Introduction
The melanocytes in vitiligo repigmentation are
derived predominantly from melanocyte precursors (MPs) of the outer
root sheath (ORS) of hair follicles (1). Thus, the in vitro culture of
pure MPs is of value in the study of the mechanisms of vitiligo
repigmentation. MPs were first successfully cultured by Tobin et
al in 1995 (2); however, with
the culture method that was utilized, it was difficult to avoid
fibroblast contamination. In addition, the culture medium that was
used promoted the differentiation and maturation of MPs. In a
previous study, we improved the culture method of Tobin et
al to reduce fibro-blast contamination; however, geneticin,
which is frequently used in experiments to remove contamination,
often leads to culture failure. In addition, the composition of our
culture medium was the same as that used by Tobin et al, and
although the cells were able to proliferate rapidly, they were not
able to maintain the undifferentiated state (3). Further improvements to the
composition of the culture medium have been reported; however, the
cultured MPs continued to produce melanin (4). Cook et al successfully
cultured human MPs established from neonatal foreskin in 2003
(5). Based on these studies, we
optimized the method for the culture of MPs from hair follicles and
performed MP identification analysis.
1,25-Dihydroxyvitamin D3 (VID) may be used for
vitiligo treatment in the clinic; however, its mode of action has
yet to be fully elucidated. In cellular models, VID has been shown
to increase tyrosinase (TYR) expression in mouse MPs and promote
melanin synthesis (6). VID has
also been demonstrated to promote melanin synthesis in B16 melanoma
cells (7). However, it has not
been revealed whether VID functions as an activator of MPs from
hair follicles. Therefore, in the present study, the activation
effects of VID on cultured MPs were observed.
Materials and methods
Major reagents and materials
MCDB-153 culture medium and trypsin were purchased
from Gibco-BRL (Grand Island, NY, USA). VID, stem cell factor
(SCF), endothelin-3 (ET-3) and basic fibroblast growth factor
(bFGF) were obtained from Peprotech, Inc. (Rocky Hill, NJ, USA),
while Chelex-100, 3,4-dihydroxy-L-phenylalanine (DOPA), cholera
toxin (CT) and dispase II were purchased from Sigma (St. Louis, MO,
USA). Fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd. (Hangzhou, China) and
the western blotting kits were obtained from Gene Company Ltd.
(Shanghai, China). The antibodies for microphthalmia-associated
transcription factor (MITF; 3F276), TYR (T311), TYR-related
protein-1 (TRP-1; SPM456), TRP-2 (C-9) and glyceraldehyde
3-phosphate dehydrogenase (sc-59540) were all purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Culture of MPs and melanocytes (MCs)
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Nanjing Medical University
(Nanjing, China), and written consent was obtained from family
members for the collection of scalp tissue from a cadaver (within 2
h of death). The donor was 21 years of age and the sample size was
6×5 cm2. The sample was disinfected with povidone-iodine
and the galea aponeurotica was excised to the greatest extent
possible. The scalp was sectioned into 0.3-cm-wide skin strips, and
the strips were cut along the upper 0.1 cm of dermis. The region
with epidermis was used for culturing MCs, while the area with
dermis was used for culturing MPs. The method for the culture of
the MPs was based on that described in our previous study (3), with certain modifications: i) Free
follicles were obtained following digestion with 1% dispase II at
4°C for 24 h, instead of using collagenase; ii) melanoblast culture
medium was used for the cell culture; and iii) a different passage
method was used. Following 7 days of culture, a large number of
cells had proliferated, including MPs. The first passage was
conducted at a ratio of 3:1 (contents of 3 bottles collected into
1) and subsequent passages were conducted every 5–7 days at a ratio
of 1:2 (contents of 1 bottle distributed into 2 bottles). Cells at
the third passage consisted solely MPs and the cells at the fourth
passage were used for experiments. The composition of the
melanoblast culture medium used was as described by Cook et
al (5). The basic medium
comprised MCDB-153, 2 mM glutamine, 10% Chelex-100-chelated FBS, 2%
FBS, 1.66 mg/l CT, 10 ng/ml SCF, 100 nM ET-3, 2.5 ng/ml bFGF, 50
U/ml penicillin and 50 g/ml streptomycin. The chelated serum was
obtained by adding 15 g Chelex-100 to 500 ml serum and continuously
mixing at 4°C for 1.5 h. MCs were cultured according to the method
described by Cook et al (5), with the same culture medium as was
used for the MPs.
