Introduction
Irritable bowel syndrome (IBS) is a chronic
widespread disease responsible for 40% of outpatient consultations
and it affects 15% of adults in western countries (1,2). IBS
often has severe consequences, with patients often having impaired
health-related quality of life (3–8). IBS
is subcategorized into three types: constipation-predominant
(C-IBS), diarrhea-predominant (D-IBS) and alternating diarrhea and
constipation.
The pathophysiology of IBS may involve alterations
in central processing, abnormal gastrointestinal motility and
visceral hypersensitivity, and the interactions of these factors
are possibly associated with the development of IBS symptoms. For
example, the majority of patients with IBS have a lower pain
threshold to colonic distension compared with that of healthy
subjects (9).
Nociceptin/orphanin FQ (N/OFQ) and the N/OFQ peptide
(NOP) receptor have been shown to be involved in the induction of
vasodilation and the regulation of reward and motivation pathways
related to substance abuse, with the NOP receptor being a candidate
target for the treatment of obesity (10–12).
N/OFQ and the NOP receptor are present in the central nervous
system (CNS) and in the periphery, playing important roles in the
modulation of gastrointestinal function and pain (13). To assess whether the NOP receptor
is involved in the pathogeneses of IBS, we measured the levels of
NOP receptor mRNA and protein in the jejunal and colonic tissues of
healthy subjects and of patients with D-IBS and C-IBS.
Subjects and methods
Subjects
A total of 50 IBS patients who underwent endoscopic
polypectomy were divided into the D-IBS group (27 cases) and the
