Introduction
The use of an optimal pacing mode for the treatment
of bradycardia is important. In patients with sick sinus syndrome
(SSS), DDD and VVI pacing modes may increase the risk of congestive
heart failure, atrial fibrillation and thromboembolism (1–3). The
main mechanism behind this may be that the electrical dyssynchrony
induced by the abnormal ventricular activation site and sequence in
the DDD and VVI pacing modes leads to left ventricular mechanical
dyssynchrony (LVMD) and left ventricular (LV) dysfunction.
Therefore, it is important that the LVMD and LV function is
objectively estimated in patients with various pacing modes.
Echocardiography is important in assessing LVMD and
LV function. At present, real-time three-dimensional
echocardiography (RT3DE) and tissue Doppler imaging (TDI) are the
most sensitive and commonly used techniques for the quantification
of LVMD. The aim of the present study was to: i) evaluate LVMD and
LV function in different pacing modes using RT3DE and TDI; and ii)
compare dyssynchrony indices derived from RT3DE with different TDI
indices when acquired from the same patients. To the best of our
knowledge, this is the first time a study of this nature has been
performed. The study may provide objective data to act as a
foundation for clinical physicians when choosing optimal pacing
modes.
Materials and methods
Study population
Twenty patients, including 12 males and 8 females
(mean age, 58±11 years), with SSS and intact intrinsic
atrioventricular (AV) conduction were enrolled during the period
from August 2011 to December 2012. All patients received
dual-chamber pacemaker implantation with the atrial leads placed in
the right atrial appendage and the right ventricular leads
positioned in the right ventricular apex. The patients had normal
cardiac function [LV ejection fraction (LVEF), 50–72%], normal
cardiac anatomy and no history of cardiovascular diseases. Any
patients with atrial fibrillation, coronary heart disease,
cardiomyopathy, AV conduction block and other rhythm disturbances
were excluded. Pacemakers were provided by Medtronic (Minneapolis,
MN, USA), Biotronik SE & Co. KG (Berlin, Germany) and St. Jude
Medical, Inc.. (St. Paul, MN, USA). All the pacemakers were
programmed with a basic rate of 70 bpm. The paced AV delay in the
dual chamber pacing was programmed to 150±23 msec. All patients
volunteered to participate in the study and were included once
spoken and written informed consent had been received. The study
protocol was approved by the Ethics Committee of the Affiliated
Wuxi People’s Hospital of Nanjing Medical University (Wuxi,
China).
Pacemaker programming
Dual-chamber pacemakers were programmed for AAI, DDD
and VVI pacing modes, respectively, i.e. the AAI pacing mode was
initially programmed in all patients, prior to the pacemakers being
programmed from AAI to DDD modes and then from DDD to VVI modes.
Subsequent to pacing being performed in each mode for 24 h, the
RT3DE and TDI images were acquired, respectively.
RT3DE acquisition
RT3DE was performed using a commercially available
echocardiography system (iE33; Philips Medical Systems, Andover,
MA, USA) by employing an X3 matrix transducer. The individuals were
asked to hold their breath and the images were coupled with an
electrocardiographic record. The images were stored in the hard
disk of the echocardiography system for further offline analysis
with special software (QLAB, version 8.1; Philips Medical Systems)
for the same equipment. The parameters evaluated using RT3DE
included LV end-diastolic volume (LVEDV), LV end-systolic volume
(LVESV), LVEF and RT3DE volume-time curves (VTCs). The left
ventricle was divided into 17 segments, from apex to base,
according to the segmentation schema of the American Heart
Association and the American Society of Echocardiography (4), and the regional VTCs were obtained
for each segment. To assess systolic dyssynchrony, the standard
deviation (SD) of time from the QRS onset to the minimal systolic
regional volume was obtained for 16 segments, i.e. 6 basal, 6
middle and 4 apical segments (Tmsv16-SD); as well as 12
segments, i.e. 6 basal and 6 middle segments
(Tmsv12-SD), and the 6 basal segments
(Tmsv6-SD) of the left ventricle in each patient. In
addition to the Tmsv index, the maximal difference (Dif) in time
from the QRS onset to the minimal regional systolic volume for the
l6, 12 and 6 segments of the left ventricle (Tmsv16-Dif,
Tmsv12-Dif and Tmsv6-Dif, respectively) was
automatically calculated. All the systolic dyssynchrony indices
were normalized as percentages of the RR-interval
(Tmsv16-SD%, Tmsv12-SD%,
Tmsv6-SD%, Tmsv16-Dif%,
Tmsv12-Dif% and Tmsv6-Dif%) (5). The cut-off value of
Tmsv16-SD% used in this study was 8.3% (6). The higher the Tmsv16-SD
value, the worse the LV synchronicity.
