Introduction
Temporal lobe epilepsy (TLE) is the most common form
of partial epilepsy in humans and is generally resistant to
treatment (1). A randomized
clinical trial has demonstrated that surgery is superior to
prolonged medical therapy for TLE patients (2). However, surgical treatments have
failed to provide a seizure-free outcome in 20–30% of TLE patients
(3). Several potential
explanations for the surgical treatment failures in TLE have been
proposed. Insufficient resectioning of the mesial temporal
structures may be a major cause of seizure recurrences following
epilepsy surgeries (4,5). Recent studies have indicated that the
coexistence of mesial TLE with hippo-campal sclerosis (HS) and a
temporal neocortical lesion (so called ‘dual pathology’) may be an
important cause of surgical failures in patients with TLE (6). Common neocortical lesions include
focal cortical dysplasia (FCD), vascular malformations (which
include arteriovenous malformations, aneurysms and cavernomas) and
benign primary brain tumors (which include gangliogliomas,
dysembryoplastic neuroepithelial tumors, pleomorphic
xanthoastrocytomas and low-grade astrocytomas) (7). ‘Triple pathology’ in TLE refers to
the coexistence of HS with two other intracranial lesions related
to the pathogenesis of epilepsy (8). TLE patients with ‘triple pathology’
have rarely been reported. In this study, we report a rare case of
the coexistence of HS, FCD and ganglioglioma in the mesial temporal
lobe in TLE patients with ‘triple pathology.’ The possible
pathogenesis of ‘triple pathology’ in epilepsy is discussed. This
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Dalian Medical University. Written informed consent was
obtained from the patients and the procedures were approved by
institutional review boards.
Case report
