Introduction
Leptin is a 16 kDa-peptide (167 amino acids) that is
synthesized and secreted predominantly by white adipose tissue
(1). One of the major effects of
this hormone is the control of energy balance via the hypothalamus,
which was previously considered to be mediated through binding to
leptin receptors in the hypothalamus (2–4).
However, leptin levels are higher in the blood leaving the brain
than in that entering it, suggesting that leptin may be synthesized
in the hypothalamus and other brain tissues (5). More than 40% of circulating leptin
originates from the brain in healthy males (6). Furthermore, the plasma leptin
concentrations contributed by the brain demonstrate a six-fold
increase in obese males compared with healthy males (935±32 ng/ml
in obese versus 160±59 ng/ml in healthy males) (6). Leptin is synthesized in the
hypothalamus (7). Leptin mRNA
expression has been detected in the hypothalamus and this
expression of leptin has been demonstrated to be suppressed by 48 h
of fasting (7). In the
hypothalamus, the leptin gene is, thus, regulated by nutrient
availability. The detection of leptin expression in the
hypothalamus by reverse transcription-polymerase chain reaction
(RT-PCR) has been confirmed with sequence analysis using rat
hypothalami (7). Leptin protein
has also been demonstrated to be present in rat hypothalami by
immunocytochemistry (7).
Therefore, leptin gene expression and leptin itself are present in
the hypothalamus.
Although leptin has been suggested to reduce
appetite, obese individuals generally exhibit high circulating
leptin levels (8) and, as
mentioned previously, a large proportion of the high leptin levels
that are apparent in obesity originate in the brain, with six-fold
more leptin secreted from the brain into the circulation of obese
versus healthy individuals (6). It
has been suggested that these high circulating levels of leptin in
obesity function pathophysiologically for the development of
hypertension (8). Epidemiological
studies have indicated that 65–75% of the risk for hypertension is
excess weight (9,10). Chronic increases in leptin levels
result in a persistent elevation in mean arterial pressure and this
hypertensive blood pressure is rapidly reversed with cessation of
leptin administration (11). Acute
infusions of leptin have also been demonstrated to lead to an
abrupt increase in blood pressure (12). Similar increases in systolic blood
pressure have been observed in transgenic mice overexpressing
leptin (13).
The heart and adipose tissue are endocrine organs
and studies have increasingly suggested that cross-talk exists
between them, although the precise mechanism is poorly defined
(14–19). The heart synthesizes four hormones,
the products of a single gene, which have significant blood
pressure-lowering effects (20).
These cardiac hormones, which are vessel dilator, long-acting
natriuretic peptide (LANP), atrial natriuretic peptide (ANP) and
kaliuretic peptide, are vasodilators, with blood pressure-lowering
properties in animals (21–26)
and humans (27–29). The original hypothesis for
hypertension was the presence of a defect in the production of the
blood pressure-lowering ANPs (30,31).
However, experimental data have revealed that, rather than being
decreased, the levels of these blood pressure-lowering peptides are
elevated in the circulation in an apparent attempt to overcome the
elevated blood pressure (30–34).
ANP levels are increased in essential hypertension (30,34).
The hypertension in obesity is also associated with increased
circulating concentrations of ANP (32), LANP (33) and vessel dilator (33), which decrease into the normal range
when the high blood pressure is reduced by weight loss (32–34).
In the present study, we hypothesized that since the levels of
cardiac hormones correlate with blood pressure in obesity (32–34),
the blood pressure-reducing effects of these hormones (20–29)
may be mediated, in part, by decreased leptin production in the
hypothalamus.
Materials and methods
Cardiac hormones
The cardiac hormones (vessel dilator, ANP,
kaliuretic peptide and LANP) were obtained from Phoenix
Pharmaceuticals, Inc., Belmont, CA, USA.
Hypothalamic cells
Hypothalamic cells (ATCC no. CRL-2005; DI TNC1) were
obtained from the American Type Culture Collection (ATCC) Manassas,
VA, USA. The ATCC authenticated this cell line.
Culture of hypothalamic cells
Propagation of the hypothalamic cells was performed
in Dulbecco’s Modified Eagle’s medium with an addition of 10%
heat-inactivated fetal bovine serum (Sigma Chemical Corporation,
St. Louis, MO, USA) and 1% penicillin, streptomycin and fungizone
at a temperature of 37°C, as recommended by the ATCC. The number of
cells in culture was 1.44×106 cells/ml. Cells were
dispensed into new flasks with subculturing every 6 days. The
growth medium was changed every 3 days.
Leptin enzyme-linked immunosorbent assay
(ELISA)
The Quantikine® leptin immunoassay ELISA
used to measure leptin levels was obtained from R&D Systems
(Minneapolis, MN, USA). This 3.5 h solid phase ELISA contained
E. coli-expressed recombinant leptin and antibodies raised
against the recombinant leptin. This quantitative sandwich enzyme
immunoassay utilized a monoclonal antibody specific for leptin. The
immunoassay has been shown to quantitate recombinant leptin
accurately. Results obtained by measuring natural leptin revealed
that the dose-response curves obtained with the recombinant
Quantikine® assay paralleled the curves with natural
leptin. The assay had a 98% recovery of leptin in the previously
mentioned cell culture media. The minimal detectable concentration
of leptin in this assay was 7.8 pg/ml. The levels of leptin
measured are the amount in the cells plus the amount of leptin
secreted into the media.
