversion into functional materials.
Chimia (Aarau). 65:81–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Men XY, Wang YF, Zheng WJ, Zhu YM, Zhang
MY and Jiang X: The synthesis of selenium nanoparticles with
polysaccharides from Undaria Pinnatifida (Charv.) Suringer
and its antivirus effects on CVB3 in vitro. Chin J Health Lab
Technol. 15:1153–1155. 2005.(In Chinese).
|
|
12.
|
Li RY and Gao JP: The primary study of
pharmacological action in vivo with abdominal cavity S180 tumor
bearing mice of the Radix Codonopsis coarse polysaccharides.
J Changzhi Med Coll. 25:94–96. 2011.(In Chinese).
|
|
13.
|
Lan MB, Guo J, Zhao HL and Yuan HH:
Antioxidant and anti-tumor activities of purified polysaccharides
with low molecular weights from Magnolia officinalis. J Med
Plant Res. 6:1025–1034. 2012.
|
|
14.
|
Tang YL, Luo Q, Ding W, Ding X and Yang
ZR: Anti-tumor activity of polysaccharides extracted from two wild
amanitas. Sichuan Da Xue Xue Bao Yi Xue Ban. 42:792–796. 2011.(In
Chinese).
|
|
15.
|
Ye CL, Hu WL and Dai DH: Extraction of
polysaccharides and the antioxidant activity from the seeds of
Plantago asiatica L. Int J Biol Macromol. 49:466–470. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jiao G, Yu G, Zhang J and Ewart HS:
Chemical structures and bioactivities of sulfated polysaccharides
from marine algae. Mar Drugs. 9:196–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang M, Cheung PC and Zhang L: Evaluation
of mushroom dietary fiber (nonstarch polysaccharides) from
sclerotia of Pleurotus tuber-regium (Fries) singer as a
potential antitumor agent. J Agric Food Chem. 49:5059–5062. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ni W, Zhang X, Wang B, et al: Antitumor
activities and immunomodulatory effects of ginseng neutral
polysaccharides in combination with 5-fluorouracil. J Med Food.
13:270–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
State Pharmacopoeia Committee: Standards
of Uyghur of Medical Ministry of the People’s Republic of China.
421999.(In Chinese).
|
|
20.
|
Wu X, Yang JS and Dong YS: Chemical
constituents of Fomes officinalis (I). Chin Tradit Herb
Drugs. 36:811–814. 2005.(In Chinese).
|
|
21.
|
Zuo L, Pa L, Bai L and Du N: The
immune-potentiating effect of Fomes officinalis
polysaccharides. J Xinjiang Med Univ. 26:563–565. 2003.(In
Chinese).
|
|
22.
|
Yi B, Ma Y, Su B, et al: Scavenging
activity of Fomes officinalis polysaccharides on oxygen free
radicals. J Xinjiang Med Univ. 29:15–17. 2006.(In Chinese).
|
|
23.
|
Xu SY: Pharmacology Experiments. People’s
Health Publishing House; Beijing: pp. 1581982, (In Chinese).
|
|
24.
|
Pharmacopoeia Commission of People’s
Republic of China: Uygur Pharmaceutical Section. Drug Standards of
Ministry of Public Health of the People’s Republic of China.
Xinjiang Science and Technology Press; Urumqi: pp. 421999, (In
Chinese).
|