A 29-year-old right-handed male had experienced
recurrent seizures for four years. The patient's seizures began
with a paroxysmal disturbance of consciousness, followed by
automatic movements such as swallowing, smacking of the lips or
glazed eyes and progressed to flexing of the limbs, shaking and
stiffening. Seizures occasionally occurred without an aura and
lasted from several seconds to minutes. Initially, the patient's
seizures only occurred three to four times a year, but this
frequency gradually increased to nearly once a week. The patient
was born prematurely and had no history of febrile convulsions,
meningitis or encephalitis. The patient had no history of other
medical conditions and the findings of the neurological examination
were normal. The patient had been treated with various drugs
including sodium valproate, carbamazepine, zonisamide, clobazam,
and Chinese herbal medicine. However, the patient's seizures became
medically intractable prior to being admitted to The Epilepsy
Center of The Second Affiliated Hospital of Dalian Medical
University.
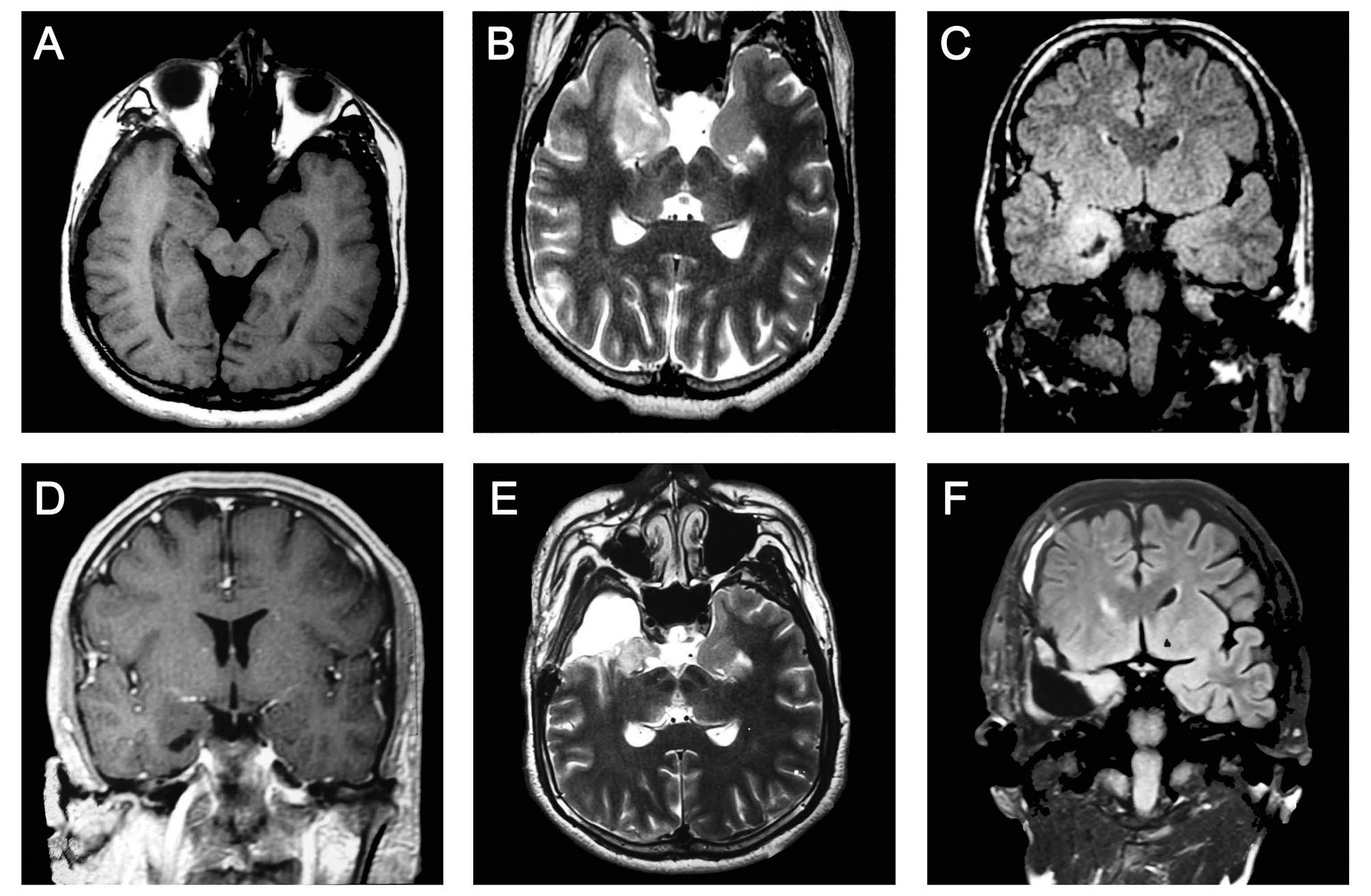
Cerebral magnetic resonance imaging (MRI) revealed a
∼2.0×1.4 cm region of abnormal signal in the right anterior and
medial temporal lobes (long T1- and T2-weighted signal).
T2-weighted and fluid-attenuated inversion recovery sequence
(FLAIR) images revealed a reduced hippocampal volume with an
increased FLAIR signal on the right side and a slightly enlarged
temporal horn, typical in patients with HS and FCD. Focal cortical
thickening with subcortical hyperintensity was noted in the right
frontal lobe. These findings indicated the coexistence of FCD in
the frontal lobe ipsilateral to the side of HS (Fig. 1A–C). Contrast-enhanced MRI revealed
no sign of enhanced signals (Fig.
1D).