Leptin protocol
The hypothalamic cells (1.44×106
cells/ml) were subcultured for 24 h, prior to 50 μl cell
culture supernatant being added to 96-well plates with 50 μl
media, containing 100 pM, 1 nM, 10 nM, 100 nM, 1 μM and 10
μM of each of the four cardiac hormones, separately (n=9 for
each concentration). The hypothalamic cells were subsequently
evaluated using the leptin ELISA from R&D Systems. Following
this, the mean of the nine measurements at each concentration of
the respective peptide hormones was then calculated and this was
compared with the mean of the leptin concentrations in the control
hypothalami, which had not been exposed to any of the cardiac
hormones. The leptin data are shown in the figures as the decrease
in the leptin level (i.e., the percentage decrease) compared with
the level of leptin in the untreated hypothalami. The standards
from R&D Systems were added to the blank wells to serve as
reference points for known leptin concentrations. In this assay,
absorbance was recorded at a 540 nm wavelength using a 96-well
BioTek Gen 5, Synergy Mx microplate reader (BioTek Instruments,
Inc., Winooski, VA, USA). There were 48 hypothalamic controls in
these experiments.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM). Statistical analysis of the data were performed by
one way analysis of variance (ANOVA) with a repeated measures
design for within-group comparisons, using a statistical module of
Excel software (Microsoft Corporation, Redmond, WA, USA). A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Vessel dilator decreases the hypothalamic
concentrations of leptin by up to 79%
Vessel dilator decreased the concentration of leptin
by a maximum of 79% (P<0.0001) from the control value of 85±4
pg/ml. The maximal reduction was obtained when the highest