Experimental grouping
VID was prepared as a 10−2 M stock
solution (using anhydrous ethanol as a solvent) and stored in the
dark; the working solution was diluted using freshly prepared
culture medium. The experimental samples were divided into four
groups according to VID concentration: 0 (blank control),
10−4, 10−6 and 10−8 M. Following
the treatment of the cells with VID for 72 h, the subsequent
experiments were performed. The 10−6 M group was used
for DOPA staining, transmission electron microscopy and western
blotting. All four groups were used for the analysis of TYR
activity and the measurement of melanin levels.
DOPA staining
The control and MC groups and the 10−6
MVID MP group were used for this experiment. Following washing with
phosphate-buffered saline (PBS), fixation with 2% paraformaldehyde
and washing with PBS a further three times, the cells were
incubated with 0.1% DOPA (dissolved in 0.1 M PBS) at 37°C for 5 h,
prior to being counterstained with nuclear fast red.
Transmission electron microscopy
The control and MC groups and the 10−6 M
VID MP group were used for this experiment. Cells were dissociated
using 0.25% trypsin and collected. Following fixation with 2.5%
glutaraldehyde, cells were conventionally embedded and sectioned.
The changes in the proportions of melanosomes were observed under a
Hitachi H-7000 transmission electron microscope (Hitachi, Tokyo,
Japan).
Western blotting
The control and MC groups and the 10−6 M
VID MP group were used for this experiment. Cells were dissociated
using 0.25% trypsin and collected; total protein was then extracted
and quantified using conventional methods. After denaturing, the
total protein was subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis with 15 μg
protein in each lane. The protein bands were transferred onto an
Immobilon-P transfer membrane (polyvinylidene fluoride). After
blocking with 3% bovine serum albumin, the membrane was incubated
with primary antibodies (3F276, T311, SPM456, C-9 and sc-59540) at
a 1:1,000 dilution overnight at 4°C. After to washing, the membrane
was incubated with secondary antibodies at room temperature for 1
h. The protein bands were developed using chemiluminescence
reagents, and the gray-scale values were quantified using an image
analysis system (Quantity One Software; Bio-Rad, Hercules, CA,
USA).
Detection of TYR activity and melanin
level
The control group and each experimental group of MPs
were used for this experiment. Cells were dissociated using 0.25%
trypsin and counted. The TYR activity and melanin level were
measured in accordance with previous studies (8,9).
Briefly, the cells were treated with both control and VID for 72 h,
washed, trypsinized and counted before pelleting. Melanin per cell
was quantified after boiling in 1 M sodium hydroxide and melanin
content in each sample was read from a calibration curve against
synthetic eumelanin (Sigma) at 490 nm and converted to means ± SE
melanin pg/cell from 3 independent experiments. For determination
of TYR activity, the cells were washed in ice-cold PBS and were
lysed with phosphate buffer (pH 6.8) containing 1% Triton X-100 and
protease inhibitors (Complete™ protease inhibitor mixture; Roche
Molecular Diagnostics, Basel, Switzerland). The lysates were
clarified by centrifugation for 10 min at 10,000 × g. After the
quantification of protein levels and adjusting concentrations using
lysis buffer, 90 μl of each lysate, containing an identical
amount of protein, was placed in 96-well plates, and 10 μl
15 mM L-DOPA was added to each well. After incubation at 37°C for
30 min, dopachrome formation was assayed by measuring absorbance at
475 nm using a microplate reader. TYR activity was reported as A475
values. Each experiment had three wells, and three independent
experiments were conducted.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were compared using the paired t-test. The
statistical analysis was performed using SPSS statistical software
version 10.0 (SPSS, Inc., Chicago, IL, USA).
