Introduction
Coronary heart disease (CHD), the most common global
cause of morbidity and mortality, is known to consume vast medical
resources (1,2). Although coronary stenting is widely
used in the treatment of patients with CHD, the high rates of late
restenosis and stent thrombosis remain the primary limitations
(3). At present, adjunctive
antiplatelet therapy has been suggested to reduce the incidence
rate of restenosis and stent thrombosis (4,5). In
addition, the utilization of antiplatelet agents has been
demonstrated to be effective in improving final outcomes (6).
Aspirin, a traditional antiplatelet agent, has been
most commonly used for the prevention of ischemic arterial events,
including coronary thrombosis; however, it has no impact on
restenosis (7). Therefore, the
introduction of an effective antiplatelet therapy to be used in
combination with aspirin and alternative antiplatelet agents
following coronary stenting is urgently required. The importance of
antiplatelet therapy with ticlopidine plus aspirin in the
prevention of subacute thrombosis following coronary artery
stenting has been demonstrated (8). However, the use of ticlopidine
presents the risk of serious side-effects, such as neutropenia or
thrombocytopenia. Cilostazol is a selective cyclic adenosine
monophosphate phosphodiesterase inhibitor that is known to inhibit
platelet aggregation and intimal hyperplasia (9,10).
In view of the fact that cilostazol use presents the risk of mild
adverse side effects, cilostazol may theoretically be a desirable
substitute for ticlopidine (11).
Thus, the adjunctive use of cilostazol plus aspirin following
coronary stenting is becoming a more respected option. Recently, it
has been demonstrated that cilostazol is able to prevent thrombosis
following coronary stenting, reduce restenosis and improve clinical
outcomes (12). Moreover,
antiplatelet therapy with cilostazol plus aspirin has been shown to
be effective in preventing late restenosis and stent thrombosis,
with less serious complications (13). However, it has also demonstrated
that cilostazol plus aspirin is not able to be statistically
distinguished from ticlopidine plus aspirin for the prevention of
adverse cardiac events following coronary stenting. Furthermore, a
prospective randomized controlled trial revealed that ticlopidine
plus aspirin resulted in a significant reduction in subacute
thrombosis compared with cilostazol plus aspirin (14).
Therefore, the aim of the present meta-analysis was
to compare the differences between cilostazol plus aspirin and
ticlopidine plus aspirin with regard to the late restenosis and
stent thrombosis rates in patients with CHD following coronary
stenting. This may be beneficial in enabling cardiologists to
select the anti-platelet therapy method with the greatest efficacy
and cost-effectiveness. Furthermore, such knowledge may be further
utilized for the accurate determination of treatment strategies for
CHD.
Materials and methods
Literature search strategy
Relevant papers (published from 1998 to March 1,
2013) were identified through a search in Pubmed, Embase, Web of
Science and Chinese BioMedicine (CBM) databases using the following
terms: (‘coronary disease’ or ‘coronary diseases’ or ‘disease,
coronary’ or ‘coronary heart disease’ or ‘heart disease, coronary’)
and (‘stents’ or ‘stent’ or ‘drug-eluting stents’ or ‘bare metal
stent’ or ‘percutaneous coronary intervention’) and (‘antiplatelet
therapy’ or ‘cilostazol’ or ‘aspirin’ or ‘ticlopidine’). This
search strategy was performed iteratively until no other relevant
articles were found. The references from the eligible articles or
textbooks were also reviewed manually to search for other potential
studies. Disagreements were resolved through discussions between
the authors.
Inclusion and exclusion criteria
The inclusion criteria for the studies included in
the present meta-analysis comprised: i) randomized controlled
trials focusing on the differences in late restenosis and stent
thrombosis between cilostazol plus aspirin and ticlopidine plus
aspirin for patients with CHD following coronary stenting; ii)
studies with follow-up periods of >1 month; iii) studies where
the published data concerning the rates of restenosis and stent
thrombosis were sufficient; iv) studies published in the English or
Chinese languages. Studies were excluded when they were: i) Not
clinically-controlled or relevant to the use of cilostazol plus
aspirin and ticlopidine plus aspirin for patients with CHD
following coronary stenting; ii) duplicates of previous
publications; iii) based on incomplete data; iv) case reports,
letters, reviews, meta-analyses or editorial articles. If more than
one study by the same authors using the same case series was
published, either the study with the largest sample size or the
most recently published study was selected.
Data extraction
Using a standardized form, data from the studies
were extracted independently by two authors. The following
information was obtained for each of the studies: First author,
year of publication, country, language, study design, numbers of
test subjects, eligible lesions, follow-up periods, antiplatelet
drug and dose or dosage, and the rates of restenosis and stent
thrombosis. In case of conflicting evaluations, an agreement was
reached following a discussion between the authors. When required,
a third review resolved any discrepancies or uncertainties with
regard to the data extraction process.
Quality assessment of the included
studies
The methodological quality of each of the included
studies was evaluated by two independent reviewers using the
Physiotherapy Evidence Database (PEDro) scale (15). Eleven assessment items matching
with the quality appraisal were used in this meta-analysis, with
scores ranging from 0 to 10. The PEDro criteria are based on the
presence/absence of 11 items: Eligibility criteria, random
allocation, allocation concealment, similar baseline
characteristics, blinding of all subjects, blinding of therapists,
blinding of outcome assessors, crossover rate of <15%,
intention-to-treat analysis, statistical comparisons between groups
and measures of variability.
Statistical analysis
The differences in late restenosis and stent
thrombosis rates between cilostazol plus aspirin and ticlopidine
plus aspirin were measured by odds ratios (ORs) or standardized
weight differences (SMDs), with 95% confidence intervals (CIs). The
statistical significance of the pooled value was examined using the
Z test. Interstudy variations and heterogeneities were estimated
using the Cochran’s Q-statistic, with P<0.05 indicating a
statistically significant heterogeneity (16,17).