concentration of vessel dilator, i.e., 10 μM was used
(Fig. 1). At the lowest
concentration of the vessel dilator (100 pM), there was a 26%
reduction in the concentration of leptin (P<0.05). The
dose-response curves indicated that vessel dilator also decreased
leptin levels by 54, 58, 55 and 73% at concentrations of 1, 10 and
100 nM and 1 μM, respectively (P<0.001 for each; Fig. 1).
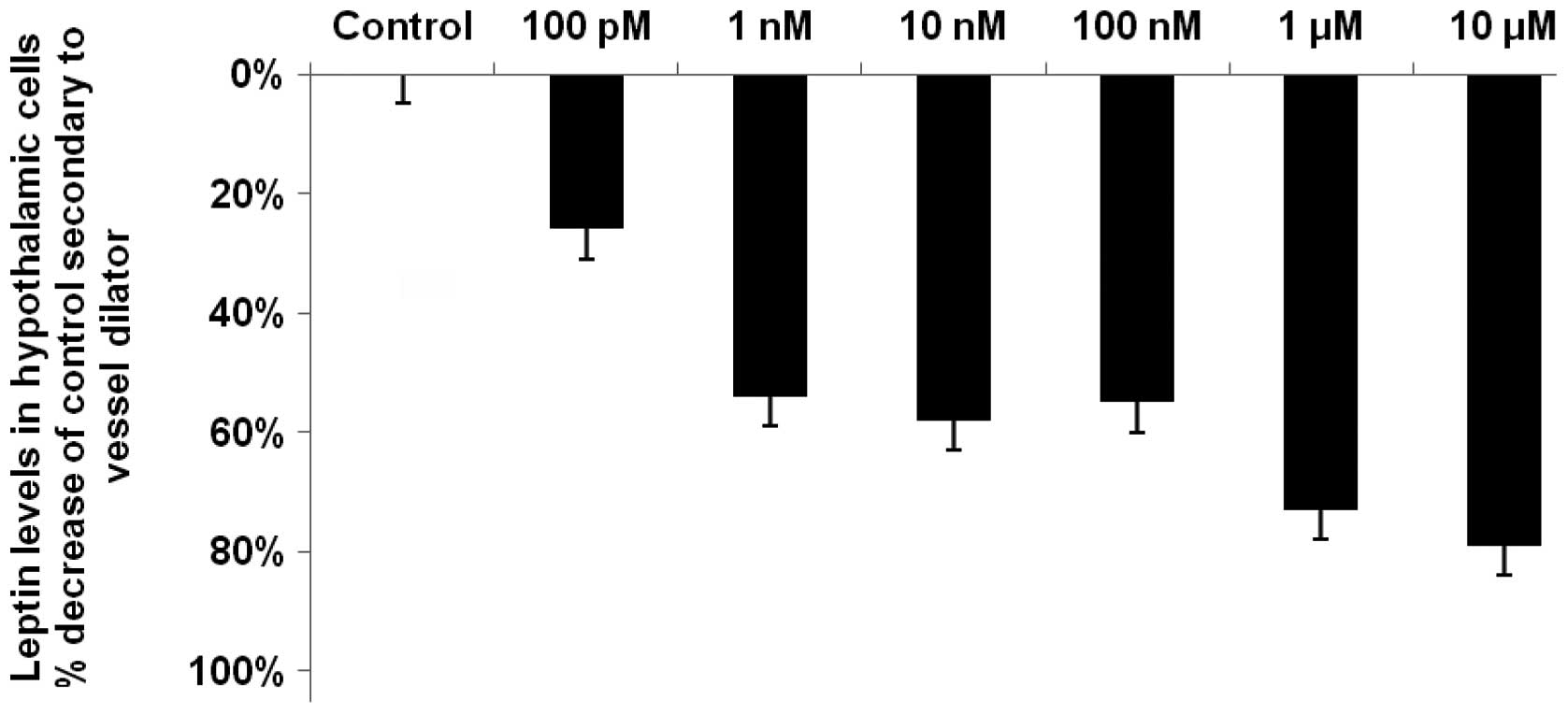 | Figure 1.Vessel dilator decreases the
hypothalamic concentration of leptin by up to 79%. Vessel dilator
maximally decreased leptin levels in the hypothalamic cells by 79%
(P<0.0001) at a concentration of 10 μM in comparison with
the control (85±4 pg/ml). Vessel dilator caused a significant
reduction in leptin levels in the hypothalamus at each of its
concentrations, with reductions of 26, 54, 58, 55 and 73% at
concentrations of 100 pM, 1 nM, 10 nM, 100 nM and 1 μM,
respectively. These results were significant at P<0.001, with
the exception of the 100 pM concentration (P<0.05), as
demonstrated by analysis of variance (ANOVA) with a repeated
measures design for within-group comparisons. n=9 for each
concentration of vessel dilator; n=48 for controls. |
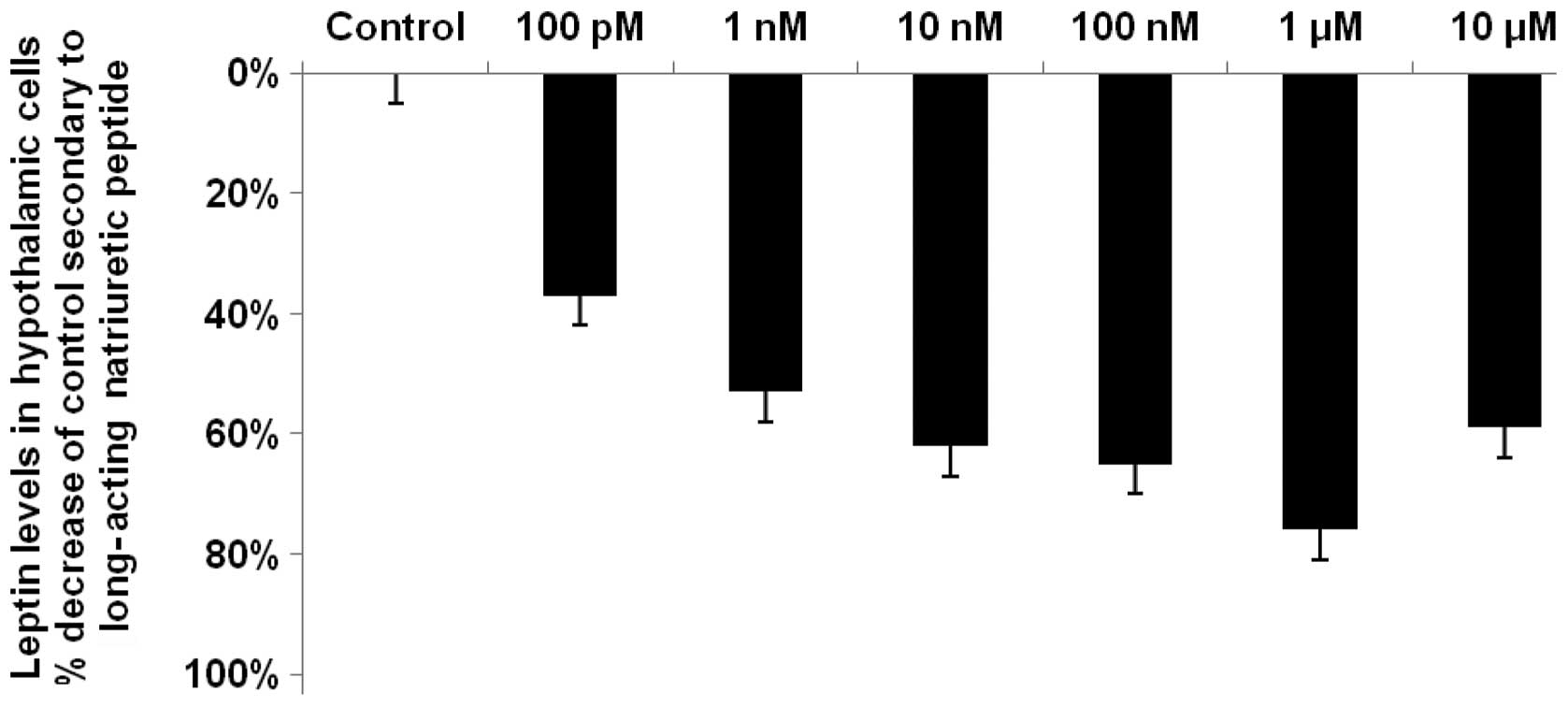
LANP decreases hypothalamic leptin by up
to 76%
LANP decreased leptin levels by up to 76%
(P<0.0001) in the hypothalami, with the maximal reduction
occurring at a LANP concentration of 1 μM (Fig. 2). LANP, similar to vessel dilator,
decreased leptin by the smallest amount at its lowest
concentration, 100 pM; however, this 37% reduction in leptin was
significant at P<0.01. There was a significant (P<0.001)
reduction in leptin levels at each of the other concentrations of
LANP, with reductions of 53, 62, 65 and 59% at concentrations of 1,
10 and 100 nM and 10 μM LANP, respectively (Fig. 2).