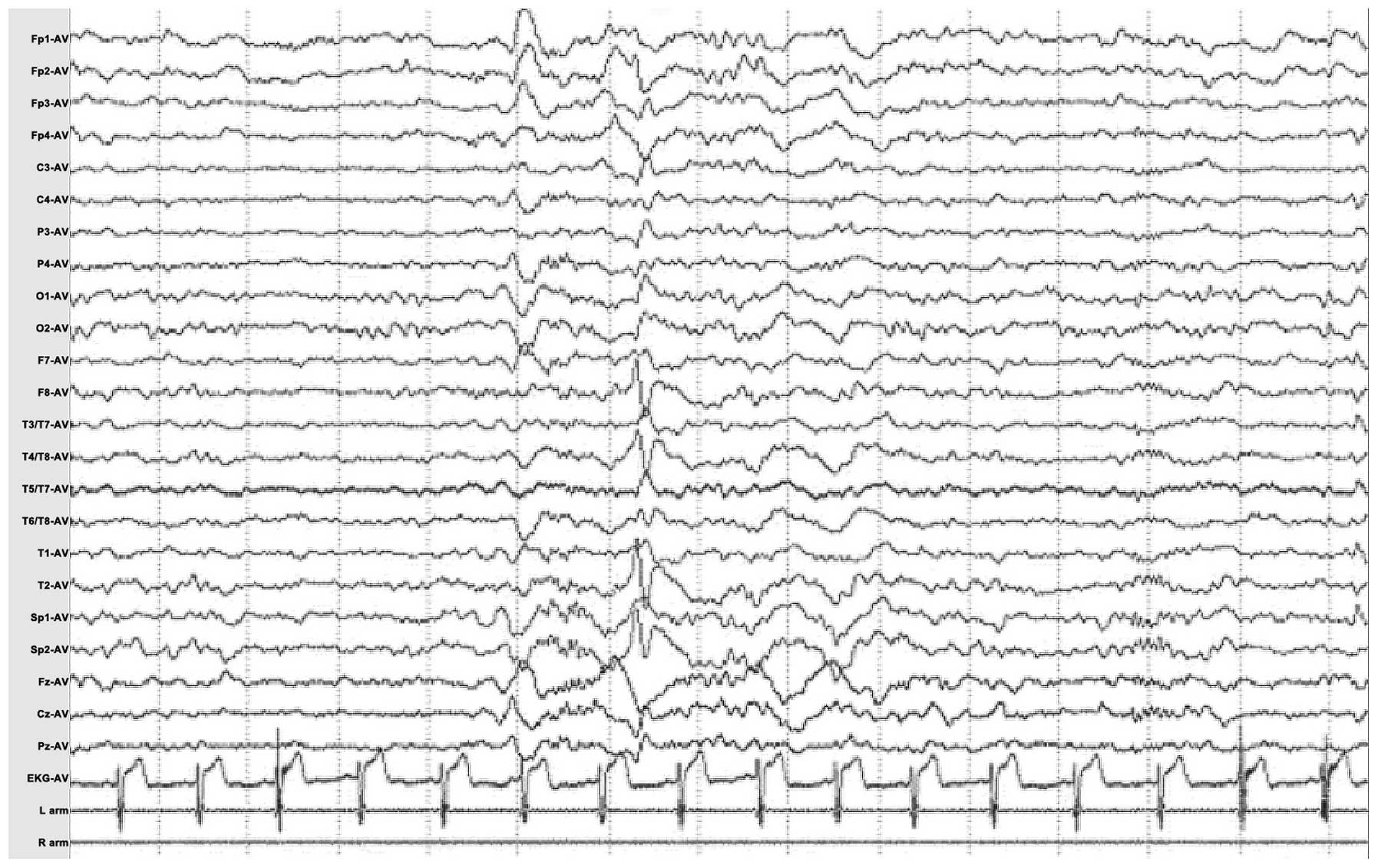
Video-electroencephalography (video-EEG) was used to
monitor one episode of a seizure attach. The attack was observed to
involve abrupt disturbances in consciousness during the daytime,
followed by left upper limb stiffening, head tilting to the left
side, blinking and
swallowing movements; lasting for a total of 70 sec. The interictal EEG indicated
multiple continuous slowing and intermittent epileptiform
discharges from the right anterior temporal region, T2 and Sp2, spreading to the ipsilateral frontal
lobe. The ictal EEG demonstrated 3–4.5 Hz slow waves rising from
the right anterior temporal lobe, then charged_6–7 Hz sharp waves
in the overall
right temporal area, gradually spreading to the
right hemisphere as continuous 3 Hz slowing spikes (Fig. 2).
