Introduction
Bilateral primary breast cancer (BPBC), a type of
primary breast cancer, has an incidence rate of between 2 and 11%
(1), of which bilateral invasive
lobular carcinoma accounts for 6–28%. Invasive lobular carcinoma
exhibits a specific pathology and the likelihood of developing
synchronal or metachronous bilateral breast cancer is two-fold that
of invasive ductal carcinoma. Common sites of invasive ductal
carcinoma metastasis are the liver, lungs and bones, whereas
invasive lobular carcinoma more readily metastasizes to the
peritoneum, retroperitoneum and the genitourinary system, in
addition to the gastrointestinal tract (2). In this case report, we describe a
case of small bowel obstruction in our hospital, which was
diagnosed as metastatic adenocarcinoma of the small intestine
caused by primary invasive lobular breast cancer.
Case report
A 41-year-old female was admitted to our hospital
(Ruikang Hospital Affiliated to Guangxi University of Chinese
Medicine, Nanning, China), presenting with recurrent abdominal pain
(apparent for >1 year) and paroxysmal abdominal pain accompanied
by bloating and loose stools, but no nausea or vomiting. The
results of the physical examination revealed abdominal distension
and visible intestinal peristaltic waves; however, there was no
whole abdominal or rebound tenderness. Orthostatic images of the
abdomen showed an incomplete intestinal obstruction, while the
results of an oral colonoscopy revealed a narrow lower end of the
jejunum, no ulceration and a new neoplasm, which was considered to
be narrow small intestine (possibly the lower end of the jejunum).
The narrow section was surgically biopsied (2011–6099) and judged
to be a chronic mucosal inflammation of the lower end of the
jejunum. Laparotomy revealed a hardening and narrowing of the small
bowel at the junction of the jejunum and ileum, with significant
expansion proximal to the jejunum and a large volume of intestinal
contents. Numerous lymph nodes were observed on the mesentery, and
the intestinal canal had significant hyperemia and edema. There was
a section of diseased small intestine ~1.5 m to the ileocecal
valve, and the lumen was hardened and narrow. The two mucous
membranes of the small intestine exhibited pathological changes and
were smooth and complete. The lesions did not penetrate the mucous
layer. Small bowel resection and anastomosis surgery were performed
on the narrow section (Fig. 1).
The postoperative pathological results revealed a poorly