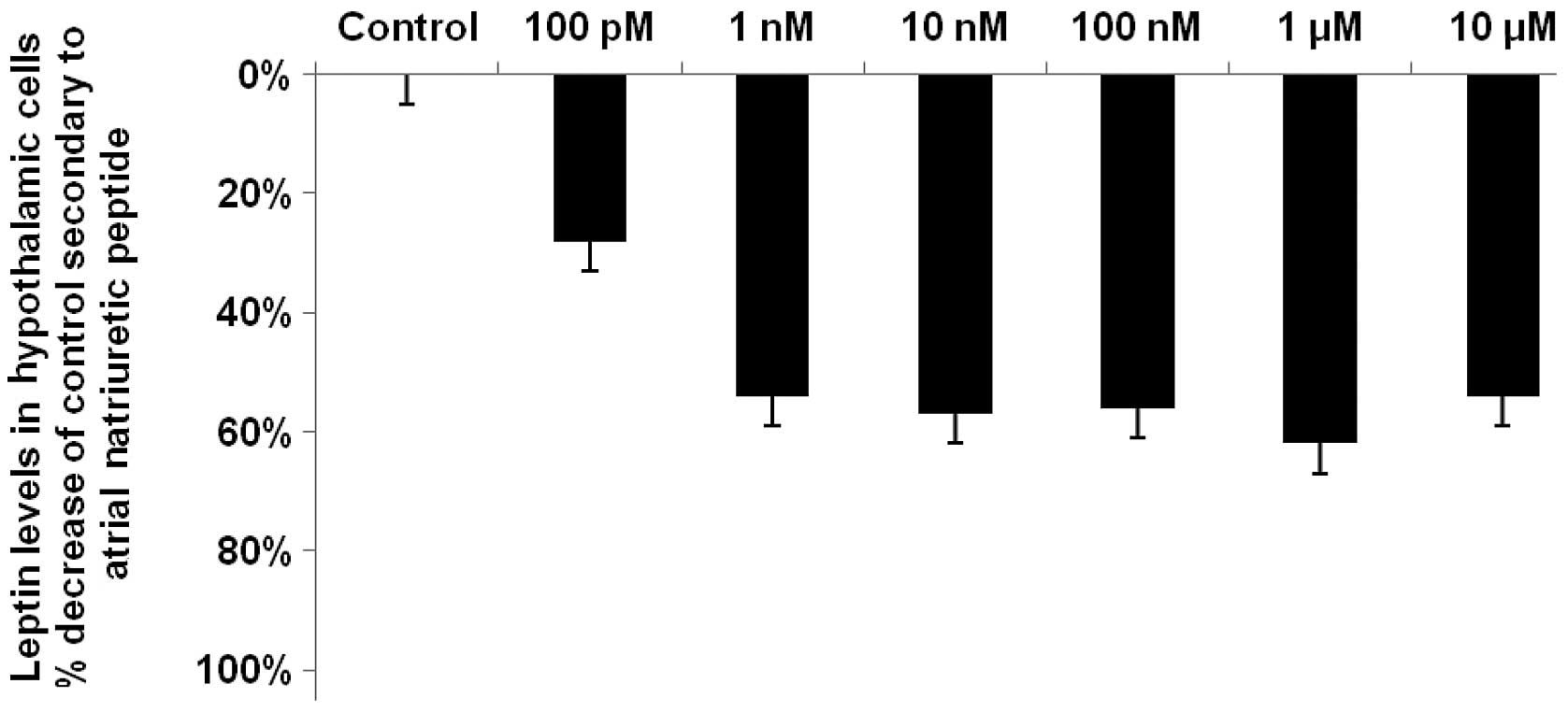
Reduction of leptin levels in the
hypothalami secondary to ANP
ANP, like LANP, caused its maximal reduction (62%;
P<0.0001) in hypothalamic leptin levels at a concentration of 1
μM and its smallest reduction (28%; P<0.05) at a
concentration of 100 pM (Fig. 3).