Due to the coexistence of HS and FCD, we predicted a
poor outcome for treatment with medication. Following complete
pre-surgical assessment, the patient underwent resectioning of the
right anterior temporal lobe, hippocampus and amygdala, in addition
to the lesion located in the medial temporal lobe. According to the
surgeon, the hippocampus, amygdala and anterior temporal lobe felt
solid, particularly the medial temporal lobe lesion, which was
located adjacent to the thalamus and basal ganglia. We failed to
achieve a complete resection of the medial temporal lobe lesion due
to its anatomical location; however, the hippocampus, amygdala and
anterior temporal lobe cortex were excised successfully (Fig. 1E and F). Pathological examination
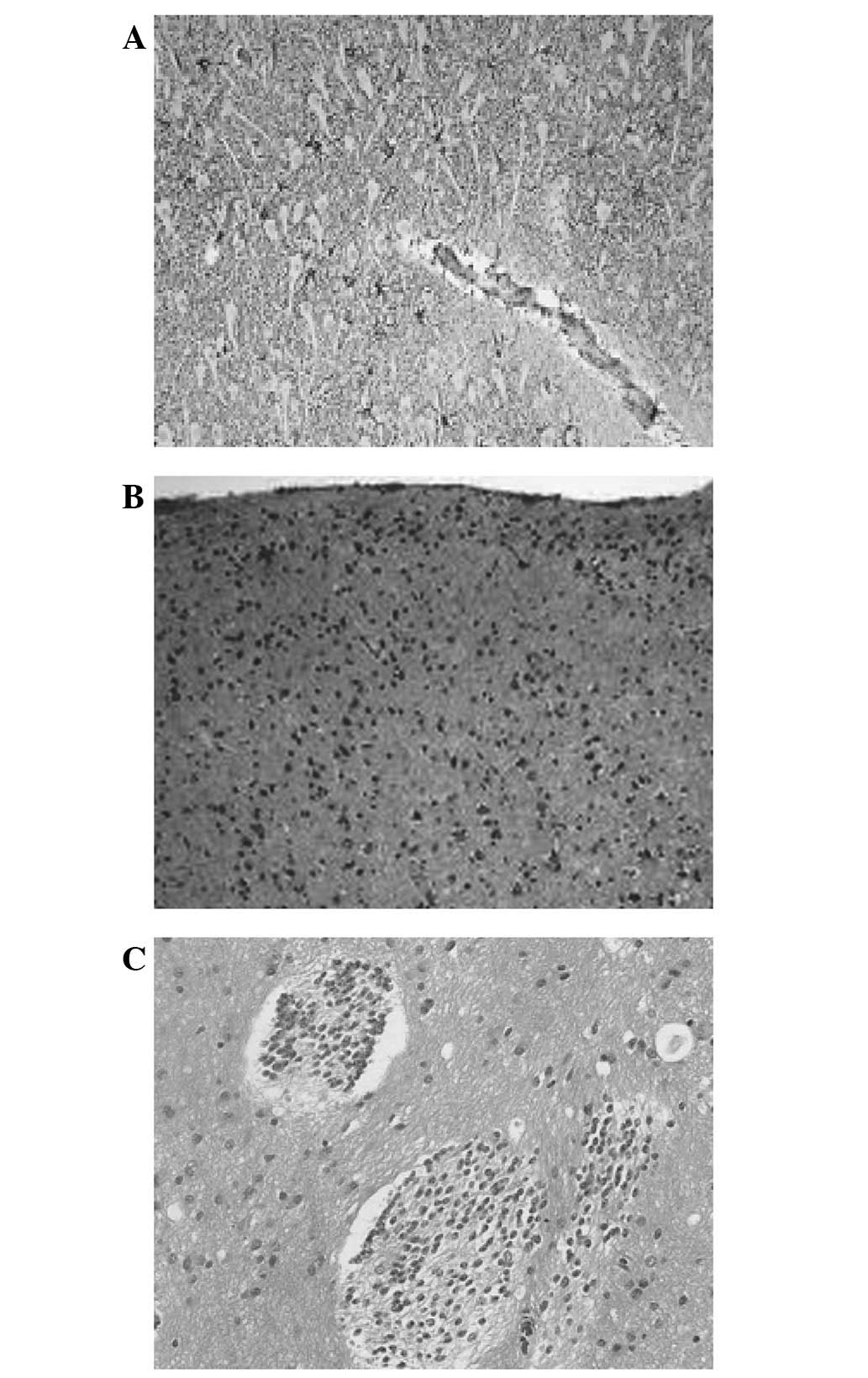
of the excised tissue revealed hippocampal sclerosis with neuronal
cell loss accompanied by astrogliosis.