C-IBS group (23 cases) following diagnosis according to the Rome
III criteria (14). Twenty healthy
volunteers were selected as the control group. Subjects were
excluded if they were <18 or >80 years of age, had a history
of abdominal surgery, were unable to undergo enteroscopy under
general anesthesia or colonoscopy, or had impaired blood
coagulation function, including a platelet count
<50×109/ml or a bleeding time >14 min.
All subjects were informed about the purpose and
methodology of the study and all provided written informed consent.
The study protocol was approved by the ethics committee of Xi’an
Jiaotong University (Xi’an, China).
Specimens
Jejunal tissue biopsy specimens were obtained during
double-balloon push enteroscopy and colon specimens were obtained
during colonoscopy. Four specimens of jejunal mucosa 10 cm distal
to the Treitz ligament were obtained from each subject who
underwent enteroscopy and four specimens of colonic mucosa were
obtained from the ascending colon 5 cm distal to the ileocecal
valve of each subject who underwent colonoscopy.
Two jejunal mucosal and two colonic mucosal
specimens from each subject were immediately frozen in liquid
nitrogen (−170°C) and stored in an ultra-low refrigerator (−80°C).
The remaining samples were fixed in 10% neutral formaldehyde
solution and embedded in paraffin wax.
RNA extraction
Total RNA was extracted from frozen specimens using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions and quantified by
measurement of absorbance at 260 nm. RNA samples were evaluated
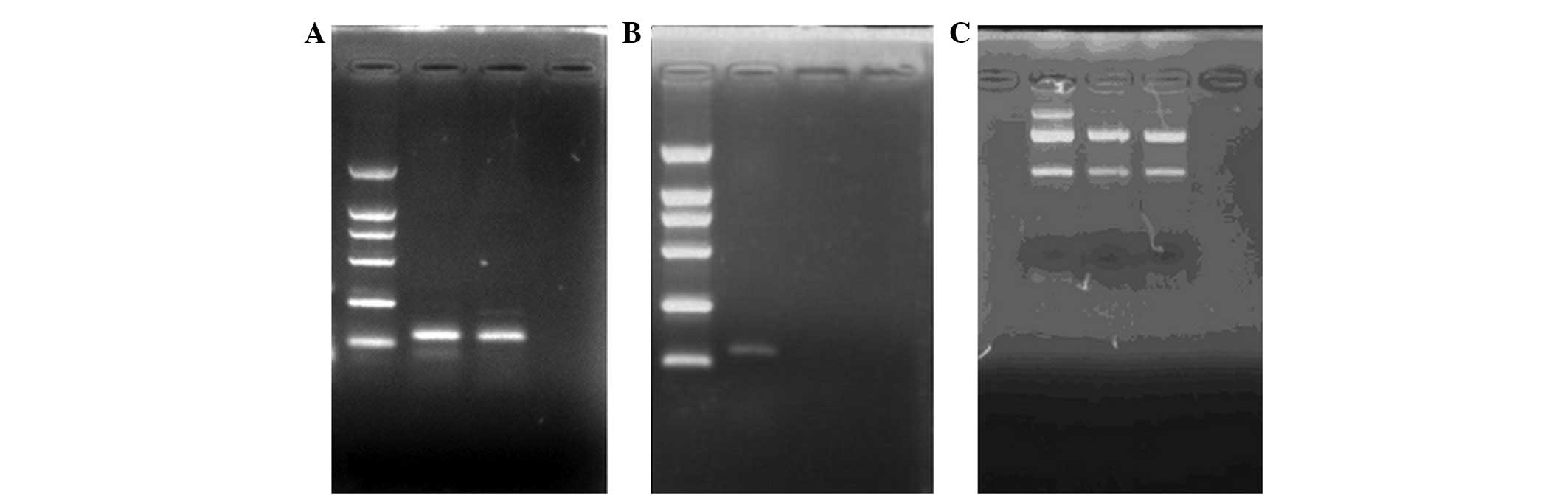
using agarose gel electrophoresis, with the presence of 28S and 18S
ribosomal RNA bands indicating the integrity of the samples