Results
Morphology of MPs
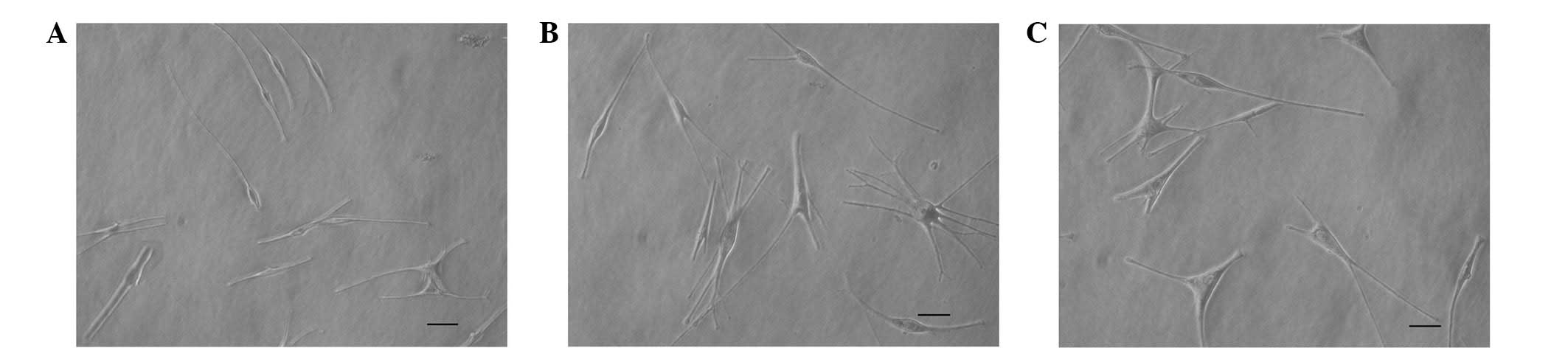
The cultured MPs were bipolar cells with small, oval
cell bodies and strong refractivity (Fig. 1A). Following VID treatment for 72
h, the cell bodies of the MPs increased in size and certain cells
had extended dendrites and/or numerous dendrites (Fig. 1B). The cultured MCs were similar to
VID-treated MPs, and they had larger cell bodies and numerous
dendrites (Fig. 1C).
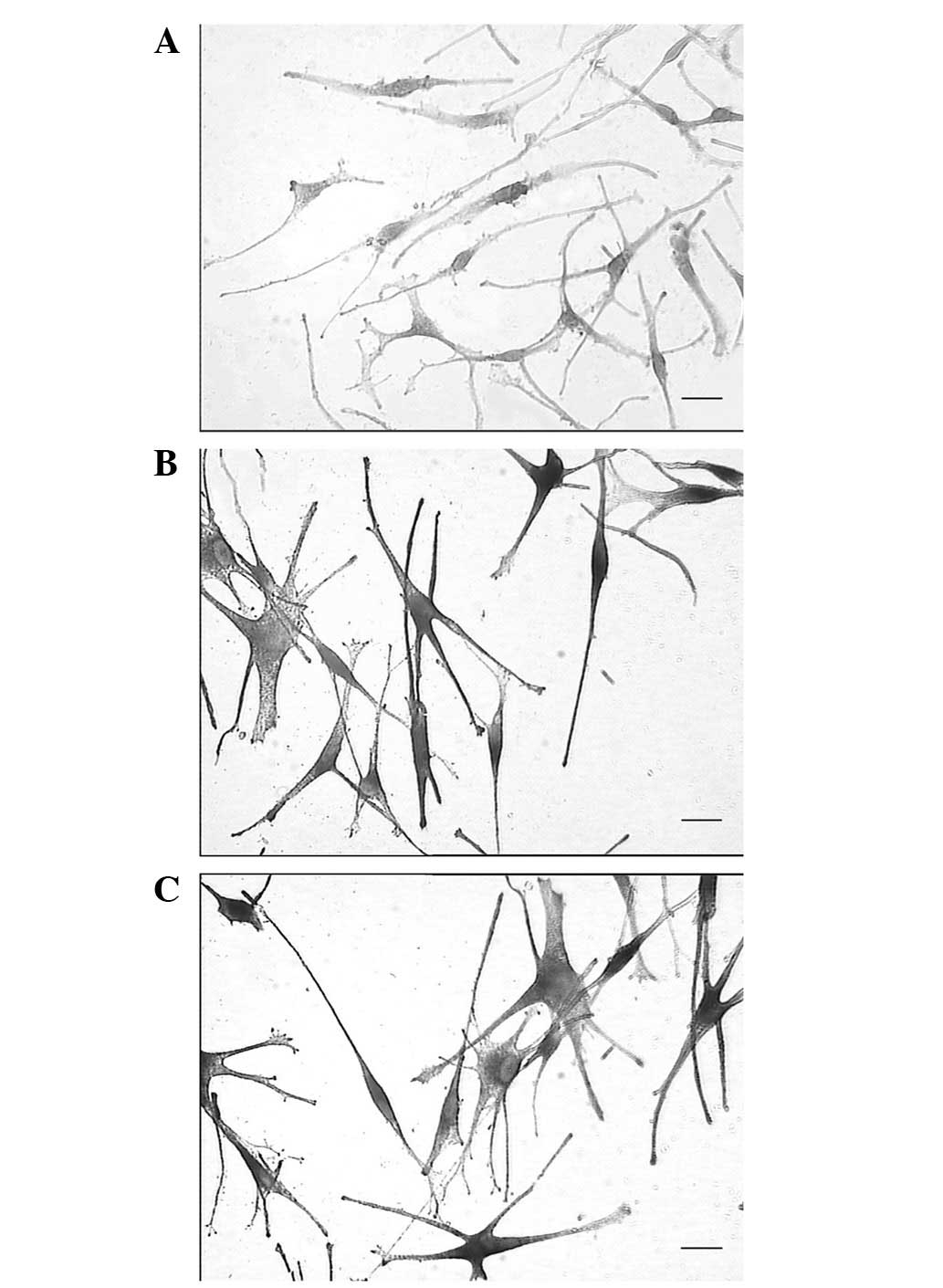
DOPA staining and transmission electron
microscopy
To further detect whether the MPs had matured,
transmission electron microscopy was performed to observe the
melanosomes, and DOPA staining was also performed to measure TYR
activity. During cell collection, the cells were washed thoroughly
with PBS five times to reduce the influence of impurities and the
color of the medium on the precipitate. The results showed that the
precipitate of the MPs was yellow-white (Fig. 2A), while the precipitate of the
VID-treated MPs was gray-black, similar to the MCs (Fig. 2B and C). Observation under an
electron microscope showed that the cytoplasm of the MPs contained
numerous stage I and II melanosomes; however, no mature stage III
or IV melanosomes were present. The melanosomes were predominantly
distributed in the perinuclear region, and the mitochondria were
regular without marked expansion (Fig.
3A). By contrast, VID treatment induced the appearance of
several stage III and IV melanosomes in the cytoplasm of the MPs,
similar to MCs. The location of the melanosomes in these cells was
further away from the nuclei and predominantly in the inner
membrane. In addition, the mitochondria in the VID-treated MPs were
observed to have significantly proliferated and expanded,
indicating that they were active (Fig.
3B and C). Therefore, these results suggested that the cultured
MPs did not have melanin synthesis functions, that VID
significantly activated the MPs and that the activated MPs had
similar functions to MCs. The DOPA staining did not show black
granules in the cytoplasm of the MPs, and the nuclear fast red
counterstain showed a light-red result in the cytoplasm (Fig. 4A). Black granules became prominent
in the cytoplasm of the VID-treated MPs, as well as in the MCs;
certain cells showed strong positive staining, which covered the
color of the nuclear fast red (Fig. 4B
and C). Therefore, the DOPA stain results further indicated
that the cultured MPs were in an undifferentiated state and that
VID significantly promoted the differentiation of MPs into mature
MCs.