differentiated/undifferentiated carcinoma of the small intestine,
with invasion of the cancerous tissue into the intestinal
submucosa, muscularis externa and placenta percreta. Extensive
invasion of the cancerous tissue was also observed inside the
mesenteric layer; however, there was no cancer present in the
mucous layer and no metastasis into the intestinal lymph node (0/2;
number of lymph nodes with metastasis/the total number of
intestinal lymph nodes). Immunohistochemical analysis showed that
the tumor cells were positive for cytokeratin (CK) (+), epithelial
membrane antigen (EMA) (+), cell proliferation nuclear antigen
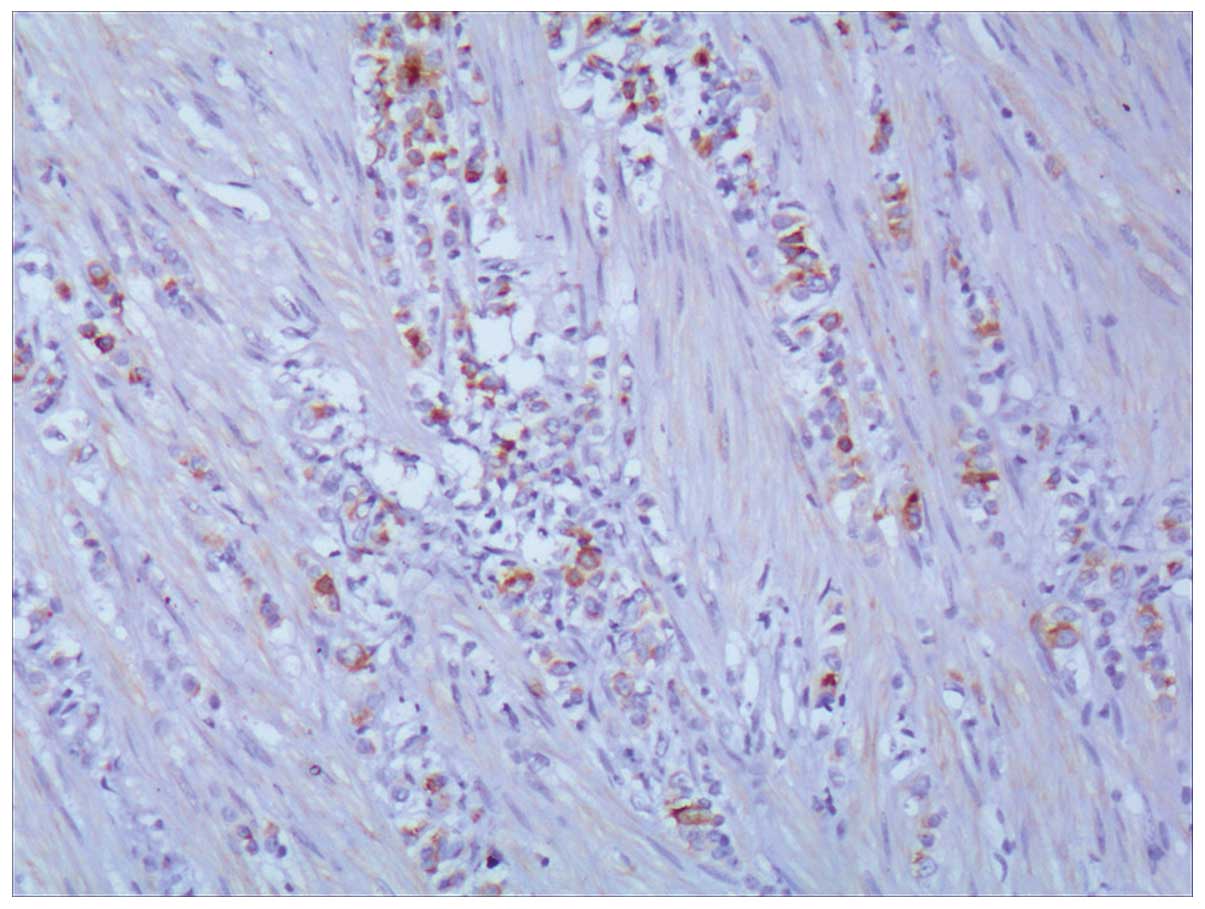
Ki-67 (+5–10%), gross cystic disease fluid protein 15 (GCDFP-15)
(+) (Fig. 2), estrogen receptor
(ER) (+) (Fig. 3) and progesterone
receptor (PR) (+) ; other indicators, such as vimentin (Vim),
synaptophysin (Syn), chromogranin A (CgA), Acid calcium binding
protein S-100, smooth muscle actin (SMA), leucocyte common antigen
(LCA) and carcinoembryonic antigen (CEA), were negative. Combined
with the immunohistochemistry results, it was recommended that the
primary tumor from the lacteal gland be located. A postoperative
examination showed that both breasts were symmetrical, while there
was a goiter measuring ~3×3 cm in the upper outer quadrant of the
right breast, and another goiter measuring ~2×1.5 cm just above it.
The two goiters were hard, without clear borders. In addition, a
4×3 cm hard goiter was felt at the right armpit. The color Doppler
results revealed a hypoechoic mass of ~15×29×7 mm in the 10 o’clock
position of the right breast, a hypoechoic mass of ~14×8 mm in the
12 o’clock position of the right breast and a hypoechoic mass of
~15×7 mm in the 12 o’clock position of the left breast. Punctiform
blood flow signals were observed inside and at the edge above the
hypoechoic mass. A hypoechoic nodule, measuring ~8×5 mm, was probed
in the right armpit, while no swelling of the lymph nodes was
detected in the left armpit. With regard to the thyroid, the
bilateral lobes were positively substantiality mass, and the
internal right lobe was fluidified and calcified. The pathology
results for the biopsy of the right breast goiter revealed that the
goiter was an infiltrating lobular carcinoma; lobular carcinoma
in situ was visible peripherally (accounting for 80%). Tests
showed ER 80%, PR 70%, CerbB-2 (−) and Ki-67 (−). The pathology
results for the biopsy of the thyroid indicated a thyroid
follicular adenoma.