(Fig. 1).
Quantitative PCR (qPCR)
Following c-DNA synthesis, the expression of NOP
receptor mRNA was assayed by amplification using the following
primers: 5′-CTC GGC TGG TGC TGG TGG TA-3′ (forward) and 5′-CGT GCA
GAA GCG CAG AAT GG-3′ (reverse). The expression was normalized
relative to the expression of β-actin mRNA, which was amplified
using the following primers: 5′-GGG TGT GAA CCA TGA GAA GTA TG-3′
(forward) and 5′-CCA TCAC GCC ACA GTT TCC-3′ (reverse). All primers
were designed and synthesized by Sangon Biology Co. (Shanghai,
China). Each 50 μl reaction contained 25 μl 2X PCR Master Mix
(Roche Diagnostics, Switzerland), 1 μl each forward and reverse
primer (final concentration, 0.2 μM each), 1 μl ROX (fluorescence
base), 1 μl cDNA and 21 μl ddH2O. The amplification
protocol consisted of 1 cycle of denaturation at 95°C for 2 min and
40 cycles of denaturation at 94°C for 30 sec, annealing at 62°C for
30 sec and extension at 72°C for 30 sec. The amplification products
were assessed by electrophoresis in 1.6% agarose gels and staining
in 0.5 μg/ml ethidium bromide.
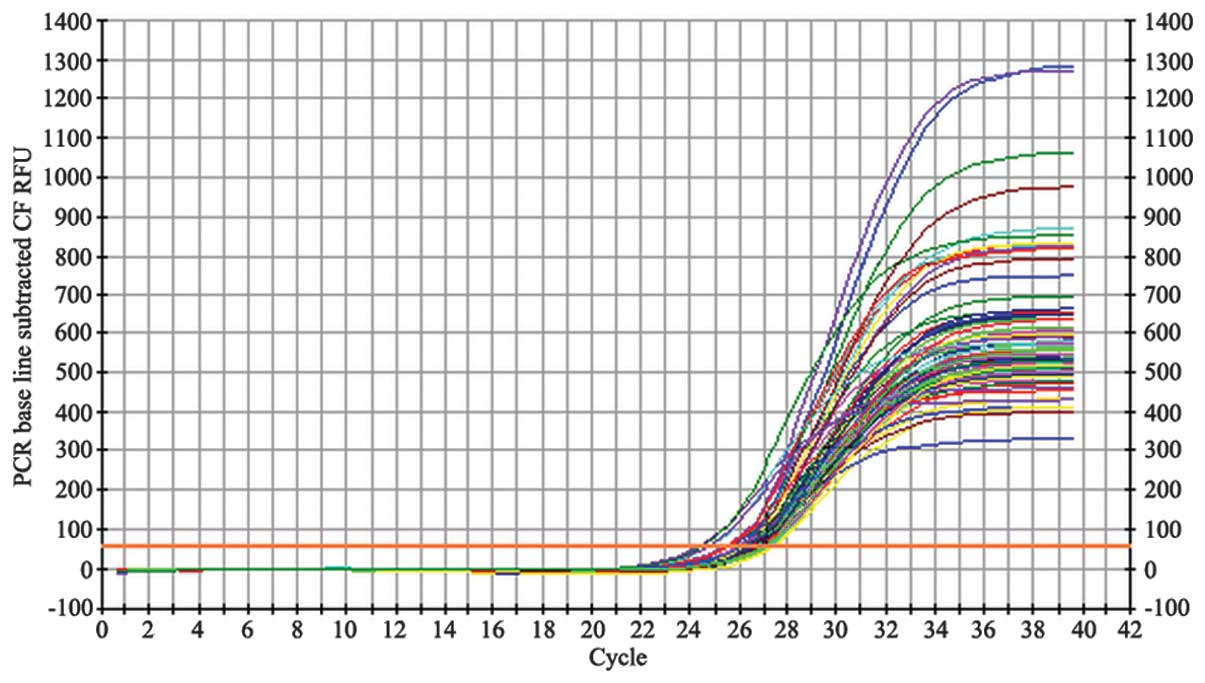
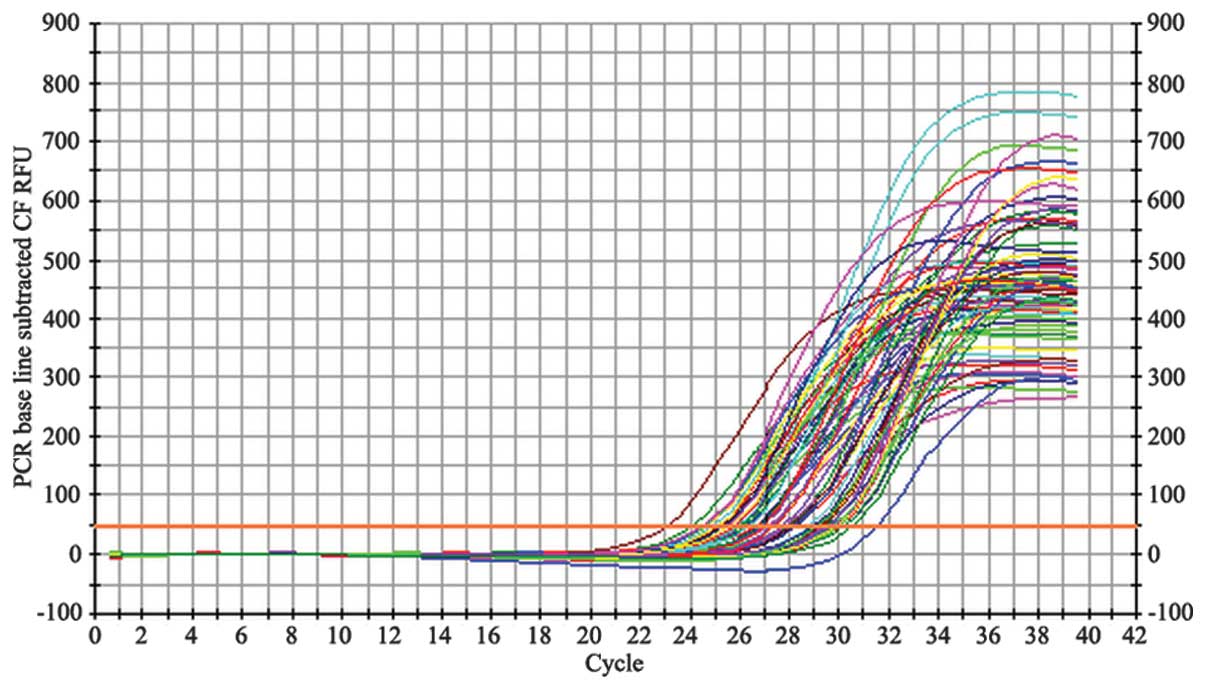
Following each PCR amplification, the fluorescence
intensity curve was generated automatically (Figs. 2 and 3). The fluorescence thresholds for
β-actin and NOP receptor mRNA retrovirus product by the maximal
curvature method were 44.2 and 56.4, respectively. The number of
cycles at which the fluorescence signal reached the threshold was
defined as the cycle threshold (Ct) value. To calibrate differences
between each specimen and the retrovirus products, the β-actin Ct
was subtracted from the NOP Ct value for each specimen.