Western blotting
The MPs and MCs expressed MITF, TYR, TRP-1 and
TRP-2. The expression levels of MITF, TYR and TRP-1 in the MCs were
higher than in the MPs, while the expression of TRP-2 in these two
cell types was similar. Following VID treatment, the MITF, TYR and
TRP-1 levels in the MPs significantly increased; however, they
remained lower than those in the MCs. The expression of TRP-2 in
the MPs was not significantly affected by VID (Fig. 5).
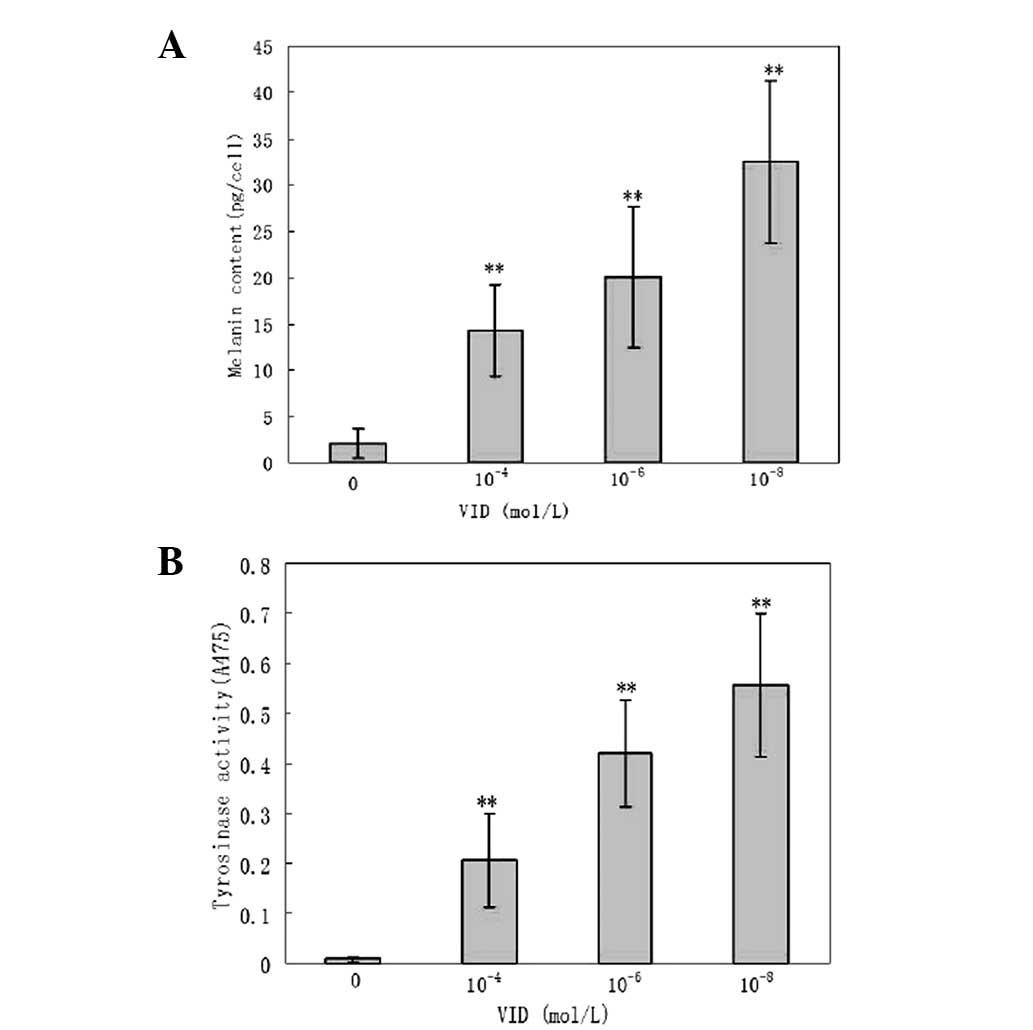
Detection of TYR activity and melanin
level
Compared with the control group, VID promoted TYR
activity and melanin synthesis in MPs in a concentration-dependent
manner (P<0.01; Fig. 6).