There was a significant reduction in leptin levels at all
concentrations of ANP, with reductions of 54, 57, 56 and 54% at
concentrations of 1, 10 and 100 nM and 10 μM, respectively
(P<0.001 for each concentration).
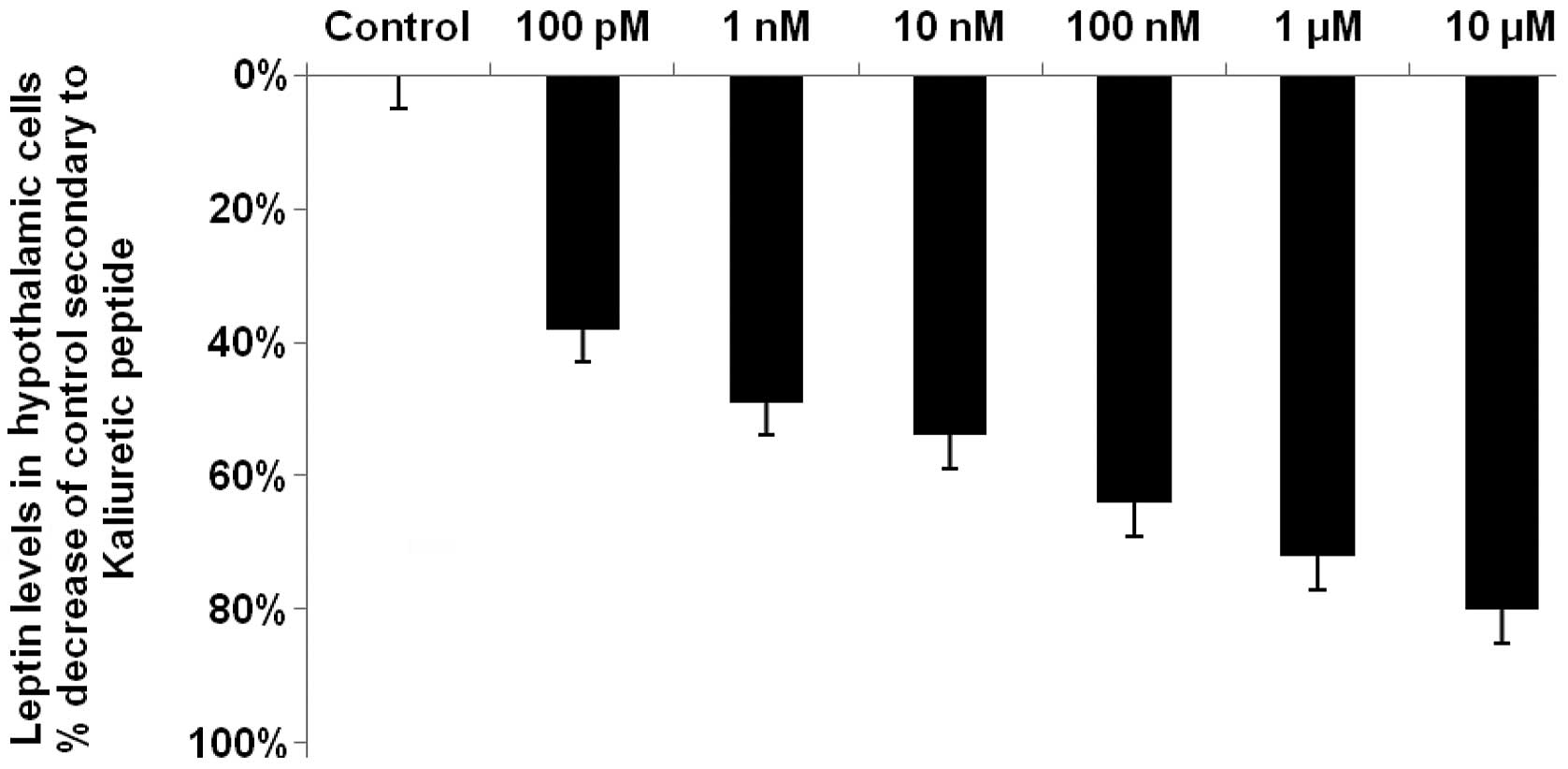
Kaliuretic peptide decreases hypothalamic
leptin by up to 80%
Kaliuretic peptide decreased leptin levels by up to
80% (P<0.0001), with a maximal reduction occurring at the
highest concentration of kaliuretic peptide, i.e., 10 μM
(Fig. 4). Kaliuretic peptide also
caused a significant (P<0.0001) 72% reduction in leptin levels
at a concentration of 1 μM. Kaliuretic peptide significantly
decreased leptin levels at each of its concentrations in the
dose-response curves, with reductions of 35, 49, 54 and 64% at
concentrations of 100 pM and 1, 10 and 100 nM (P<0.001 for each,
with the exception of the 100 pM concentration where P<0.01).