Standardized values were analyzed using the ΔΔCt method to
determine the relative amount of NOP receptor mRNA in each
specimen.
Immunohistochemistry
Paraffin sections were dewaxed at room temperature
using dimethylbenzene, twice for 15 min and then hydrated in an
ethyl alcohol series (100, 95, 90, 80 and 70%) for 6 min. The
samples were washed in 1X phosphate-buffered saline (PBS) for 35
min and the tissues were then blocked by incubation in 3%
H2O2 for 15 min at 37°C. Then, the samples
were washed three times for 1 min each in 1X PBS, incubated with
goat serum for 15 min at 37°C and incubated with rabbit
anti-nociceptin (1:200; Phoenix Pharmaceuticals, Inc., Burlingame,
CA, USA) for 2–3 h at room temperature. The samples were washed
three times for 5 min each with PBS and incubated with 50 μl goat
anti-rabbit IgG-horseradish peroxidase (HRP) for 40 min at 37°C.
Following three washes in PBS for 5 min each, the samples were
incubated in HRP-labeled streptomycin-avidin working solution
(S-A/HRP) for 30 min at 37°C. Then, the samples were washed three
times for 5 min each with PBS and the color was developed using
3,3′-diaminobenzidine (DAB) for 1–2 min. The sections were rinsed
in running water, duplicated with hematin, rinsed again in running
water and dehydrated with laddered density alcohol. The samples
were made transparent by incubation in dimethybenzene for 10 min,
sealed with neutral tree glue and viewed by optical microscopy.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean (SEM) and analyzed using the multi-independent sample
Kruskal-Wallis H-test, with the Nemenyi test used to identify
significant differences among the three groups. All statistical
analyses were performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic data
We assessed 20 healthy subjects as the control
group, 27 patients diagnosed with D-IBS and 23 patients diagnosed
with C-IBS. All subjects underwent colonoscopy or double-balloon
pushed enteroscopy to obtain specimens of the colon or jejunum
(Table I).
 | Table IDemographic characteristics of the
study subjects. |
Table I
Demographic characteristics of the
study subjects.
| Control | D-IBS | C-IBS |
|---|
|
|
|
|
|---|
| Jejunum | Colon | Jejunum | Colon | Jejunum | Colon |
|---|
| Number of
subjects | 9 | 11 | 15 | 12 | 10 | 13 |
| Gender
(male/female) | 5/4 | 5/6 | 9/6 | 5/7 | 4/6 | 7/6 |
| Age range (mean,
years) | 21–54 (34.0) | 20–65 (34.0) | 24–60 (36.0) | 25–63 (37.2) | 19–53 (34.4) | 19–68 (38.3) |
| Course of disease
(months) | | | 45.4±24.6 | 52.8±29.3 | 89.7±62.8 | 82.1±56.4 |
Expression of NOP receptor mRNA
Using specific primers, we obtained a qPCR product
for NOP mRNA of ~130 bp; the presence of 28S and 18S rRNA bands
demonstrated that the total mRNA remained intact (Fig. 1). The fluorescence intensity curves
for β-actin (Fig. 2) and the NOP
receptor (Fig. 3) illustrate the
quality of the mRNA samples.