Discussion
Numerous studies have demonstrated the successful
culture of MPs (2–4). However, there are two main problems
that remain unsolved: contamination by fibroblasts and, most
importantly, the maintenance of the MPs in an undifferentiated
state and without any melanin-synthesizing activity.
The fibroblasts that contaminate the MP culture
mainly originate from the connective tissue sheath outside the ORS.
Contamination may be reduced using two strategies: the first
focusing on the separation of the hair follicles and the second
focusing on the selection of the culture medium. During the
separation of hair follicles, it is necessary to reduce the
contamination with connective tissues to the minimum. In a previous
study, we used dispase II combined with collagenase IV to separate
the hair follicles, which greatly reduced connective tissue
contamination; however, the experiments continued to fail
occasionally due to contamination (3). Dispase II digests the basement
membrane surrounding the hair follicles, reducing the adhesion of
connective tissues to free hair follicles. Collagenase IV, however,
digests the basement membrane and the dermis, thereby facilitating
the adhesion of connective tissues to the free hair follicles. In
the present study, we used 1% dispase II to digest the follicles
for 24 h at 4°C, followed by 1 h at 37°C to eliminate the
collagenase IV step. The results showed that this method had
significant benefits: There was less adhesion between connective
tissues and isolated hair follicles and no fibroblast contamination
in the cultures, and the proliferation of cultured MPs was not
blocked.
The second method of reducing fibroblast
contamination entails using medium components that do not support
the growth of fibroblasts. The growth of fibroblasts is
Ca2+ dependent (10);
thus, in order to reduce contamination, it is necessary to use a
low-calcium culture medium. The culture medium used in the present
study was MCDB-153. The difference between this medium and the
Minimum Essential Medium (MEM)/RPMI-1640 media that have been used
previously is that it has a lower Ca2+ concentration
(RPMI-1640 medium: 0.42 mM; MCB-153: 0.03 nM). In addition, the FBS
in the culture medium contributes Ca2+. In our
experiment, the serum was reacted with the divalent metal
ion-chelating agent, Chelex-100, prior to use, thereby reducing the
increase in Ca2+ concentration caused by the serum. Due
to the fact that some divalent cations are necessary for MP
culture, 2% non-chelated serum was added to provide essential
nutrients. Through these two modifications, it was observed that
there was no fibroblast contamination in the culture and the MPs
actively proliferated.
Since MPs only accounted for a small percentage of
the confluent cells, the first passage during the culture process
was performed at a 3:1 ratio. There were two advantages to this
method: i) the MPs remained relatively highly concentrated, which
was conducive to proliferation and ii) mature melanocytes died
after passaging. Using these two features combined with the
differential trypsinization method to remove keratinocytes, the
percentage of MPs in the second generation reached 90%, while the
mature melanocytes decreased in number and gradually died.
Following 7–8 days, cells regained confluence and the passaging was
conducted at the ratio of 1:2. There were no mature melanocytes or
keratinocytes in the third generation of cells and there were only
pure MPs.