Histopathological examination confirmed FCD (type
IIA) of the anterior temporal lobe with dysmorphic neurons and a
malformation of the cortical structure. Immunohistochemical
findings revealed disorganized neurons, glial fibrillary acidic
protein (GFAP), oligo-2 glial cells, a small quantity of CD34 and a
Ki-67 index <1%. The medial temporal lobe lesion was confirmed
as ganglioglioma (WHO grade I). Immunohistochemistry results for
the lesion revealed that the tumor cells were positive for GFAP,
NeuN and CD34 and had a Ki-67 index <1%. Following surgery, the
patient was seizure-free for eight months (Fig. 3). During the first eight months
following surgery, the patient's seizures were controlled with
zonisamide and phenytoin. An EEG assessment eight months
post-surgery confirmed that there had been no epileptic discharges
or relapses.
Discussion
The coexistence of three intracranial lesions
related to epileptic pathogenesis is known as ‘triple pathology’.
The occurrence of ‘triple pathology’ in epilepsy patients was
initially proposed by Maciunas et al (9) and Samura et al (10). Samura et al reported a case
of TLE with the coexistence of HS, FCD and cavernoma (CA) (10). The authors theorized that FCD was
the epileptic lesion responsible for secondary hippocampal damage
and gradually induced intractable epilepsy, while the existence of
a CA was not an aggravating factor. Thus, they conducted a
resection of the right anterior temporal lobe, hippocampus and
amygdala, in addition to a biopsy on the FCD lesion; no treatment
for CA was performed. The patient achieved a 12-month seizure-free
outcome, which preliminarily fitted with their assumption. Maciunas
et al reported two cases of TLE with ‘triple pathology’: one
case had CA, FCD and HS, while the other had CA, FCD and venous
angioma (9). By reviewing their
cases, the authors concluded that FCD is heterogeneous with various
lesions appearing to coexist with other pathologies.
FCD has been reported with a high frequency of
coexistence with other pathological lesions in TLE (11). Recent studies have indicated that
FCD has molecular similarities to tuberous sclerosis, ganglioglioma
and hemimegalencephaly (12). In
the current study, we demonstrated that FCD and ganglioglioma were
different histological expressions due to the same pathogenesis,
that is, heteromorphism, causing recurrent seizures, which
gradually induced hippocampal damage or HS. Therefore, we conclude
that ‘triple pathology’ lesions may have similar pathological
origins.
A ganglioglioma is a neoplasm comprised of neurons
and glial cells, which has a slow growth process, occasional
anaplasia and an incidence rate of 0.4–4% among central nervous
system (CNS) tumors (WHO grade I–II) (13). Although a ganglioglioma is not a
major cause of refractory seizures, it is normally present in
intractable epilepsy cases and considered to be a major cause of
secondary epilepsy (14,15). Clinicians and researchers
acknowledge that the chronic growth process of a ganglioglioma may
stimulate and oppress its surrounding cortex, thereby inducing
seizure attacks (16). In the
current study we failed to perform a complete resection, but the
patient had a successful outcome, which confirmed our two initial
assumptions. Firstly, FCD and HS may be the lesions responsible for
epilepsy while the ganglioglioma had only a minor role in the
seizure network; therefore, the complete resection of HS and FCD
would result in a good recovery. Secondly, the ganglioglioma
induced seizures were likely to be due to the surrounding cortex. A
surgical resection of the ganglioglioma was incomplete and certain
surgical injuries were sustained to the surrounding cortex, which
may have jeopardized the primary conduction network and led to the
intractable seizures.