Expression of NOP receptor mRNA in the
gut mucosa of healthy subjects
We observed that NOP receptor mRNA was expressed in
all mucosal specimens from the control group, and the relative
quantification was 7.86±4.66 in jejunum specimens and 1.04±0.33 in
colon specimens respectively. This indicated that, the normalized
level of expression was significantly higher in jejunum specimens
than in colon specimens (Table
II).
 | Table IIRelative quantification of NOP
receptor mRNA in control subjects and IBS patients. |
Table II
Relative quantification of NOP
receptor mRNA in control subjects and IBS patients.
| Control | D-IBS | C-IBS |
|---|
|
|
|
|
|---|
| Jejunum | Colon | Jejunum | Colon | Jejunum | Colon |
|---|
| Number of
subjects | 9 | 11 | 15 | 12 | 10 | 13 |
| Relative
quantification | 7.86±4.66 | 1.04±0.33a | 2.71±2.31b | 0.32±0.11c | 6.66±4.94 | 1.05±1.26 |
Expression of NOP receptor mRNA in
patients with IBS
The results showed that the relative expression of
NOP receptor mRNA was 2.71±2.31 in jejunum specimens and 0.32±0.11
in colon specimens of D-IBS patients. These data were significantly
lower than in samples from healthy controls. However, the relative
expression of NOP receptor mRNA was 6.66±4.94 in jejunum specimens
and 1.05±1.26 in colon specimens of C-IBS patients, and no
difference was observed between C-IBS and the control (Table II).
Immunohistochemistry
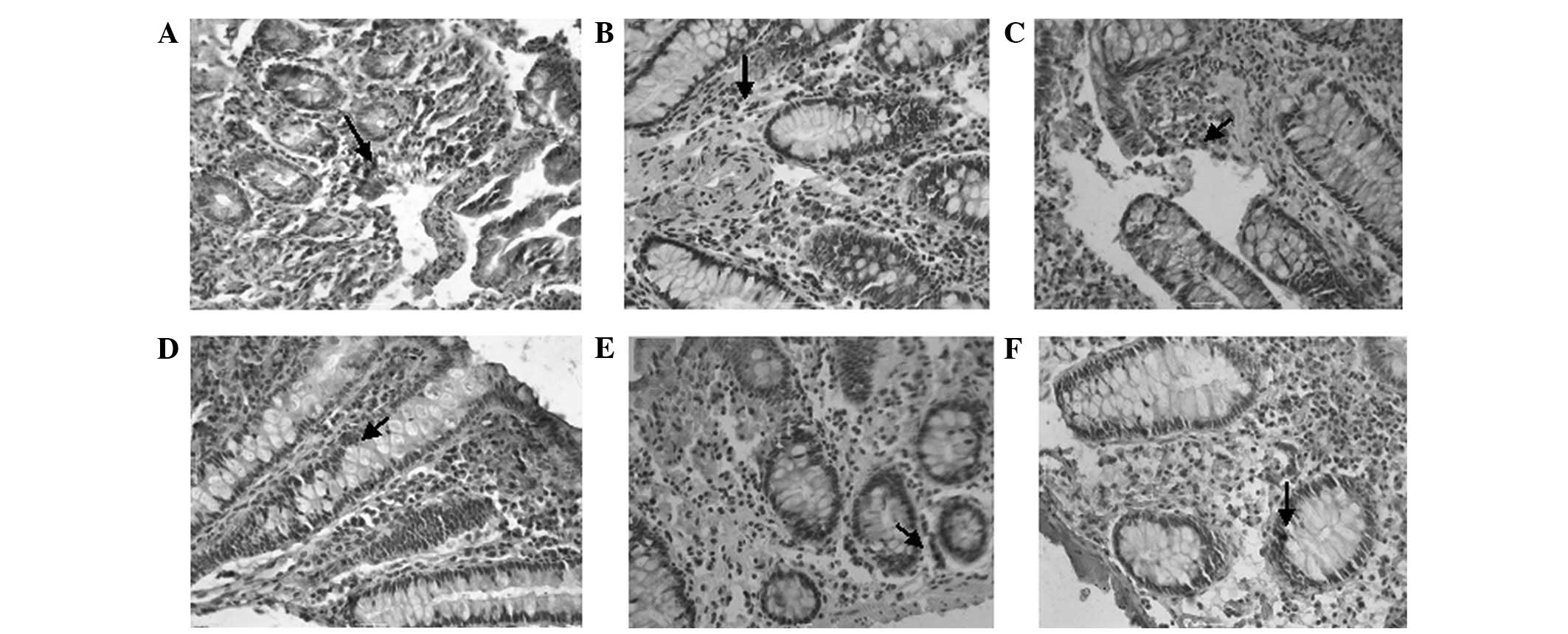
The OFQ-positive cells were stained dark brown in
color following incubation with anti-nociceptin antibody (Fig. 4). We observed no differences in the
number of OFQ-positive cells among the control, D-IBS and C-IBS
groups, in the colonic or jejunal mucosa.