The culture medium used in the present study was
prepared according to the method described by Cook et al
(5). The major additives were 10
ng/ml SCF, 100 nM ET-3, 2.5 ng/ml bFGF and 1.66 μg/l CT. SCF
is important in the survival, differentiation, proliferation and
migration of MCs and appears to be required for MC culture. When
binding to its ligand, c-kit, SCF is able to regulate the
expression of MITF, thereby promoting MC proliferation and
inhibiting melanin synthesis in MCs. Therefore, it is crucial in
maintaining the undifferentiated state of MCs. Kawa et al
(11) cultured the mouse
melanocyte precursor cell line, NCC-4.1, and SCF was the only
cytokine used to maintain cell proliferation and the
undifferentiated state. ET-3 is also frequently used for
melanoblast culture; it is necessary for the differentiation of
neural crest cells into MCs and is able to significantly promote
proliferation (10). The function
of ET-3 in the differentiation of MPs remains inconclusive. It has
been demonstrated that ET-3 is able to combine with SCF to promote
the differentiation of neural crest cells into mature MCs (11). However, a different study showed
that ET-3 was able to induce the dedifferentiation of MPs and
transformation into glial cells (12). bFGF promotes MC proliferation, has
mitogenic functions and has been used as an additive in a number of
MP cultures. These cytokines are all able to significantly promote
the proliferation of MCs and melanoblasts. However, they have
bidirectional effects on cell differentiation: In certain
conditions, they promote differentiation, while in other
conditions, they inhibit differentiation. We hypothesized that
these bidirectional effects on differentiation are predominantly
associated with two factors: the status of the cells and the
interaction of the components in the culture medium. With regard to
the status of the cells, if MPs are at the neural crest cell stage,
then all the previously mentioned factors promote differentiation;
however, when the MPs are going to enter the mature melanocyte
stage, SCF and ET-3 inhibit melanin synthesis. The other factor is
the interaction of all components in the culture medium. The
culture medium for the culture of epidermal melanoblasts used in a
previous study (5) contained four
commonly used proliferation-promoting agents: SCF, ET-3, bFGF and
CT. Those four components cooperated to not only promote
proliferation but also to maintain cells in an immature state. By
applying this method, we successfully cultured MPs from hair
follicles.
The DOPA staining of MCs in vivo is positive,
indicating that MCs have the ability to synthesize melanin, while
the DOPA staining of MPs is negative, indicating that MPs have no
melanin synthesizing ability (13). In the present study, under an
electron microscope, stage III and IV melanosomes were observed in
MCs while MPs contained only stage I and II melanosomes in the
absence of VID. The methods used to culture MPs from hair follicles
in previous studies all promoted melanin synthesis in MPs (2–4).
Therefore, although those cultured MPs were different from
epidermal melanocytes, they had functionally transformed into
mature melanocytes. The cultured MPs in the present study were
DOPA-negative and did not contain melanosomes above stage II under
an electron microscope, indicating that they were in the MP state
and were closer to true MPs in vivo (13,14).
MPs express MITF, TRP-2 and TRP-1 but not TYR in vivo. The
present western blotting results showed that the cultured MPs
expressed MITF, TRP-2, TRP-1 and TYR. The expression levels of
TRP-2 in the MPs and MCs were not significantly different; by
contrast, the expression levels of MITF, TRP-1 and TYR in the MPs
were significantly lower than those in the MCs. Due to the fact
that epidermal MCs and MPs used the same culture medium, these
results further indicated that the cultured MPs and MCs were
biologically distinct, despite the fact that the MPs showed some
differentiation.
The topical treatment of vitiligo using VID in
clinical practice produces excellent results. However, its
mechanism of action has not been studied in depth. The
repigmentation of vitiligo is mainly dependent on the activation of
MPs in the ORS of hair follicles and their migration to the
epidermis (1). Therefore, an aim
of this study was to confirm whether VID had an activating effect
on MPs derived from the ORS of hair follicles. The results showed
that VID promoted dendrite formation in the MPs, increased cell
body size, increased the expression of enzymes involved in melanin
synthesis and catalyzed melanin synthesis. Electron microscopy and
chemical detection methods demonstrated that VID promoted melanin
synthesis in MPs.
VID is able to increase TYR activity in B16 melanoma
cells and promote melanin synthesis (7); however, it is still controversial
whether VID is able to promote melanin synthesis in normal
epidermal melanocytes. Mansur et al (15) showed that VID did not affect
melanin synthesis in MCs (15);
however, Tomita et al (16)
demonstrated that VID promoted TYR expression in melanocytes
(16). VID is able to
significantly induce the differentiation of mouse MPs (6); this was further confirmed by the
present results. Abdel-Malek et al (17) reported that the topical application
of VID increased the number of DOPA-positive melanocytes in the
epidermis (17). Therefore, based
on these previous and current results, we propose that the
mechanism by which VID counters vitiligo involves the activation of
MPs from the ORS, followed by the entry of the MPs into the
epidermis and their differentiation into MCs.