Following two cycles of XT chemotherapy (paclitaxel,
210 mg, 175 mg/m2 and Xeloda, 2,500 mg, 1,250
mg/m2), MRI results indicated that the goiter on the
upper outer quadrant of right breast was patchy, measuring
~17×35×34 mm, with inhomogeneous enhancement, and the time-signal
intensity curve was of the rising type. MRI examination following
five cycles of chemotherapy revealed that the goiter on the upper
outer quadrant of the right breast was irregular, with a trend of
fusion, ~17×35 cm and 17×16 cm. The time-signal intensity curve was
of the rising type. According to the Response Evaluation Criteria
in Solid Tumors (RECIST), the evaluation was stable disease (SD). A
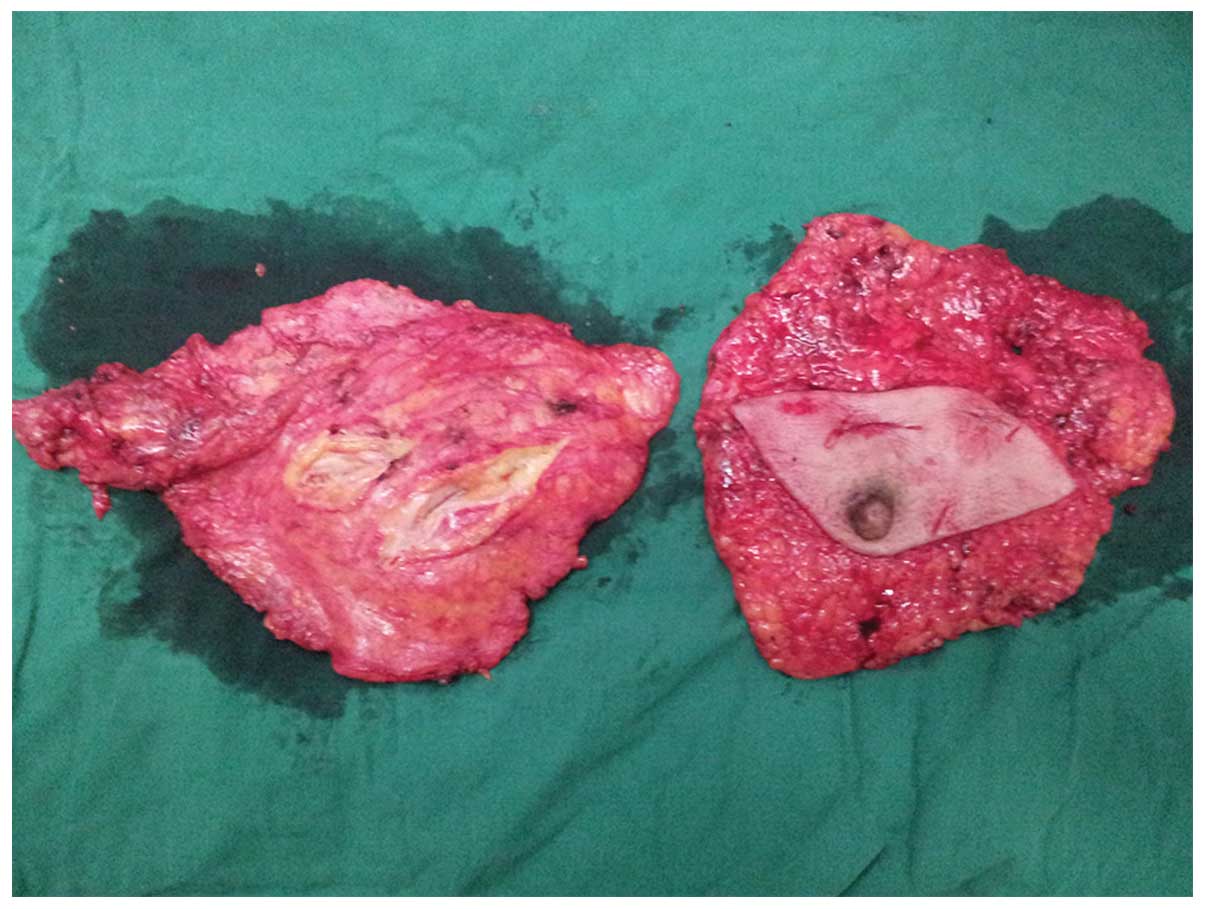
bilateral modified radical mastectomy was performed (Fig. 4), and the pathology results
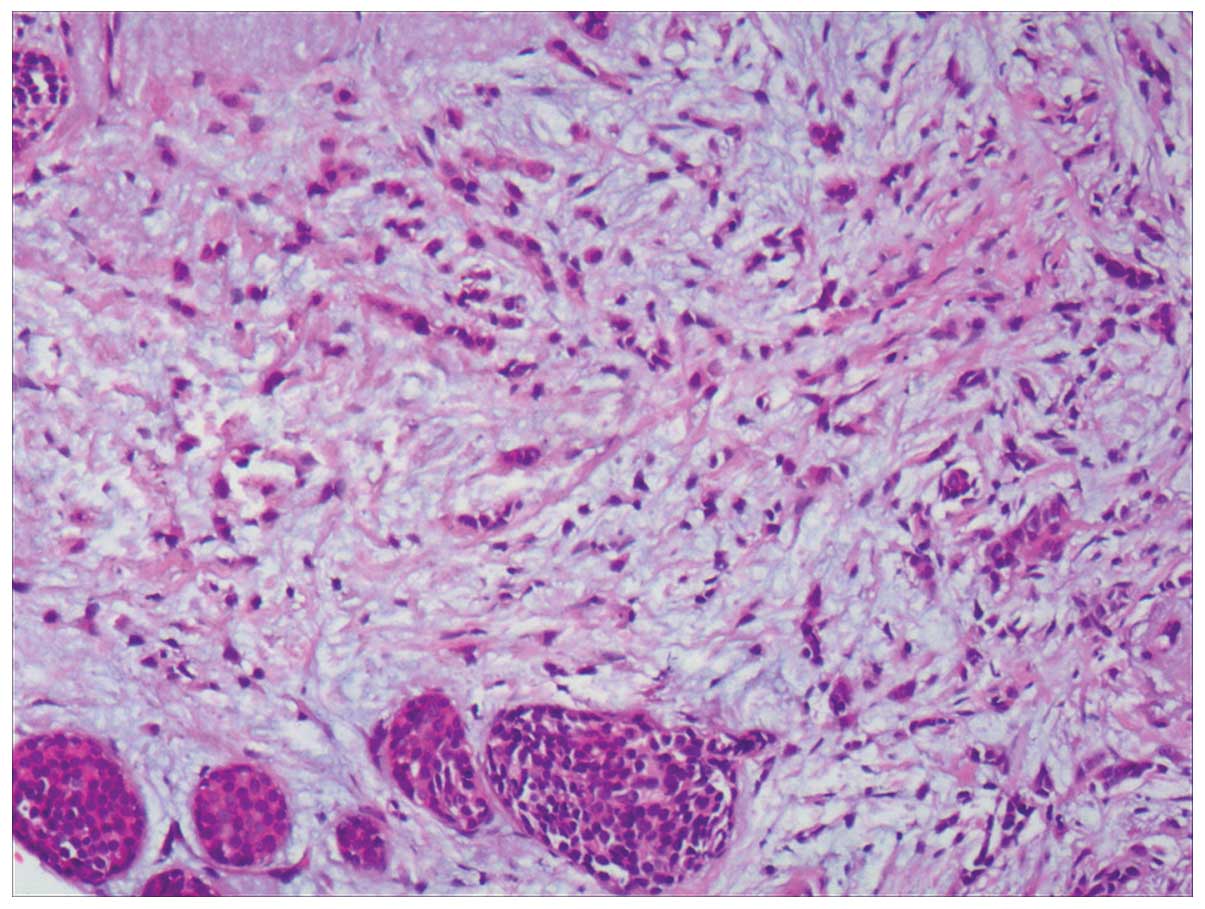
demonstrated that the right breast had an invasive lobular
carcinoma (Fig. 5) with two
lesions (~3×2×2 cm and 5×4×3 cm, respectively), axillary lymph node
29/31 metastasis, ER 8%, PR 90%, CerbB-2 (+/−) and Ki-67 <3%.