Discussion
The heptadecapeptide N/OFQ is the endogenous ligand
for the NOP receptor and shares significant homology with classical
opioid receptors (15,16). The N/OFQ system is widely
distributed throughout the CNS and in peripheral organs of various
species (15,17–21).
Using qPCR, the N/OFQ system has been detected in the alimentary
tracts of rats, pigs and guinea pigs (19,22).
Using immunohistochemistry, N/OFQ has been shown to be localized in
the rat enteric nervous system, with the mRNA encoding its
precursor (prepro-OFQ/-N) and the cognate receptor ORL-1 expressed
in the intestinal tract. Similar to classical opioids (23,24),
N/OFQ has been shown to affect gastrointestinal motor and secretory
responses, in vitro and in vivo. Unlike opioids,
however, N/OFQ is insensitive to naloxone (25–27).
Although N/OFQ has been observed to inhibit neurogenic contractions
of the stomach and small intestine in vitro, it has also
been shown to contract the rodent colon. In vivo, N/OFQ acts
at sites in the central and peripheral nervous systems stimulating
mechanical activity in the stomach and inhibiting this activity in
the colon (25,28). Thus, N/OFQ acts as a neuromodulator
of gastrointestinal motility and may have additional roles in the
regulation of intestinal blood flow, active ion transport and
immunity (25). Furthermore, the
distribution and level of expression of the N/OFQ system differs
among different species, as does the mechanism of regulation and
functional sites (22–25).
In the current study, we demonstrated that NOP
receptor mRNA and protein were present in the mucosal cells of the
jejunum and colon of healthy subjects and patients with IBS,
suggesting that the N/OFQ system plays an important role in
regulating the pathophysiological onset of intestinal function.
qPCR demonstrated that the level of expression of NOP receptor mRNA
was higher in the jejunum than in the colon in healthy subjects,
suggesting that the regulation of gastrointestinal motion,
secretion and immunity is more complex in the jejunum than in the
colon (29,30) and that the involvement of the NOP
receptor in regulation is proportional to its level of
expression.
Using immunohistochemical staining, we observed no
differences in the number of OFQ-positive cells in the colonic or
jejunal mucosa among the three groups of subjects, suggesting that
OFQ may not be involved in the pathogenesis of IBS. These findings
may also indicate, however, that the sensitivity of our
immunohistochemical methods is insufficient to detect differences
in protein expression (31,32).
The etiopathogenesis of IBS remains unclear.
Although the majority of patients with IBS also have psychiatric
symptoms, causality has not been demonstrated. Thus, although
antidepressants are effective in the treatment of these patients,
IBS is considered a functional gastrointestinal disease (33). Moreover, the pathogenesis of IBS is
closely related to disturbances in the brain-gut axis (34).
The level of NOP mRNA in the colon and jejunum was
lower in patients with D-IBS than in healthy subjects, whereas no
differences were observed between patients with C-IBS and healthy
subjects. It is not clear, however, whether these differences
between D-IBS and C-IBS patients are related to the
pathophysiological mechanisms of these types of IBS, whether an
imbalance in the N/OFQ system is partly responsible for the
oversensitivity of the intestinal tract in IBS patients or whether
NOP overexpression (25,28) induces contraction of the human
small intestine and colon in IBS patients.
In the central and peripheral nervous systems, the
N/OFQ system regulates the release and transit of
neurotransmitters, including norepinephrine, dopamine,
5-hydroxytryptamine and γ-aminobutyric acid (35,36).
Our results suggest that the N/OFQ system also regulates the
expression of neurotransmitters that act on intestinal motion and
that the brain-gut axis is involved in these processes by an
undetermined mechanism. Investigation of the N/OFQ system may be
important in determining the pathogenesis of functional
gastroenteropathies, including IBS.
Acknowledgements
This study was supported by the Hospital Innovation
Funds of Xi’an Jiaotong University. The authors are grateful to
Professor Jianli Wang for the assistance in statistical analyses
and to Professor Jun Yang for technical assistance.