The effect of heterogeneity was also quantified using the
I2 test (ranges from 0 to 100%), which represented the
proportion of interstudy variability that may be contributed to
heterogeneity rather than chance. When a significant Q-statistic
(P<0.05) or I2>50% indicated that heterogeneity
existed among the studies, the random effects model (DerSimonian
Laird method) was conducted for the meta-analysis; otherwise, the
fixed effects model (Mantel-Haenszel method) was used. An analysis
of sensitivity was performed by omitting each study in turn to
assess the quality and consistency of the results. Begger’s funnel
plots were used to detect publication biases. In addition, the
Egger’s linear regression test, which measures funnel plot
asymmetry using a natural logarithm scale of OR, was used to
evaluate the publication biases (18). To ensure the reliability and the
accuracy of the results, two authors assessed the data in the
statistical software programs independently and obtained the same
results. All the P-values were two-sided and all analyses were
calculated using Stata statistical software, version 12.0 (Stata
Corp., College Station, TX, USA).
Results
Characteristics of the included
studies
According to the inclusion criteria, 12 randomized
controlled trials were included in this meta-analysis (3–5,8,14,19–25).
The publication year of the included studies ranged from 1999 to
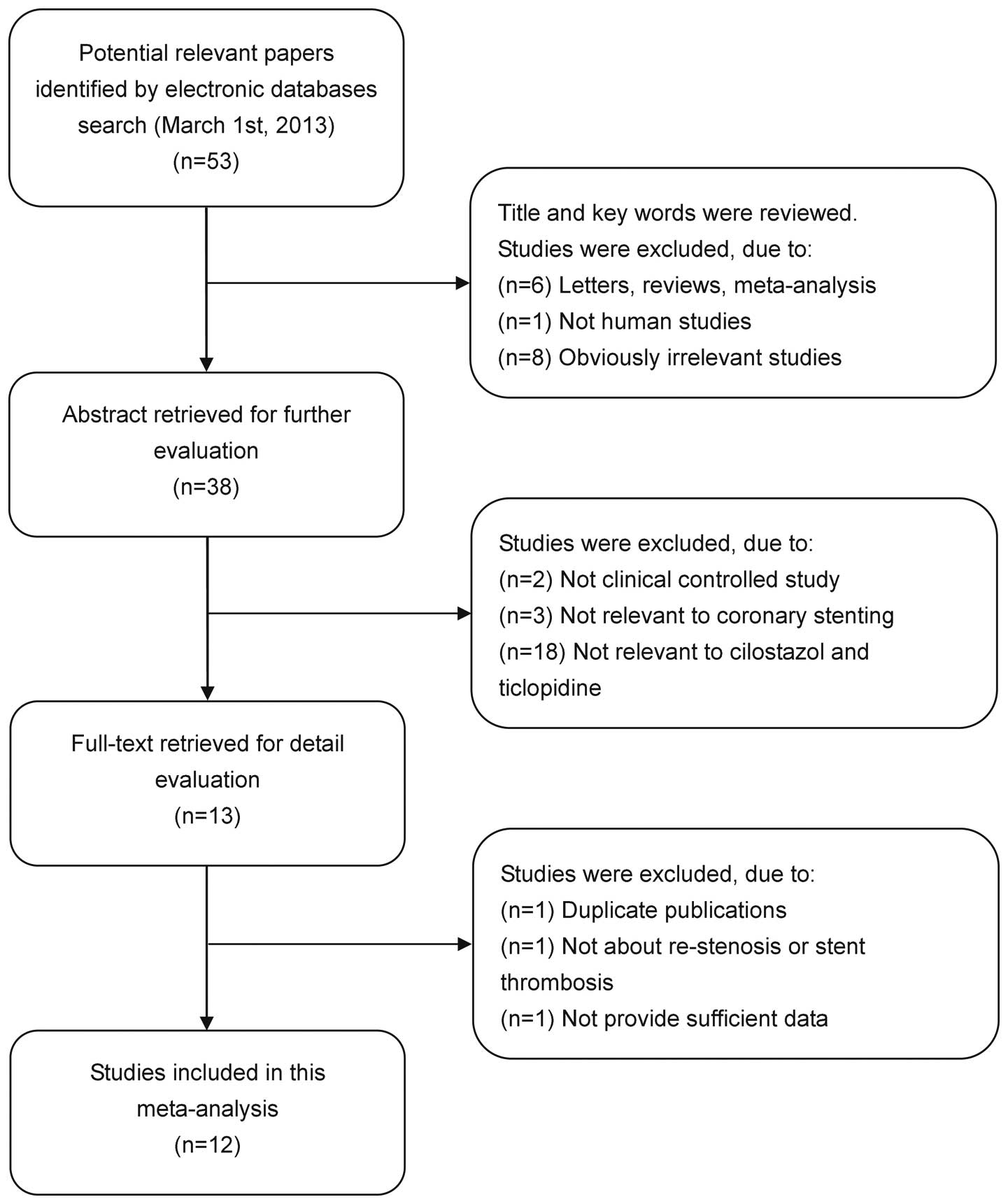
2006. The flow chart of study selection is shown in Fig. 1. The meta-analysis comprised a
total of 2,708 patients with CHD following coronary stenting,
including 1,371 patients treated with cilostazol plus aspirin and
1,337 patients treated with ticlopidine plus aspirin. The doses of
aspirin ranged from 80 to 243 mg/day, while ticlopidine ranged from
200 to 500 mg/day and cilostazol was administered at 100 mg/bid.