TDI acquisition
TDI echocardiograms were acquired using the iE33
echocardiography system with a broadband transducer (S5-1,2-5 MHz).
The images were coupled with an electrocardiographic record. The
TDI echocardiographic examinations were performed in accordance
with the guidelines of the American Society of Echocardiography
(7). The parameters evaluated
using TDI were Ts-SD and Ts-Dif, which were defined as the standard
deviation and the maximal difference in time, respectively, from
the QRS onset to the peak systolic tissue velocity for 12 segments
of the left ventricle, i.e. 6 basal and 6 middle segments obtained
from two, three and four-chamber apical views. In addition, TDI
analysis of 6 basal and 6 middle segments was obtained from two,
three and four-chamber apical views. The cut-off value of Ts-SD
used in this study was 32.6 msec (8,9). The
higher this value, the worse the LV synchronicity. The peak speed
of the early diastolic phase in the mitral valve annulus (E) was
measured using pulsed Doppler. In addition, the peak speed of the
early diastolic phase in the mitral valve annulus (Em) was measured
using TDI and E/Em was calculated.
The RT3DE and TDI analysis of each patient was
undertaken by three different observers and the data shown are the
mean of three consecutive measurements.
Statistical analysis
Continuous variables are presented as the mean ± SD.
A one-way analysis of variance (ANOVA) test was used to evaluate
the differences among the three modes and a Student-Newman-Keuls
test was performed to make a comparison between two modes.
Non-parametric tests were used if the data were abnormally
distributed or showed heterogeneous variance. To evaluate the
correlation analysis, Pearson’s correlation method (r) was employed
and the χ2 test was used to compare categorical
variables. Data were processed with a statistical analysis software
(SPSS for Windows version 19.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Evaluations of LVMD using RT3DE