References
|
1
|
Neal KR, Hebden J and Spiller R:
Prevalence of gastrointestinal symptoms six months after bacterial
gastroenteritis and risk factors for development of the irritable
bowel syndrome: postal survey of patients. BMJ. 314:779–782. 1997.
View Article : Google Scholar
|
|
2
|
Drossman DA, Li Z, Andruzzi E, et al: U.S.
householder survey of functional gastrointestinal disorders.
Prevalence, sociodemography, and health impact. Dig Dis Sci.
38:1569–1580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dean BB, Aguilar D, Barghout V, et al:
Impairment in work productivity and health-related quality of life
in patients with IBS. Am J Manag Care. 11(Suppl 1): S17–S26.
2005.PubMed/NCBI
|
|
4
|
El-Serag HB: Impact of irritable bowel
syndrome: prevalence and effect on health-related quality of life.
Rev Gastroenterol Disord. 3(Suppl 2): S3–S11. 2003.PubMed/NCBI
|
|
5
|
El-Serag HB, Olden K and Bjorkman D:
Health-related quality of life among persons with irritable bowel
syndrome: a systematic review. Aliment Pharmacol Ther.
16:1171–1185. 2002. View Article : Google Scholar
|
|
6
|
Longstreth GF, Bolus R, Naliboff B, et al:
Impact of irritable bowel syndrome on patients’ lives: development
and psychometric documentation of a disease-specific measure for
use in clinical trials. Eur J Gastroenterol Hepatol. 17:411–420.
2005.
|
|
7
|
Hulisz D: The burden of illness of
irritable bowel syndrome: current challenges and hope for the
future. J Manag Care Pharm. 10:299–309. 2004.PubMed/NCBI
|
|
8
|
Pace F, Molteni P, Bollani S, et al:
Inflammatory bowel disease versus irritable bowel syndrome: a
hospital-based, case-control study of disease impact on quality of
life. Scand J Gastroentrol. 38:1031–1038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanazawa M, Palsson OS, Thiwan SI, et al:
Contributions of pain sensitivity and colonic motility to IBS
symptom severity and predominant bowel habits. Am J Gastroenterol.
103:2550–2561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olszewski PK, Grace MK, Fard SS, et al:
Central nociceptin/orphanin FQ system elevates food consumption by
both increasing energy intake and reducing aversive responsiveness.
Am J Physiol Regul Integr Comp Physiol. 299:R655–R663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Przydzial MJ and Heisler LK:
Nociceptin/orphanin FQ peptide receptor as a therapeutic target for
obesity. Mini Rev Med Chem. 8:796–811. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushita H, Ishihara A, Mashiko S, et
al: Chronic intracerebroventricular infusion of nociceptin/orphanin
FQ produces body weight gain by affecting both feeding and energy
metabolism in mice. Endocrinology. 150:2668–2673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agostini S, Eutamene H, Broccardo M, et
al: Peripheral anti-nociceptive effect of nociceptin/orphanin FQ in
inflammation and stress-induced colonic hyperalgesia in rats. Pain.
141:292–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drossman DA and Dumitrascu DL: Rome III:
New standard for functional gastrointestinal disorders. J
Gastrointestin Liver Dis. 15:237–241. 2006.
|
|
15
|
Bunzow JR, Saez C, Mortrud M, et al:
Molecular cloning and tissue distribution of a putative member of
the rat opioid receptor gene family that is not a mu, delta or
kappa opioid receptor type. FEBS Lett. 347:284–288. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mogil JS and Pasternak GW: The molecular
and behavioral pharmacology of the orphanin FQ/nociceptin peptide
and receptor family. Pharmacol Rev. 53:381–415. 2001.PubMed/NCBI
|
|
17
|
Anton B, Fein J, To T, Li X, Silberstein L
and Evans CJ: Immunohistochemical localization of ORL-1 in the
central nervous system of the rat. J Comp Neurol. 368:229–251.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Florin S, Leroux-Nicollet I, Meunier JC
and Costentin J: Autoradiographic localization of [3H]
nociceptin binding sites from telencephalic to mesencephalic
regions of the mouse brain. Neurosci Lett. 230:33–36. 1997.