Thus, with respect to the maximal reduction in leptin levels, the
effects of vessel dilator, LANP and kaliuretic peptide were
approximately equal and each of these cardiac hormones had a
stronger ability than ANP to decrease leptin levels.
Discussion
Vessel dilator, LANP, kaliuretic peptide and ANP
each significantly decreased leptin levels in the hypothalamus, an
area of the brain that synthesizes leptin (7). The brain contributes more than 40% of
the leptin in the circulation (6).
The hypothalamus and brain contribute approximately six-fold more
to the circulating concentration of leptin in obese individuals in
comparison with the concentration in the circulation of healthy
individuals (6), which may be the
reason that leptin is elevated in the circulation of obese
individuals (8). This suggests
that there is an increase of leptin being synthesized in the
hypothalamus in obese individuals. Furthermore, this indicates that
the hypothalamus is significant in the elevation of leptin levels
in the circulation of obese individuals with hypertension (6). With regard to hypertension in
obesity, the present results indicated that the hypertension may be
treated with the vasodilatory cardiac hormones investigated in the
present study, since increased levels of leptin are correlated with
the development of hypertension in obese individuals (8,11,12).
The ability of all four cardiac hormones to markedly decrease
leptin levels was suggestive of a novel potential treatment target
for hypertension in obesity, since these four cardiac hormones have
demonstrated blood pressure-lowering properties (21–29).
The circulating concentrations of these four cardiac hormones
increase in individuals with high blood pressure in an apparent
attempt to overcome the constriction of the blood vessels (34). In calorie-restricted weight
reduction, the four cardiac hormones have been demonstrated to be
correlated in a linear fashion (P<0.0001) with blood pressure
reduction (34). During this blood
pressure reduction, plasma aldosterone and plasma renin were
inversely correlated with the concentration of the four cardiac
hormones and blood pressure (34).
These results were consistent with the ability of ANP to inhibit
renin release and aldosterone secretion from the adrenal gland, as
well as the strong inhibition of renin release mediated by vessel
dilator (25,34). The ability of these hormones to
decrease leptin levels thus suggests one mechanism for the known
correlation with blood pressure in obesity (32–34).
In the present study, the cardiac hormones were
demonstrated to directly decrease leptin levels in the
hypothalamus. It may be expected that the cardiac hormones also
have the ability to decrease leptin levels in other
leptin-synthesizing tissues, as leptin promotes angiogenesis by
increasing vascular endothelial growth factor (VEGF) levels
(35) and the cardiac hormones
have been demonstrated to decrease levels of VEGF and the VEGFR-2
receptor by up to 92% (36). Thus,
one of the mediators (VEGF) by which leptin causes vascular
permeability and angiogenesis (35) is inhibited by each of the cardiac
hormones (36). This suggests that
the effects of leptin on blood vessels may also be decreased by the
four cardiac hormones. In addition, ANP has been shown to inhibit
leptin release from adipose tissues (37), with receptors for ANP being present
in adipose tissues (38).
Hormone-sensitive lipase breaks down triglycerides
into non-essential fatty acids and glycerol (14). This hydrolysis is commonly termed
lipolysis (14). ANP activates
hormone-sensitive lipase through an increase in cyclic guanosine
3′,5′-monophosphate (cGMP) production, via the enhancement of
guanylyl cyclase (15).
Furthermore, the three other cardiac hormones synthesized by the
ANP prohormone gene also markedly enhance cGMP production by
stimulating guanylyl cyclase (39). The application of ANP via a
microdialysis probe has been shown to increase lipolysis in
abdominal subcutaneous adipose tissue of healthy young males
(14,16), while a systemic ANP infusion
increases lipolysis (17,18), even at physiological concentrations
(19). Prior to the demonstration
that cardiac hormones were able to cause lipid mobilization,
catecholamines and insulin were considered the major acute
regulators of lipid mobilization and they act via a cyclic
adenosine 5′-phosphate (AMP)-dependent regulation of lipolysis
(14). By contrast, the cardiac
hormones activate the guanylyl cyclase-cGMP pathway (15,20,39),
which is completely independent from the cyclic AMP-dependent
pathway in adipose cells (15).
Resistance to catecholamine-induced lipolysis in subcutaneous
adipose tissue has been demonstrated in obese adults (40) and obese children (41). Since the cardiac hormones increase
lipolysis in obese subjects, as well as helping to alleviate
obesity-interrelated hypertension mediated by leptin, this suggests
they may be a multi-targeted novel therapy for obesity.
Acknowledgements
The authors would like to thank Karen
Murphy for excellent secretarial assistance. The contents of this
publication do not represent the views of the Department of
Veterans Affairs or the United States Government. This work was
supported in part by grants from the James and Esther King Florida
Biomedical Research Program, the Florida Department of Health, and
the Mama Mare Breast Cancer Foundation.