The descriptive analysis of RT3DE-derived LV
dyssynchrony indices in patients with AAI, DDD and VVI pacing modes
are shown in Table I. The
Tmsv16-SD% (2.9±1.6) and Tmsv16-Dif%
(5.8±2.6) in the AAI mode were significantly lower than those in
the DDD mode (9.1±3.3%, P<0.05 and 12.8±6.2%, respectively;
P<0.05) and the VVI mode (11.2±3.9%, P<0.05 and 15.6±5.3%,
respectively; P<0.05). The same trends were also observed for
Tmsv12-SD%, Tmsv12-Dif%, Tmsv6-SD%
and Tmsv6-Dif%. These results showed that LV systolic
synchronization in the AAI mode was superior to that in the DDD and
VVI modes. However, no significant difference between the DDD and
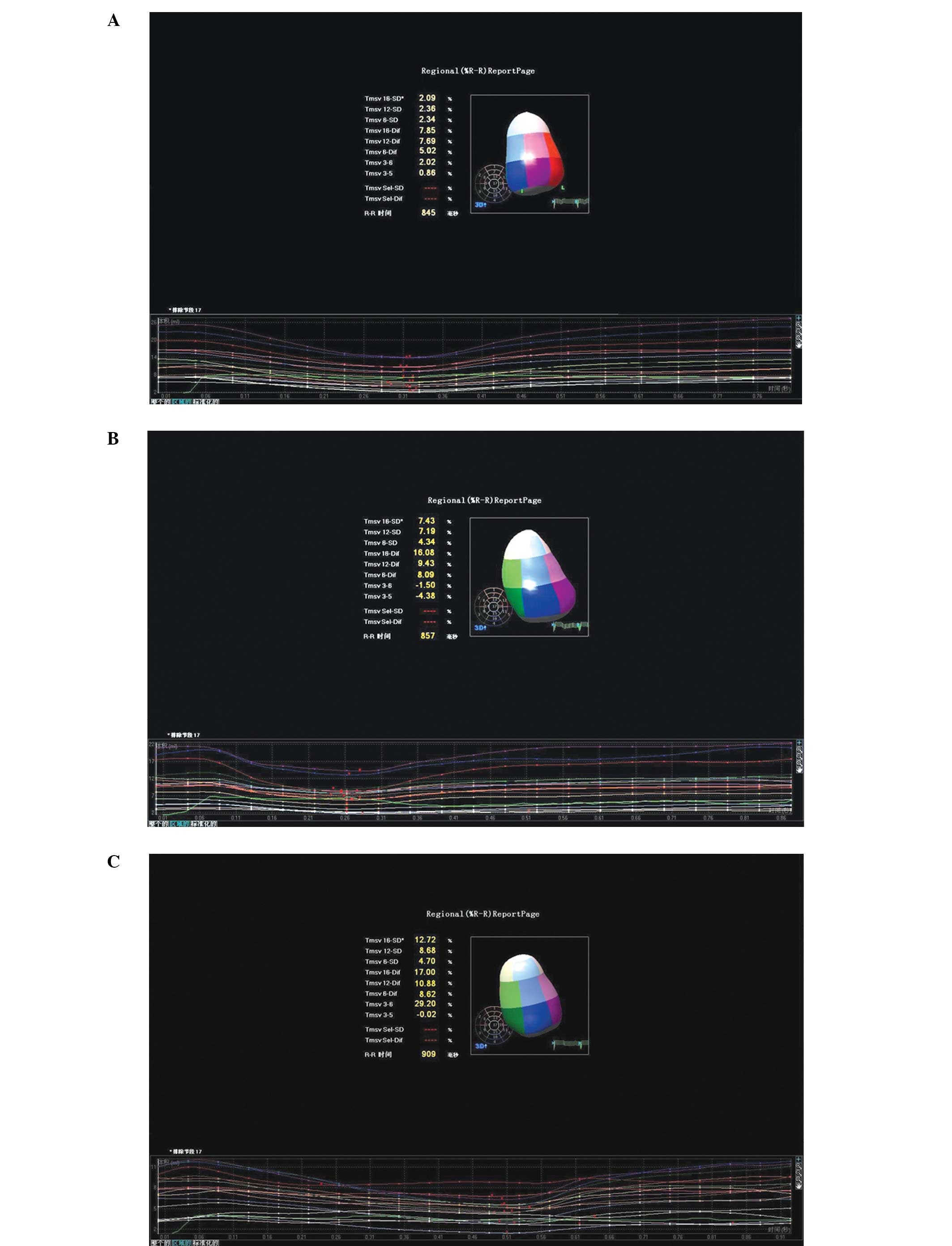
VVI modes was observed (P>0.05). The VTCs of the AAI, DDD and
VVI pacing modes are shown in Fig.
1.
 | Table I.Different dyssynchrony indices in AAI,
DDD and VVI pacing modes. |
Table I.
Different dyssynchrony indices in AAI,
DDD and VVI pacing modes.
| Dyssynchrony
indices | AAI mode (n=20) | DDD mode (n=20) | VVI mode (n=20) |
|---|
|
Tmsv16-SD% | 2.9±1.6 | 9.1±3.3a | 11.2±3.9a,b |
|
Tmsv12-SD% | 2.7±0.9 | 7.5±2.6a | 8.2±3.1a,b |
|
Tmsv6-SD% | 2.3±1.2 | 5.7±2.5a | 6.3±2.8a,b |
| Tmsv16-Dif
% | 5.8±2.6 | 12.8±6.2a | 15.6±5.3a,b |
|
Tmsv12-Dif% | 4.9±2.2 | 12.0±3.8a | 13.9±5.1a,b |
|
Tmsv6-Dif% | 3.7±1.9 | 7.5±2.6a | 8.2±5.2a,b |
| Ts-SD (msec) | 23.6±4.9 | 42.3±9.7a | 46.1±5.6a,b |
| Ts-Dif (msec) | 37.9±12.6 | 106±23.6a | 112±28.7a,b |
Evaluations of LVMD using TDI
The descriptive analysis of TDI-derived LV
dyssynchrony indices in patients with AAI, DDD and VVI pacing modes
are shown in Table I. The Ts-SD in
the AAI, DDD and VVI modes was 23.6±4.9, 42.3±9.7 and 46.1±5.6
msec, respectively, while the Ts-Dif in the AAI, DDD and VVI modes
was 37.9±12.6, 106±23.6 and 112±28.7 msec, respectively. The Ts-SD
and Ts-Dif values for the DDD and VVI modes were significantly
different from those for the AAI mode (P<0.05). However, there
was no significant difference between the DDD and VVI modes
(P>0.05).