Acknowledgements
This study was supported by the
Chinese Natural Science Foundation (grant nos. 3017086 and
81000703, China) and the Jiangsu Province Natural Science
Foundation (grant no. BK2009437).
References
|
1.
|
Cui J, Shen L and Wang G: Role of the hair
follicles in the repig-mention of vitiligo. J Invest Dermatol.
97:410–416. 1991. View Article : Google Scholar
|
|
2.
|
Tobin DJ, Colen SR and Bystryn JC:
Isolation and long-term culture of human hair follicle melanocytes.
J Invest Dermatol. 104:86–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhu WY, Zhang RZ, Ma HJ and Wang DG:
Isolation and culture of amelanotic melanocytes from human hair
follicles. Pigment Cell Res. 18:668–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kauser S, Thody AG, Schallreuter KU, et
al: A fully-functional POMC/MC-1R system regulates the
differentiation of human scalp hair follicle melanocytes.
Endocrinology. 146:532–543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cook AL, Donatien PD, Smith AG, et al:
Human melanoblasts in culture: expression of BRN2 and synergistic
regulation by fibroblast growth factor-2, stem cell factor, and
endothelin-3. J Invest Dermatol. 121:1150–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Watabe H, Soma Y, Kawa Y, et al:
Differentiation of murine melanocyte precursors induced by
1,25-dihydroxyvitamin D3 is associated with the stimulation of
endothelin B receptor expression. J Invest Dermatol. 118:583–589.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Oikawa A and Nakayasu M: Stimulation of
melanogenesis in cultured melanoma cells by calciferols. FEBS Lett.
42:32–35. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Masoodi M, Nicolaou A, Gledhill K, Rhodes
LE, Tobin DJ and Thody AJ: Prostaglandin D production in FM55
melanoma cells is regulated by alpha-melanocyte-stimulating hormone
and is not related to melanin production. Exp Dermatol. 19:751–753.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ando H, Itoh A, Mishima Y and Ichihashi M:
Correlation between the number of melanosomes, tyrosinase mRNA
levels, and tyrosinase activity in cultured murine melanoma cells
in response to various melanogenesis regulatory agents. J Cell
Physiol. 163:608–614. 1995. View Article : Google Scholar
|
|
10.
|
Fang D, Leishear K, Nguyen TK, et al:
Defining the conditions for the generation of melanocytes from
human embryonic stem cells. Stem Cells. 24:1668–1677. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kawa Y, Ito M, Ono H, et al: Stem cell
factor and/or endothelin-3 dependent immortal melanoblast and
melanocyte populations derived from mouse neural crest cells.
Pigment Cell Res. 13(Suppl 8): S73–S80. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dupin E, Glavieu C, Vaigot P and Le
Douarin NM: Endothelin 3 induces the reversion of melanocytes to
glia through a neural crest-derived glial-melanocutic progenitor.
Proc Natl Acad Sci USA. 97:7882–7887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Horikawa T, Norris DA, Johnson TW, et al:
Dopa-negative melanocytes in the outer root sheath of human hair
follicles express premelanosomal antigens but not a melanosomal
antigen or the melanosome-associated glycoprotein tyrosinase,
TRP-1, and TRP-2. J Invest Dermatol. 106:28–35. 1996. View Article : Google Scholar
|
|
14.
|
Osawa M, Egawa G, Mak SS, et al: Molecular
characterization of melanocyte stem cells in their niche.
Development. 132:5589–5599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mansur CP, Gordon PR, Ray S, et al:
Vitamin D, its precursors, and metabolites do not affect
melanization of cultured human melanocytes. J Invest Dermatol.
91:16–21. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tomita Y, Torinuki W and Tagami H:
Stimulation of human melanocytes by vitamin D3 possibly mediates
skin pigmentation after sun exposure. J Invest Dermatol.
90:882–884. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Abdel-Malek ZA, Ross R, Trinkle L, et al:
Hormonal effects of vitamin D3 on epidermal melanocytes. J Cell
Physiol. 136:273–280. 1988. View Article : Google Scholar : PubMed/NCBI
|