References
|
1.
|
Kshatriya S, Lui K, Salah A, Szombathy T,
Freeman RH, Reams GP, Spear RM and Villarreal D: Obesity
hypertension: the regulatory role of leptin. Int J Hypertens.
2011:2706242011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lönnqvist F: The obese (ob) gene
and its product leptin - a new route toward obesity treatment in
man? QJ Med. 89:327–332. 1996.PubMed/NCBI
|
|
3.
|
Misra A and Garg A: Leptin, its receptor
and obesity. J Investig Med. 44:540–548. 1996.PubMed/NCBI
|
|
4.
|
Tartaglia LA, Dembski M, Weng X, Deng N,
Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J,
et al: Identification and expression cloning of a leptin receptor,
OB-R. Cell. 83:1263–1271. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Esler M, Vaz M, Collier G, Nester P,
Jennings G, Kaye D, Seals D and Lambert G: Leptin in human plasma
is derived in part from the brain, and cleared by the kidneys.
Lancet. 351:879–880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Eikelis N, Lambert G, Wiesner G, Kaye D,
Schlaich M, Morris M, Hastings J, Socratous F and Esler M:
Extra-adipocyte leptin release in human obesity and its relation to
sympathoadrenal function. Am J Physiol Endocrinol Metab.
286:E774–E752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Morash B, Li A, Murphy PR, Wilkinson M and
Ur E: Leptin gene expression in the brain and pituitary gland.
Endocrinology. 140:5995–5998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Considine RV, Sinha MK, Heiman ML,
Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee
LJ, Bauer TL and Caro JF: Serum immunoreactive-leptin
concentrations in normal-weight and obese humans. New Engl J Med.
334:292–295. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Garrison RJ, Kannel WB, Stokes J III and
Castelli WP: Incidence and precursors of hypertension in young
adults: the Framingham Offspring Study. Prev Med. 16:235–251. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wofford MR and Hall JE: Pathophysiology
and treatment of obesity hypertension. Curr Pharm Des.
10:3621–3637. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shek EW, Brands MW and Hall JE: Chronic
leptin infusion increases arterial pressure. Hypertension.
31:409–414. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yuan K, Yu J, Shah A, Gao S, Kim SY, Kim
SZ, Park BH and Kim SH: Leptin reduces plasma ANP level via nitric
oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol.
298:R1007–R1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Huang F, Xiong X, Wang H, You S and Zeng
H: Leptin-induced vascular smooth muscle cell proliferation via
regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta
Biochim Biophys Sin (Shanghai). 42:325–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sengenès C, Berlan M, De Glisezinski I,
Lafontan M and Galitzky J: Natriuretic peptides: a new lipolytic
pathway in human adipocytes. FASEB J. 14:1345–1351. 2000.
|
|
15.
|
Sengenès C, Bouloumie A, Hauner H, Berlan
M, Busse R, Lafontan M and Galitzky J: Involvement of a
cGMP-dependent pathway in the natriuretic peptide-mediated
hormone-sensitive lipase phosphorylation in human adipocytes. J
Biol Chem. 278:48617–48626. 2003.PubMed/NCBI
|
|
16.
|
Moro CC, Galitzky J, Sengenès C, Crampes
F, Lafontan M and Berlan M: Functional and pharmacological
characterization of the natriuretic peptide-dependent lipolytic
pathway in human fat cells. J Pharmacol Exp Ther. 308:984–992.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Uehlinger DE, Weidemann P, Gnädinger MP,
Hasler L, Bachmann C, Shaw S, Hellmüller B and Lang RE: Increase in
circulating insulin induced by atrial natriuretic peptide in normal
humans. J Cardiovasc Pharmacol. 8:1122–1129. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Galitzky J, Sengenès C, Thalamus C,
Marques MA, Senard JM, Lafontan M and Berlan M: The
lipid-mobilizing effect of atrial natriuretic peptide is unrelated
to sympathetic nervous system activation or obesity in young men. J
Lipid Res. 42:536–544. 2001.
|
|
19.
|
Birkenfeld AL, Boschmann M, Moro C, Adams
F, Heusser K, Franke G, Berlan M, Luft FC, Lafontan M and Jordan J:
Lipid mobilization with physiological atrial natriuretic peptide
concentrations in humans. J Clin Endocrinol Metab. 90:3622–3628.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vesely DL: Natriuretic hormones. Seldin
and Giebisch’s, The Kidney: Physiology and Pathophysiology. Alpern
RJ, Moe OW and Caplan M: 5th edition. Elsevier/North - Holland
Biomedical Press; Amsterdam: pp. 1241–1281. 2013
|
|
21.
|
Martin DR, Pevahouse JB, Trigg DJ, Vesely
DL and Buerket JE: Three peptides from the ANF prohormone
NH2-terminus are natriuretic and/or kaliuretic. Am J
Physiol. 258:F1401–F1408. 1990.PubMed/NCBI
|
|
22.
|
Gunning ME, Brady HR, Otuechere G, Brenner
BM and Ziedel ML: Atrial natriuretic peptide(31–67) inhibits
Na+ transport in rabbit inner medullary collecting duct
cells. Role of prostaglandin E2. J Clin Invest. 89:1411–1417.