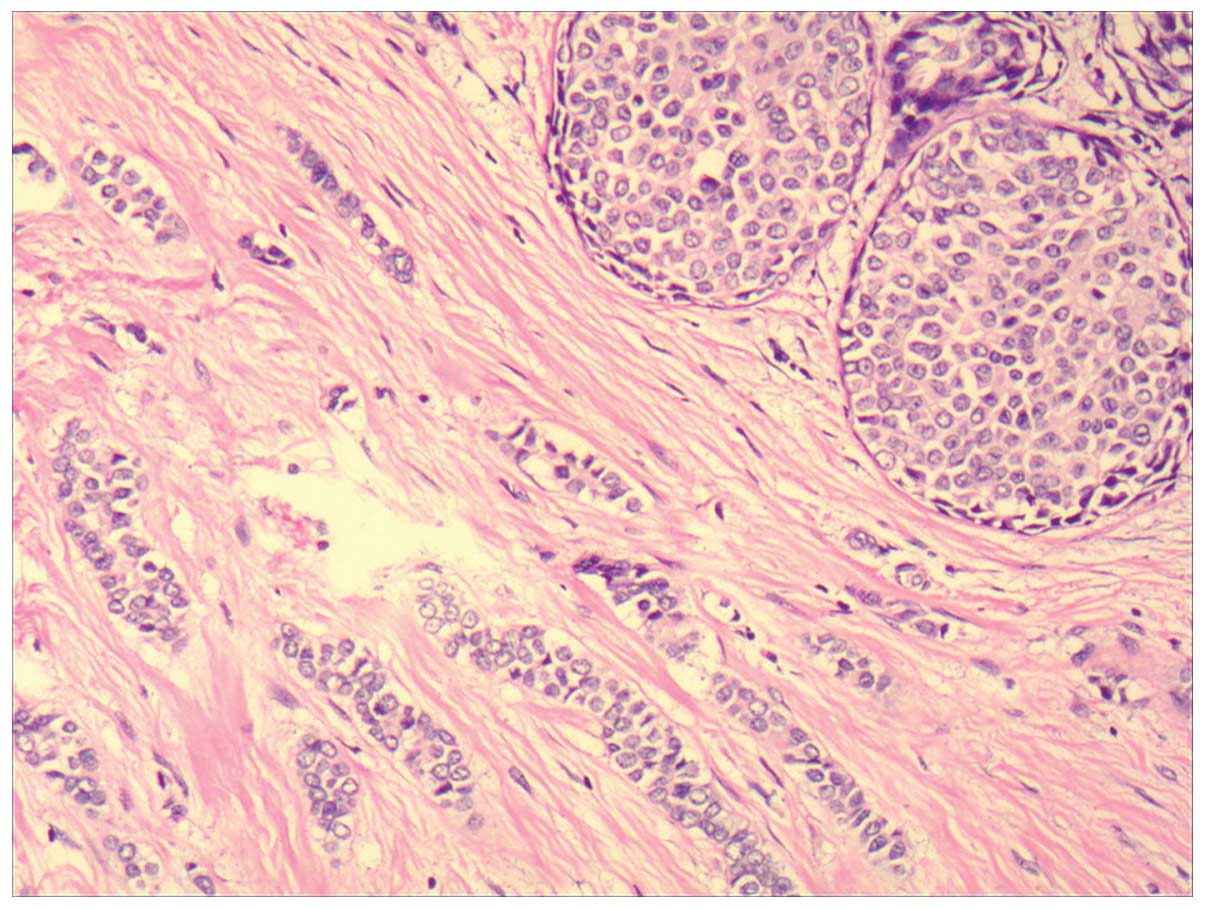
The left breast had an invasive lobular carcinoma (Fig. 6) with two lesions, there was no
metastasis in all 17 axillary lymph nodes, ER (−), PR 50~75%,
CerbB-2 (−) and Ki-67<5%. It was therefore recommended that the
patient undergo an oophorectomy and commence endocrine therapy.