|
|
19
|
Wang JB, Johnson PS, Imai Y, et al: cDNA
cloning of an orphan opiate receptor gene family member and its
splice variant. FEBS Lett. 348:75–79. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nothacker HP, Reinscheid RK, Mansour A, et
al: Primary structure and tissue distribution of the orphanin FQ
precursor. Proc Natl Acad Sci USA. 93:8677–8682. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mollereau C, Simons MJ, Soularue P, et al:
Structure, tissue distribution, and chromosomal localization of the
prepronociceptin gene. Proc Natl Acad Sci USA. 93:8666–8670. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calo’ G, Guerrini R, Rizzi A, Salvadori S
and Regoli D: Pharmacology of nociceptin and its receptor: a novel
therapeutic target. Br J Pharmacol. 129:1261–1283. 2000.PubMed/NCBI
|
|
23
|
Fox DA and Burks TF: Roles of central and
peripheral mu, delta and kappa receptors in the mediation of
gastric acid secretory effects in the rat. J Pharmacol Exp Ther.
244:456–462. 1988.PubMed/NCBI
|
|
24
|
Improta G and Broccardo M: Effect of
selective mu 1, mu 2 and delta 2 opioid receptor agonists on
gastric functions in the rat. Neuropharmacology. 33:977–981. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osinski MA and Brown DR: Orphanin
FQ/nociceptin: a novel neuromodulator of gastrointestinal function.
Peptides. 21:999–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HY, Yan X, Xue QL, et al: Effects of
nociceptin/orphanin FQ on rats with cathartic colon. World J
Gastroenterol. 13:141–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Broccardo M, Guerrini R, Petrella C and
Improta G: Gastrointestinal effects of intracerebroventricularly
injected nociceptin/orphaninFQ in rats. Peptides. 25:1013–1020.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi T, Mizuta Y and Owyang C:
Orphanin FQ, but not dynorphin A, accelerates colonic transit in
rats. Gastroenterology. 119:71–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunkel EJ, Campbell JJ, Haraldsen G, et
al: Lymphocyte CC chemokine receptor 9 and epithelial
thymus-expressed chemokine (TECK) expression distinguish the small
intestinal immune compartment: Epithelial expression of
tissue-specific chemokines as an organizing principle in regional
immunity. J Exp Med. 192:761–768. 2000. View Article : Google Scholar
|
|
30
|
Alemayehu A, Lock KR, Coatney RW and Chou
CC: L-NAME, nitric oxide and jejunal motility, blood flow and
oxygen uptake in dogs. Br J Pharmacol. 111:205–212. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kamel HM, Willmott N, McNicol AM and Toner
PG: The use of electron microscopy and immunocytochemistry to
characterise spontaneously-arising, transplantable rat tumors.
Virchows Arch B Cell Pathol Incl Mol Pathol. 57:11–18. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Looi LM, Yap SF and Cheah PL: Correlation
between oestrogen receptor protein expression in infiltrating
ductal carcinoma of the breast by immunohistochemistry and cytosol
measurements. Ann Acad Med Singap. 26:750–753. 1997.
|
|
33
|
Ford AC, Talley NJ, Schoenfeld PS, Quigley
EM and Moayyedi P: Efficacy of antidepressants and psychological
therapies in irritable bowel syndrome: systematic review and
meta-analysis. Gut. 58:367–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohman L and Simrén M: Pathogenesis of IBS:
role of inflammation, immunity and neuroimmune interactions. Nat
Rev Gastroenterol Hepatol. 7:163–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lü N, Han M, Yang ZL, Wang YQ, Wu GC and
Zhang YQ: Nociceptin/orphanin FQ in PAG modulates the release of
amino acids, serotonin and norepinephrine in the rostral
ventromedial medulla and spinal cord in rats. Pain. 148:414–425.
2010.PubMed/NCBI
|
|
36
|
Yazdani A, Takahashi T, Bagnol D, Watson
SJ and Owyang C: Functional significance of a newly discovered
neuropeptide, orphanin FQ, in rat gastrointestinal motility.
Gastroenterology. 116:108–117. 1999. View Article : Google Scholar : PubMed/NCBI
|