The follow-up periods ranged from 1 to 12 months. The main
characteristics of all the eligible studies are listed in Table I.
 | Table ICharacteristics of included studies in
this meta-analysis. |
Table I
Characteristics of included studies in
this meta-analysis.
| First author
(ref) | Year | Country | Number of cases | Follow-up
(months) | Drug doses (mg/dosage
frequency) | PEDro score |
|---|
|
|
|---|
| Cilostazol plus
aspirin | Ticlopidine plus
aspirin | Cilostazol plus
aspirin | Ticlopidine plus
aspirin |
|---|
| Yoon et
al(25) | 1999 | Korea | 147 | 149 | 1 | aspirin 100
(mg/day) | aspirin 100
mg/day | 5 |
| | | | | | cilostazol 100
(mg/bid), 1 month | ticlopidine 250
mg/bid | |
| Park et
al(23) | 2000 | Korea | 208 | 201 | 6 | aspirin 200
(mg/day) | aspirin 200
mg/day | 6 |
| | | | | | cilostazol 100
(bid) | ticlopidine 250
mg/bid | |
| Kozuma et
al(22) | 2001 | Japan | 65 | 65 | 12 | aspirin 200
mg/day | aspirin 200
mg/day | 7 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid | |
| Nagaoka et
al(5) | 2001 | Japan | 18 | 17 | 4 | aspirin 81
mg/day | aspirin 81
mg/day | 6 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid, 4 months | |
| Tanabe et
al(8) | 2001 | Japan | 54 | 50 | 6 | aspirin 81
mg/day | aspirin 243
mg/day | 5 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid | |
| Kamishirado et
al(21) | 2002 | Japan | 54 | 57 | 6 | aspirin 81
mg/day | aspirin 81
mg/day | 8 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid | |
| Inoue et
al(20) | 2004 | Japan | 34 | 32 | - | aspirin 81
mg/day | aspirin 81
mg/day | 6 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid | |
| Sekiguchi et
al(14) | 2004 | Japan | 144 | 138 | 6 | aspirin 81
mg/day | aspirin 81
mg/day | 7 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/bid | |
| Ge et
al(3) | 2005 | China | 201 | 196 | 9 | aspirin 100
mg/day | aspirin 100
mg/day | 8 |
| | | | | | cilostazol 100
mg/bid | ticlopidine 250
mg/bid | |
| Han et
al(4) | 2005 | China | 50 | 50 | 6 | aspirin 100
mg/day | aspirin 100
mg/day | 6 |
| | | | | | cilostazol 100
mg/bid | ticlopidine 250
mg/bid | |
| Takeyasu et
al(24) | 2005 | Japan | 321 | 321 | 6 | aspirin 80–200
mg/day | aspirin 80–200
mg/day | 6 |
| | | | | | cilostazol 200
mg/day | ticlopidine 200
mg/day | |
| Hong et
al(19) | 2006 | China | 75 | 61 | 6 | aspirin 100
mg/day | aspirin 100
mg/day | 8 |
| | | | | | cilostazol 200
mg/day | ticlopidine 500
mg/day, 1 month | |
Quantitative data synthesis
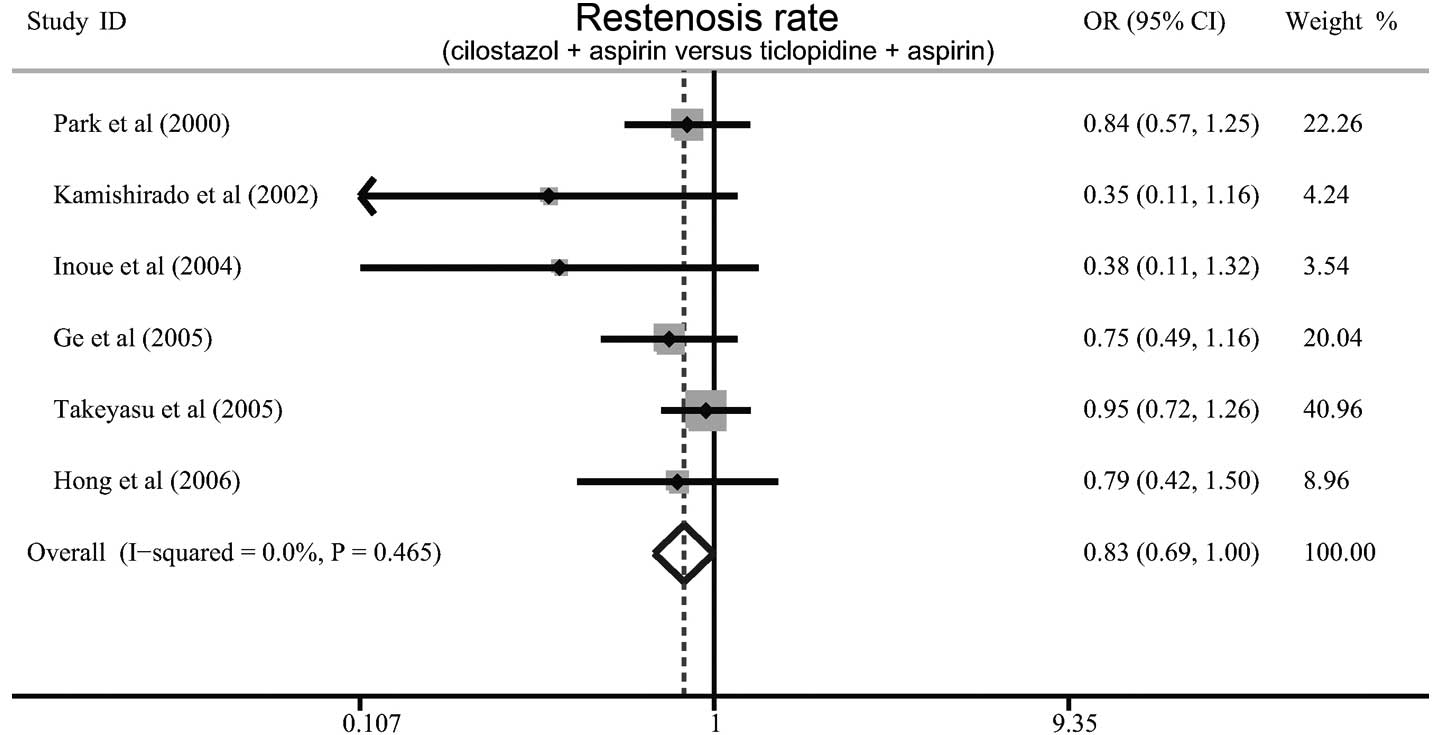
Six studies referred to the differences between
cilostazol plus aspirin and ticlopidine plus aspirin with regard to
the rates of restenosis in patients with CHD following coronary
stenting. There was no evident heterogeneity (P=0.465,
I2=0%), and therefore the fixed effects model was used.