Discussion
Bilateral primary breast cancer (BPBC), a type of
primary breast cancer, has an incidence rate of between 2 and 11%
(1) and between 1.4 and 7.7% in
China (3). The pathology includes
invasive lobular carcinoma (4.8%) (4). A retrospective analysis from McLemore
et al(5) revealed that
1,516 out of 12,550 cases of breast cancer in a 15-year period were
invasive lobular carcinoma, accounting for 12%. Invasive lobular
carcinoma has specific cancer characteristics and is relatively
rare, compared with other types of invasive breast cancer. Invasive
lobular carcinoma has an ipsilateral multifocal breast
characteristic, and bilateral cancers are frequently observed
(6). It has negative
characteristics of PR+, ER+, HER-2, P53 and EGFR (7). In the current case, the breast tumor
biopsy results prior to chemotherapy were PR+, ER+ and HER-2 (−),
which were consistent with the literature. The postoperative
pathology indicated ER (−), and the lobular carcinoma in
situ prior to chemotherapy accounted for 80%. Following the
chemotherapy, there was no obvious in situ carcinoma
component. These changes were considered to result from the impact
of chemotherapy on the breast cancer cells. It has been suggested
that gastrointestinal metastasis occurs more commonly in breast
invasive lobular carcinoma and in the PR+, rather than in the
non-ER+ subtype (8).
The cells of invasive lobular carcinoma are small
with poor tumor cell adhesion and cohesion. However, they
demonstrate strong invasive power and may easily break into the
vasculature, penetrate the basement membrane and enter the lymph or
the blood circulation. Therefore, lymphatic and distant metastases
of invasive lobular carcinoma are more common, resulting in poor
prognosis. In the review by McLemore et al(5), out of 12,001 cases of metastatic
breast cancer, only 23 cases had gastrointestinal metastasis alone.
Primary invasive lobular carcinoma accounted for 54% of these
cases, a significantly higher proportion than the other
pathological types (P<0.001). In addition, there were two cases
(3%) of bilateral breast cancer and six cases (8%) where
contralateral secondary breast cancer occurred prior to metastasis.
In 45% of cases there was metastasis to the colon and rectum, while
metastasis to the stomach and small intestine occurred in 28 and
19% of cases, respectively. Borst and Ingold analyzed the pathology
of more than 2,500 cases of metastatic breast cancer in 18 years,
and revealed that gastrointestinal metastasis was apparent in only
17 cases (<1%) (9). Therefore,
this suggests that gastrointestinal metastasis from breast cancer
is rare.
It has been demonstrated that invasive lobular
carcinoma and invasive ductal carcinoma exhibit different
metastatic characteristics, with lobular carcinoma metastasizing
more rarely than ductal carcinoma, particularly to the lungs, liver
and brain parenchyma (2). Instead,
lobular carcinoma has the tendency to metastasize to the pia mater,
peritoneal surface, retroperitoneum and the gastrointestinal and
reproductive organs. Cifuentes and Pickren (10) identified 112 (16%) cases with
gastrointestinal metastasis of primary breast cancer in 707
autopsies (10), among which the
small intestine accounted for 64 cases (9%), the stomach for 69
cases (10%) and the large intestine for 57 cases (8%). Therefore,
this study suggests that gastrointestinal metastasis of breast
cancer is not uncommon. The clinical manifestations of the majority
of gastrointestinal diseases include abdominal pain, diarrhea,
gastrointestinal bleeding, intestinal obstruction and
intussusception. Since these manifestations are also apparent in
patients with gastrointestinal metastasis, there may be problems
diagnosing gastrointestinal metastasis, which may lead to treatment
difficulties (11). The patient
admitted into hospital in the present case exhibited symptoms of
acute intestinal obstruction and the doctors and patient ignored
the breast disease; therefore, breast disease was not diagnosed
preoperatively. Postoperative pathology prompted the discovery that
the primary disease responsible for the small intestine metastasis
was in the breast. We concluded that there were two main errors in
the case. One factor was a lack of awareness of the disease in the
patient: The patient had known for several years that breast and
thyroid masses were present; however, since they did not affect the
patient’s life, they were not considered to be a disease and the
patient did not report them to a doctor. The gastrointestinal
obstructive symptoms severely impacted the patient’s life, and it
was only this situation that caused the patient to go to the
hospital. Another factor was that the doctor did not demonstrate
sufficient understanding: When the preoperative colonoscopy
revealed no evidence of a tumor, the bowel obstruction was
considered to be mere inflammation. Therefore a thorough and
comprehensive medical history and physical examination was
necessary.