1992.
|
|
23.
|
Benjamin BA and Peterson TV: Effects of
proANF-(31–67) on sodium excretion in conscious monkeys. Am J
Physiol. 269:R1351–R1355. 1995.
|
|
24.
|
Zeidel ML: Regulation of collecting duct
Na+ reabsorption by ANP 31-67. Clin Exp Pharmacol
Physiol. 22:121–124. 1995. View Article : Google Scholar
|
|
25.
|
Villarreal D, Reams GP, Taraben A and
Freeman RH: Hemodynamic and renal effects of proANF31-67 in
hypertensive rats. Proc Soc Exp Biol Med. 221:166–170. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Dietz JR, Scott DY, Landon CS and Nazian
SJ: Evidence supporting a physiological role for proANP-(1–30) in
the regulation of renal excretion. Am J Physiol Regul Integr Comp
Physiol. 280:R1510–R1517. 2001.PubMed/NCBI
|
|
27.
|
Vesely DL, Douglass MA, Dietz JR, Gower WR
Jr, McCormick MT, Rodriguez-Paz G and Schocken DD: Three peptides
from the atrial natriuretic factor prohormone amino terminus lower
blood pressure and produce diuresis, natriuresis and/or kaliuresis
in humans. Circulation. 90:1129–1140. 1994. View Article : Google Scholar
|
|
28.
|
Vesely DL, Douglass MA, Dietz JR, Giordano
AT, McCormick MT, Rodriguez-Paz G and Schocken DD: Negative
feedback of atrial natriuretic peptides. J Clin Endocrinol Metab.
78:1128–1134. 1994.PubMed/NCBI
|
|
29.
|
Vesely DL, Dietz JR, Parks JR, Baig M,
McCormick MT, Cintron G and Schocken DD: Vessel dilator enhances
sodium and water excretion and has beneficial hemodynamic effects
in persons with congestive heart failure. Circulation. 98:323–329.
1998. View Article : Google Scholar
|
|
30.
|
Sugarawa A, Nakao K, Sakamoto M, Morii N,
Yamada T, Itoh H, Shiono S and Imura H: Plasma concentration of
atrial natriuretic polypeptide in essential hypertension. Lancet.
2:1426–1427. 1985. View Article : Google Scholar
|
|
31.
|
Arendt R, Gerbes A, Ritter D, Stangl E and
Zähringer J: Atrial natriuretic factors in plasma of patients with
arterial hypertension, heart failure or cirrhosis of the liver. J
Hypertens Suppl. 4:S131–S135. 1986.PubMed/NCBI
|
|
32.
|
McMurray RW Jr and Vesely DL: Weight
reduction decreases atrial natriuretic factor and blood pressure in
obese patients. Metabolism. 38:1231–1237. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
McMurray RW Jr and Vesely DL: Weight
reduction decreases the circulating concentration of the N-terminus
of the ANF prohormone. Am J Med Sci. 303:2–8. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
McMurray RW Jr and Vesely DL:
Calorie-restricted weight reduction, blood pressure, and atrial
natriuretic peptides. Nutrition. 9:178–182. 1993.PubMed/NCBI
|
|
35.
|
Cao R, Brakenhielm E, Wahlestedt C,
Thyberg J and Cao Y: Leptin induces vascular permeability and
synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc
Natl Acad Sci USA. 98:6390–6395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nguyen JP, Frost CD, Lane ML, Skelton lv
WP, Skelton M and Vesely DL: Novel dual inhibitors of vascular
endothelial growth factor and VEGFR2 receptor. Eur J Clin Invest.
42:1061–1067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fain JN, Kanu A, Bahouth SW, Gowan GS and
Lloyd Hiler M: Inhibition of leptin release by atrial natriuretic
peptide (ANP) in human adipocytes. Biochem Pharmacol. 65:1883–1888.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sarzani R, Dessì-Fulgheri P, Paci VM,
Espinosa E and Rappelli A: Expression of natriuretic peptide
receptors in human adipose and other tissues. J Endocrinol Invest.
19:581–585. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Vesely DL: Peptides from the N-terminus of
the atrial natriuretic factor prohormone enhance guanylate cyclase
activity and increase cyclic GMP levels in a wide variety of
tissues. Mol Cell Biochem. 109:43–50. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Jensen MD, Haymond MW, Rizza RA, Cryer PE
and Miles JM: Influence of body fat distribution on free fatty acid
metabolism in obesity. J Clin Invest. 80:1168–1173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Bougnères P, Stunff CL, Pecqueur C,
Pinglier E, Adnot P and Ricquier D: In vivo resistance of lipolysis
to epinephrine. A new feature of childhood onset obesity. J Clin
Invest. 99:2568–2573. 1997.PubMed/NCBI
|