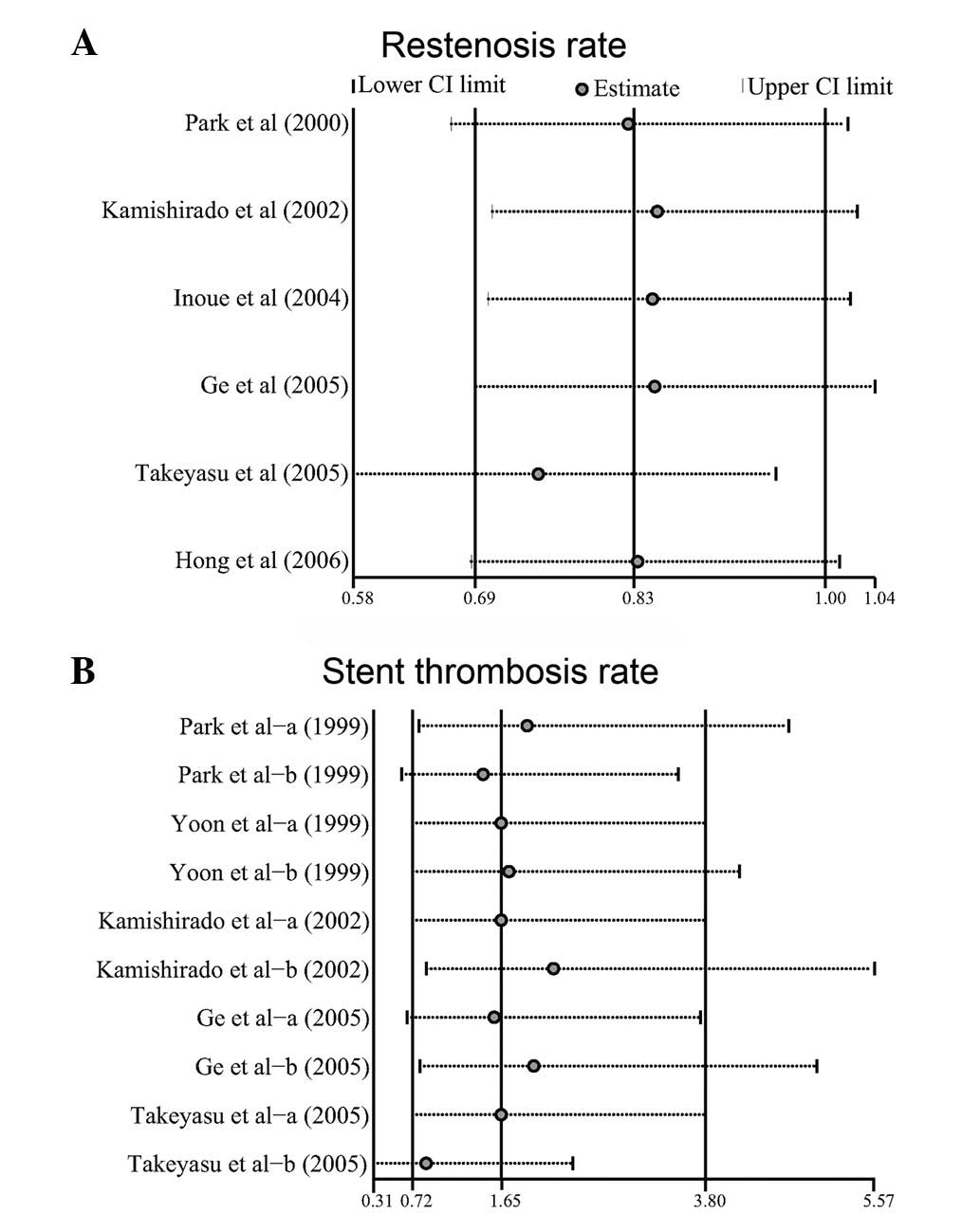
When all the eligible studies were pooled into the meta-analysis,
the results showed that the patients treated with cilostazol plus
aspirin exhibited a lower rate of restenosis than those with
ticlopidine plus aspirin (OR=0.83, 95% CI=0.69–0.99, P=0.047;
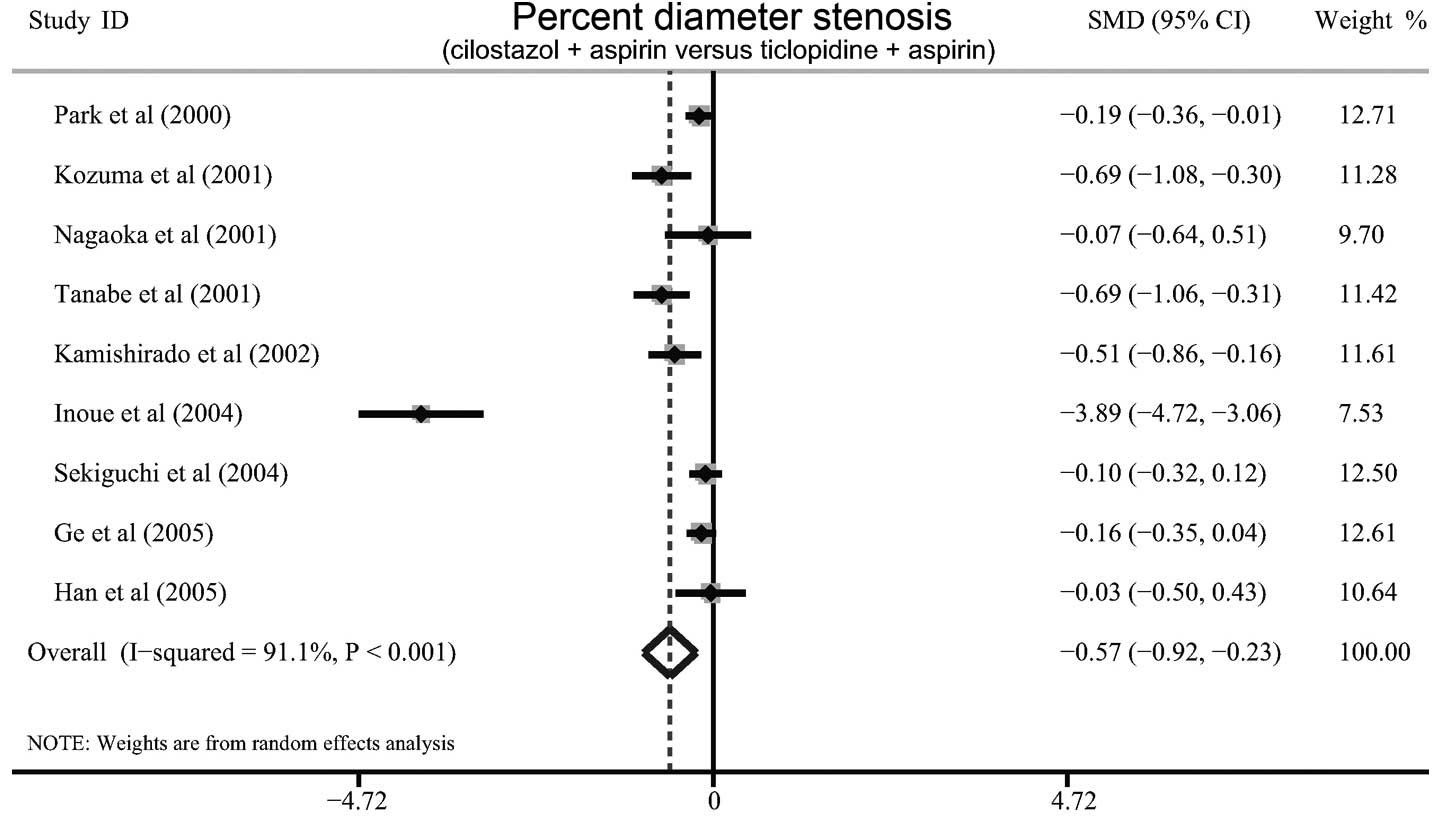
Fig. 2). Furthermore, a
significant difference was observed in the average percent diameter
stenosis between cilostazol plus aspirin and ticlopidine plus
aspirin (SMD=−0.57, 95% CI=−0.92 - −0.23, P=0.001; Fig. 3).
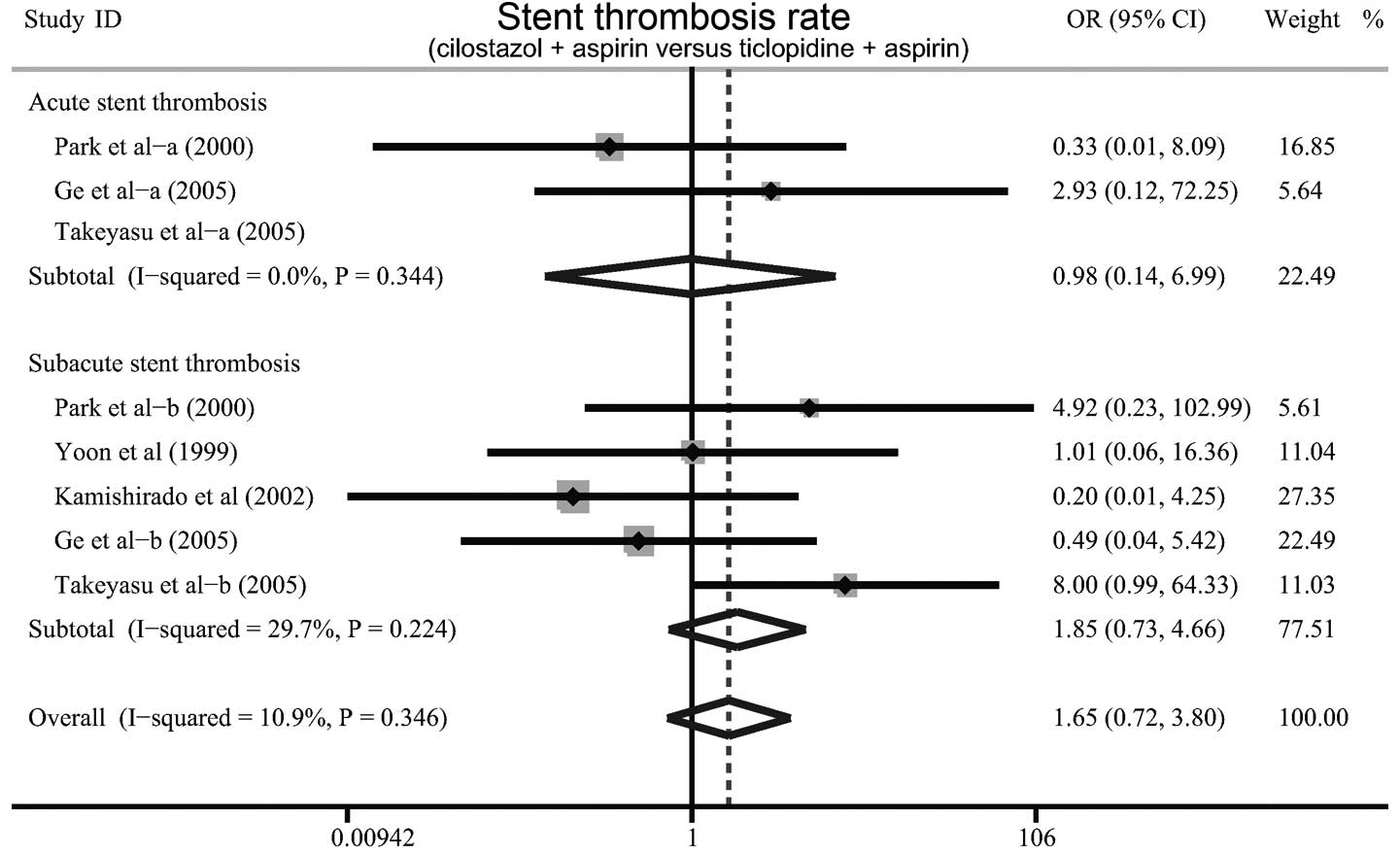
The difference in the rate of stent thrombosis
between cilostazol plus aspirin and ticlopidine plus aspirin was
discussed in six studies. Since no significant heterogeneity was