Following the small intestine tumor resection, and
according to the pathology of the breast tumor puncture, the
patient was diagnosed with advanced breast cancer with symptomatic
visceral metastasis. Subsequent to the surgery, chemotherapy was
initially considered to control the systemic metastasis, and the
patient was treated with paclitaxel in combination with
capecitabine (paclitaxel, 175 mg/m2 day 1, capecitabine,
1,250 mg/m2 days 1–14, 21 days for one cycle). Following
five cycles of chemotherapy, the breast mass of the patient was
evaluated to be SD, leading to a bilateral modified radical
mastectomy being proposed. The failure of chemotherapy was due to
the breast mass being lumimal A type, and, therefore, not sensitive
to chemotherapy, while sensitive to hormone therapy. Seewaldt et
al(12) described four cases
where intestinal perforation occurred when small intestinal
metastatic tumors were treated with paclitaxel chemotherapy
(12). The perforation was
revealed not to be caused by the dissolution of the tumor, since
three cases progressed. McLemore et al(5) concluded that the overall survival
following gastrointestinal metastasis of breast cancer was 28
months (5), with age and diagnosis
of gastrointestinal metastasis having no effect on overall
survival; however, systematic chemotherapy and tamoxifen treatment
were considered to be influential factors. The patient in the
present case underwent an oophorectomy and was treated with
letrozole for endocrine therapy following the bilateral modified
radical mastectomy. It was indicated that the endocrine therapy was
more efficacious than the chemotherapy.
The clinical symptoms and signs of small intestine
metastatic tumors have no identifiable features when compared with
other diseases of the small intestine, leading to certain
difficulties in the diagnosis. Gastrointestinal tract metastasis
may be apparent in cases of invasive lobular carcinoma; however,
intestinal metastasis in cases of bilateral breast infiltrating
lobular carcinoma is clinically rare. As a consequence, doctors and
patients have a tendency to ignore the signs and symptoms of
intestinal metastasis of invasive lobular cancer. This case
reinforced the fact that it is necessary to consider the
possibility of metastasis when diagnosing gastrointestinal
diseases, and that there is potential for gastrointestinal tract
metastasis in cases of invasive breast lobular carcinoma.
References
|
1
|
Hartman M, Czene K, Reilly M, et al:
Genetic implications of bilateral breast cancer: a population based
cohort study. Lancet Oncol. 6:377–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Théraux J, Bretagnol F, Guedj N, et al:
Colorectal breast carcinoma metastasis diagnosed as an obstructive
colonic primary tumor. A case report and review of the literature.
Gastroenterol Clin Biol. 33:1114–1117. 2009.PubMed/NCBI
|
|
3
|
Xu Binghe: Breast Cancer. Peking
University Medical Press; Beijing: pp. 257–258. 2005
|
|
4
|
He JJ: Bilateral primary breast cancer: an
analysis of 2942 cases. China Modern Doctor. 48(5): 8–10. 2010.(In
Chinese).
|
|
5
|
McLemore EC, Pockaj BA, Reynolds C, et al:
Breast cancer: presentation and intervention in women with
gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol.
12:886–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dixon AR, Ellis IO, Elston CW and Blamey
RW: A comparison of the clinical metastatic patterns of invasive
lobular and ductal carcinomas of the breast. Br J Cancer.
63:634–635. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doyle DJ, Relihan N, Redmond HP and Barry
JE: Metastatic manifestations of invasive lobular breast carcinoma.
Clin Radiol. 60:271–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borst MJ and Ingold JA: Metastatic
patterns of invasive lobular versus invasive ductal carcinoma of
the breast. Surgery. 114:637–642. 1993.PubMed/NCBI
|
|
9
|
Schwarz RE, Klimstra DS and Turnbull AD:
Metastatic breast cancer masquerading as gastrointestinal primary.
Am J Gastroenterol. 93:111–114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cifuentes N and Pickren JW: Metastases
from carcinoma of mammary gland: an autopsy study. J Surg Oncol.
11:193–205. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nazareno J, Taves D and Preiksaitis HG:
Metastatic breast cancer to the gastrointestinal tract: a case
series and review of the literature. World J Gastroenterol.
12:6219–6224. 2006.PubMed/NCBI
|
|
12
|
Seewaldt V, Cain JM, Greer BE, Tamimi H
and Figge DC: Bowel complications with taxol therapy. J Clin Oncol.
11:11981993.PubMed/NCBI
|