In conclusion, based on the literature review and a
detailed review of this case, we postulate two possible
explanations for the pathogenesis of ‘triple pathology’: i) ‘triple
pathology’ is a combination of pathological progression; and ii)
‘triple pathology’ lesions have similar pathological origins.
References
|
1.
|
Tamber MS and Mountz JM: Advances in the
diagnosis and treatment of epilepsy. Semin Nucl Med. 42:371–386.
2012. View Article : Google Scholar
|
|
2.
|
Wiebe S, Blume WT, Girvin JP and Eliasziw
M: A randomized, controlled trial of surgery for temporal-lobe
epilepsy. N Engl J Med. 345:311–318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Harroud A, Bouthillier A, Weil AG and
Nguyen DK: Temporal lobe epilepsy surgery failures: a review.
Epilepsy Res Treat. 2012:2016512012.PubMed/NCBI
|
|
4.
|
Hennessy MJ, Elwes RD, Binnie CD and
Polkey CE: Failed surgery for epilepsy. A study of persistence and
recurrence of seizures following temporal resection. Brain. 123(Pt
12): 2445–2466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Guye M, Régis J, Tamura M, et al: The role
of corticothalamic coupling in human temporal lobe epilepsy. Brain.
129:1917–1928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jung R, Aull-Watschinger S, Moser D, et
al: Is reoperation an option for patients with temporal lobe
epilepsy after failure of surgery? Seizure. Dec 26–2012.(E-pub
ahead of print).
|
|
7.
|
Engel J Jr, Wiebe S, French J, et al:
Practice parameter: temporal lobe and localized neocortical
resections for epilepsy: report of the Quality Standards
Subcommittee of the American Academy of Neurology, in association
with the American Epilepsy Society and the American Association of
Neurological Surgeons. Neurology. 60:538–547. 2003.
|
|
8.
|
Cheong JY, Wong C, Bleasel A, Varikatt W,
Ng T and Dexter MA: Late onset Rasmussen's encephalitis with triple
pathology. J Clin Neurosci. 16:1677–1681. 2009.
|
|
9.
|
Maciunas JA, Syed TU, Cohen ML, Werz MA,
Maciunas RJ and Koubeissi MZ: Triple pathology in epilepsy:
coexistence of cavernous angiomas and cortical dysplasias with
other lesions. Epilepsy Res. 91:106–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Samura K, Morioka T, Hashiguchi K, et al:
Temporal lobe epilepsy associated with ‘triple pathology’ of
hippocampal sclerosis, focal cortical dysplasia and cavernoma in
the ipsilateral frontal lobe. Epilepsy & Seizure. 2:34–41.
2009.
|
|
11.
|
Tassi L, Garbelli R, Colombo N, et al:
Type I focal cortical dysplasia: surgical outcome is related to
histopathology. Epileptic Disord. 12:181–191. 2010.PubMed/NCBI
|
|
12.
|
Crino PB: Focal brain malformations:
seizures, signaling, sequencing. Epilepsia. 50(Suppl 9): 3–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nishio S, Morioka T, Mihara F, Gondo K and
Fukui M: Cerebral ganglioglioma with epilepsy: neuroimaging
features and treatment. Neurosurg Rev. 24:14–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Im SH, Chung CK, Cho BK and Lee SK:
Supratentorial ganglioglioma and epilepsy: postoperative seizure
outcome. J Neurooncol. 57:59–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Maehara T, Shimizu H, Oda M and Arai N:
Coexistence of ganglioglioma and cortical dysplasia in a patient
with intractable epilepsy - case report. Neurol Med Chir (Tokyo).
37:752–756. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zaghloul KA and Schramm J: Surgical
management of glioneuronal tumors with drug-resistant epilepsy.
Acta Neurochir (Wien). 153:1551–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|