observed, the fixed effects model was used. The results of the
meta-analysis showed that the incidence of stent thrombosis in
patients treated with cilostazol plus aspirin was not significantly
lower than that in those treated with ticlopidine plus aspirin
(OR=1.66, 95% CI=0.72–3.80, P=0.235). Furthermore, there were no
significant differences in the incidences of acute or subacute
stent thrombosis in patients treated with cilostazol plus aspirin
compared with those treated with ticlopidine plus aspirin (OR=0.98,
95% CI=0.14–6.99, P=0.983; OR=1.85, 95% CI=0.73–6.99, P=0.467,
respectively; Fig. 4).
Sensitivity analysis and publication
bias
A sensitivity analysis was performed to assess the
influence of each individual study on the pooled ORs by omitting
each of the individual studies in turn. The analysis results
suggested that no individual study significantly affected the
pooled values of the rates of restenosis or stent thrombosis
(Fig. 5), indicating statistically
robust results.
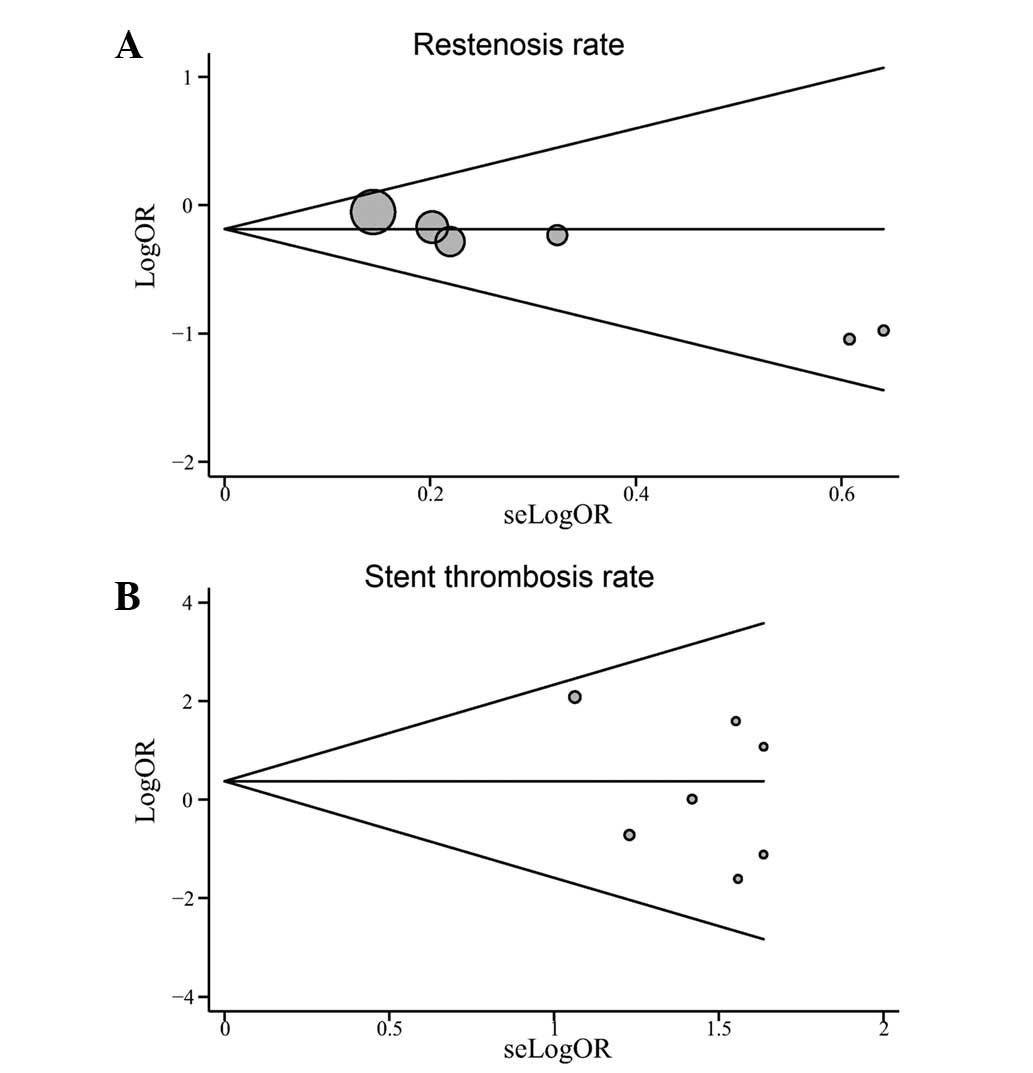
Publication bias exists to the extent that the
available results for a study are unrepresentative of all the
results for that study. Begger’s funnel plots and Egger’s linear
regression tests were performed to assess the publication bias of
the included studies. The shapes of the funnel plots did not reveal
any indication of obvious asymmetry (Fig. 6). The Egger’s tests also showed
that there was no statistically significant evidence of publication
bias for the rates of restenosis and stent thrombosis (t=−2.04,
P=0.111; t=−1.18, P=0.292, respectively).
Discussion
Cilostazol, a selective phosphodiesterase III
inhibitor, has been demonstrated to be effective in reducing the
incidence of restenosis following coronary stenting (26). At present, the combination of
cilostazol and aspirin is regarded as the most acceptable option
for the antithrombotic treatment of patients with CHD undergoing
coronary stenting (27). However,
certain studies have shown that aspirin plus ticlopidine is
superior to the combination of cilostazol and aspirin with regard
to the midterm occurrence of adverse side-effects (28). Ticlopidine is a potent inhibitor of
collagen-induced platelet aggregation, which has been demonstrated
to decrease the incidence of clinical events following coronary
stenting (14). By activating
platelet adenylate cyclase, ticlopidine is able to enhance the
stimulatory action of prostaglandin E1 (PGE1) on the cyclase and
block the inhibitory action of PGE2 on the cyclase (5). However, compared with cilostazol, the
use of ticlopidine may result in more severe side-effects,
therefore leading to a shorter course of treatment (29). Numerous studies have been designed
to compare the effectiveness of cilostazol plus aspirin with
ticlopidine plus aspirin (3,5,21).
However, the definite outcomes of the quantitative angiographic
analyses of the two antithrombotic regimens remain in dispute.
Thus, there is a requirement for a comprehensive well-defined
comparison of the two groups of antithrombotic regimens to be
implemented. As a powerful statistical method, a meta-analysis
provides a quantitative approach for pooling the results of
different studies on the same topic. Therefore, a systematic review
and meta-analysis of the differences between cilostazol plus
aspirin and ticlopidine plus aspirin, with regard to the rates of
restenosis and stent thrombosis, was of great value.
In this meta-analysis, 12 randomized controlled
studies were included with a total of 2,708 patients with CHD
following coronary stenting. The patient population comprised 1,371
patients treated with cilostazol plus aspirin and 1,337 patients
treated with ticlopidine plus aspirin. The predominant finding of
this meta-analysis was that the rates of restenosis in patients
treated with cilostazol plus aspirin were significantly lower than
those in patients treated with ticlopidine plus aspirin, suggesting
that cilostazol may be more effective than ticlopidine in reducing
restenosis. A possible reason may be the different functional
mechanisms of the two antithrombotic agents. However, no
significant differences were observed in the rates of acute or
subacute stent thrombosis between cilostazol plus aspirin and
ticlopidine plus aspirin. These results suggested that there was no
difference between cilostazol and ticlopidine with regard to their
efficacy as an adjunctive therapy to coronary stenting or in the
prevention of stent-associated thrombosis. This is despite the fact
that cilostazol demonstrates a different anti-platelet mechanism to
ticlopidine, which may lead to a suppression of platelet
aggregation. These results were inconsistent with the outcomes
published by Hashiguchi et al and Schleinitz et
al(6,29), which may be due to the limited
number of included studies.
In the interpretation of the results of the present
meta-analysis, it is necessary for certain specific issues
pertinent to the study to be addressed. The sample size included in
the meta-analysis is relatively small and may not provide
sufficient statistical power to estimate the differences between
cilostazol plus aspirin and ticlopidine plus aspirin. Furthermore,
potential heterogeneity and bias may exist due to the differences
in the inclusion criteria, follow-up periods, doses of antiplatelet
drugs and the severity of disease. In addition, as previously
mentioned, each pretreatment regimen was not always identical and
the doses of aspirin, ticlopidine or cilostazol were variable.
Further limitations included the facts that the type of stent used
in each patient was not always identical and there may have been
differences with regard to the efficacy of the antiplatelet agents.
Moreover, although all participants of each study were well defined
with similar inclusion criteria, there may be factors that were not
taken into account and that may have influenced our results. There
is thus a requirement for the present results to be interpreted
with caution due to the potential heterogeneity among trials.
In conclusion, this meta-analysis suggests that the
use of cilostazol plus aspirin may result in lower restenosis rates
and percent diameter stenosis than ticlopidine plus aspirin for
patients with CHD following coronary stenting. However, further
well-designed clinical trials are required to investigate the
differences between cilostazol and ticlopidine.
References
|
1
|
Fatima S, Ahmad SI and Ahmad HR: The
intercept and slope of breathlessness/chest pain-heart rate
relationship in patients with coronary artery disease using
exercise tolerance test. J Pak Med Assoc. 62:382–385.
2012.PubMed/NCBI
|
|
2
|
Kang YH, Lao HY, Yu XY, Chen JY and Zhong
SL: Progress in genetic and epigenetic research on in-stent
restenosis after percutaneous coronary interventions. Zhonghua Yi
Xue Yi Chuan Xue Za Zhi. 29:38–42. 2012.(In Chinese).
|
|
3
|
Ge J, Han Y, Jiang H, et al; RACTS
(Randomized Prospective Antiplatelet Trial of Cilostazol Versus
Ticlopidine in Patients Undergoing Coronary Stenting) Trial
Investigators. RACTS: a prospective randomized antiplatelet trial
of cilostazol versus ticlopidine in patients undergoing coronary
stenting: long-term clinical and angiographic outcome. J Cardiovasc
Pharmacol. 46:162–166. 2005. View Article : Google Scholar
|
|
4
|
Han Y, Wang S, Li Y, et al: Cilostazol
improves long-term outcomes after coronary stent implantation. Am
Heart J. 150:5682005.PubMed/NCBI
|
|
5
|
Nagaoka N, Matsubara T, Okazaki K, Masuda
N, Shikaura K and Hotta A: Comparison of ticlopidine and cilostazol
for the prevention of restenosis after percutaneous transluminal
coronary angioplasty. Jpn Heart J. 42:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashiguchi M, Ohno K, Nakazawa R, Kishino
S, Mochizuki M and Shiga T: Comparison of cilostazol and
ticlopidine for one-month effectiveness and safety after elective
coronary stenting. Cardiovasc Drugs Ther. 18:211–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harding SA, Walters DL, Palacios IF and
Oesterle SN: Adjunctive pharmacotherapy for coronary stenting. Curr
Opin Cardiol. 16:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanabe Y, Ito E, Nakagawa I and Suzuki K:
Effect of cilostazol on restenosis after coronary angioplasty and
stenting in comparison to conventional coronary artery stenting
with ticlopidine. Int J Cardiol. 78:285–291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang JS, Jin HY, Seo JS, et al: A
meta-analysis of randomized controlled trials appraising the
efficacy and safety of cilostazol after coronary artery stent
implantation. Cardiology. 122:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura T, Tsuruta S and Uchiyama S:
Cilostazol combined with aspirin prevents early neurological
deterioration in patients with acute ischemic stroke: a pilot
study. J Neurol Sci. 313:22–26. 2012. View Article : Google Scholar
|
|
11
|
Dangas G, Mehran R, Abizaid AS, et al:
Combination therapy with aspirin plus clopidogrel versus aspirin
plus ticlopidine for prevention of subacute thrombosis after
successful native coronary stenting. Am J Cardiol. 87:470–472.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rogers KC, Faircloth JM and Finks SW: Use
of cilostazol in percutaneous coronary interventions. Ann
Pharmacother. 46:839–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng DF, Liu M, Jin DM, Wu W, Deng J and
Wang JF: Cilostazol-based triple antiplatelet therapy compared to
dual antiplatelet therapy in patients with coronary stent
implantation: a meta-analysis of 5,821 patients. Cardiology.
122:148–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sekiguchi M, Hoshizaki H, Adachi H,
Ohshima S, Taniguchi K and Kurabayashi M: Effects of antiplatelet
agents on subacute thrombosis and restenosis after successful
coronary stenting: a randomized comparison of ticlopidine and
cilostazol. Circ J. 68:610–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maher CG, Sherrington C, Herbert RD,
Moseley AM and Elkins M: Reliability of the PEDro scale for rating
quality of randomized controlled trials. Phys Ther. 83:713–721.
2003.PubMed/NCBI
|
|
16
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong B, Qian JY, Fan B, Jin XJ and Ge JB:
A randomized trial of cilostazol versus ticlopidine for
anti-platelet therapy after coronary artery stenting for prevention
of restenosis. Chin J Intervent Cardiol. 14:170–172. 2006.(In
Chinese).
|
|
20
|
Inoue T, Uchida T, Sakuma M, et al:
Cilostazol inhibits leukocyte integrin Mac-1, leading to a
potential reduction in restenosis after coronary stent
implantation. J Am Coll Cardiol. 44:1408–1414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamishirado H, Inoue T, Mizoguchi K, et
al: Randomized comparison of cilostazol versus ticlopidine
hydrochloride for antiplatelet therapy after coronary stent
implantation for prevention of late restenosis. Am Heart J.
144:303–308. 2002. View Article : Google Scholar
|
|
22
|
Kozuma K, Hara K, Yamasaki M, et al:
Effects of cilostazol on late lumen loss and repeat
revascularization after Palmaz-Schatz coronary stent implantation.
Am Heart J. 141:124–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SW, Lee CW, Kim HS, et al: Effects of
cilostazol on angiographic restenosis after coronary stent
placement. Am J Cardiol. 86:499–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takeyasu N, Watanabe S, Noguchi Y,
Ishikawa K, Fumikura Y and Yamaguchi I: Randomized comparison of
cilostazol vs ticlopidine for antiplatelet therapy after coronary
stenting. Circ J. 69:780–785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon Y, Shim WH, Lee DH, et al: Usefulness
of cilostazol versus ticlopidine in coronary artery stenting. Am J
Cardiol. 84:1375–1380. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jennings DL and Kalus JS: Addition of
cilostazol to aspirin and a thienopyridine for prevention of
restenosis after coronary artery stenting: a meta-analysis. J Clin
Pharmacol. 50:415–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubboli A, Milandri M, Castelvetri C and
Cosmi B: Meta-analysis of trials comparing oral anticoagulation and
aspirin versus dual antiplatelet therapy after coronary stenting.
Clues for the management of patients with an indication for
long-term anticoagulation undergoing coronary stenting. Cardiology.
104:101–106. 2005. View Article : Google Scholar
|
|
28
|
Cosmi B, Rubboli A, Castelvetri C and
Milandri M: Ticlopidine versus oral anticoagulation for coronary
stenting. Cochrane Database Syst Rev. CD0021332001.PubMed/NCBI
|
|
29
|
Schleinitz MD, Olkin I and Heidenreich PA:
Cilostazol, clopidogrel or ticlopidine to prevent sub-acute stent
thrombosis: a meta-analysis of randomized trials. Am Heart J.
148:990–997. 2004. View Article : Google Scholar : PubMed/NCBI
|