LV systolic and diastolic function
The changes in LV systolic and diastolic function in
patients with AAI, DDD and VVI pacing modes are shown in Table II. The LVEF in the AAI, DDD and VVI
modes was 63.1±8.9, 58.6±11.2 and 57.9±7.6%, respectively
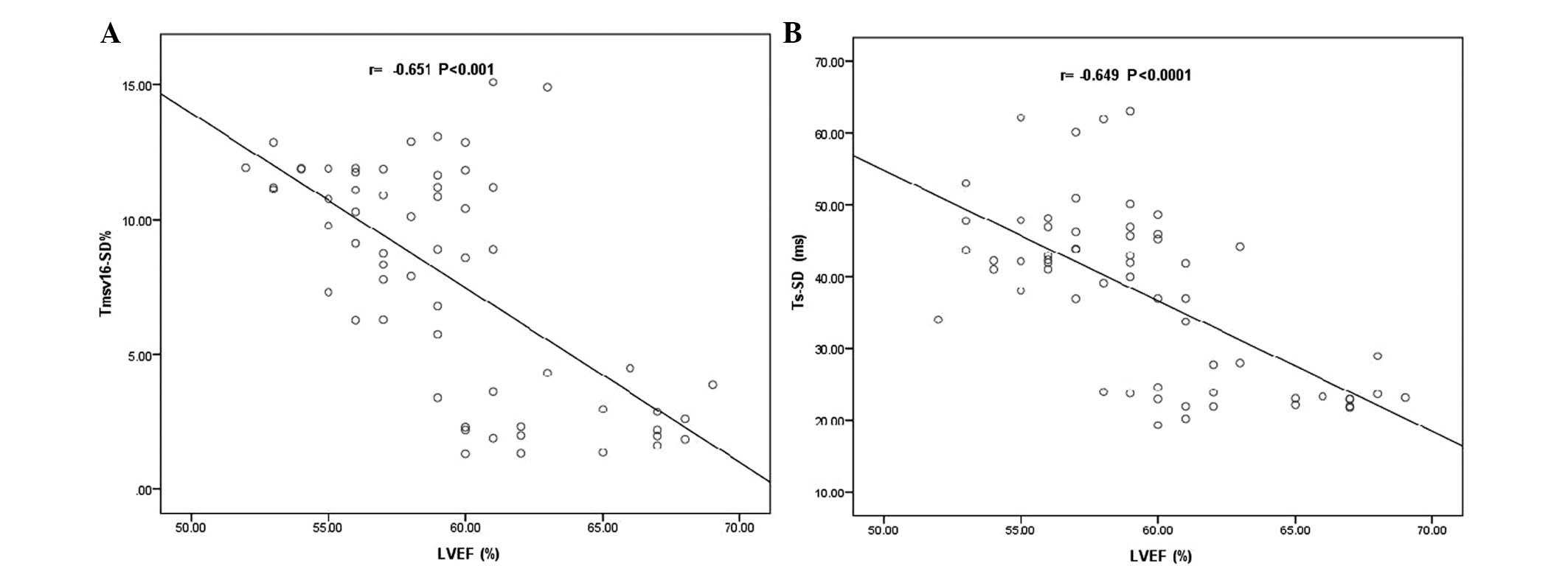
(P>0.05). Pearson’s correlation analysis showed that the LVEF
was inversely correlated with the RT3DE and TDI-derived LV
dyssynchrony indices. The descriptive analysis is shown in Table III. The coefficient of correlation
(r) between the LVEF and Tmsv16-SD% was −0.651
(P<0.001; Fig. 2A), while the
correlation between the LVEF and Ts-SD was −0.649 (P<0.0001;
Fig. 2B). No significant
differences were identified in LVEDV, LVESV and E/Em among the AAI,
DDD and VVI pacing modes (P>0.05).
 | Table II.Left ventricular systolic and
diastolic function in AAI, DDD and VVI pacing modes. |
Table II.
Left ventricular systolic and
diastolic function in AAI, DDD and VVI pacing modes.
| Echocardiographic
parameters | AAI mode
(n=20) | DDD mode
(n=20) | VVI mode
(n=20) |
|---|
| LVEDV (ml) | 99.7±8.3a | 99.9±8.3a | 98.1±7.7a |
| LVESV (ml) | 36.8±5.1b | 39.7±4.5b | 43.2±4.6b |
| LVEF (%) | 63.1±8.9c | 58.6±11.2c | 57.9±7.6c |
| E/Em | 4.92±0.96d | 5.82±0.74d | 5.70±0.63d |
 | Table III.Correlations of RT3DE- and
TDI-derived LV dyssynchrony indices and LVEF. |
Table III.
Correlations of RT3DE- and
TDI-derived LV dyssynchrony indices and LVEF.
| RT3DE | Ts-SD (TDI)
| Ts-Dif (TDI)
| LVEF
|
|---|
| r | P-value | r | P-value | r | P-value |
|---|
|
Tmsv16-SD% | 0.698 | <0.0001 | 0.612 | <0.001 | −0.651 | <0.001 |
|
Tmsv12-SD% | 0.639 | <0.001 | 0.586 | <0.001 | −0.632 | <0.001 |
|
Tmsv6-SD% | 0.368 | ns | 0.322 | ns | −0.398 | ns |
|
Tmsv16-Dif% | 0.636 | <0.0001 | 0.532 | <0.05 | −0.614 | <0.001 |
|
Tmsv12-Dif% | 0.585 | <0.001 | 0.509 | <0.05 | −0.594 | <0.001 |
|
Tmsv6-Dif% | 0.328 | ns | 0.296 | ns | −0.382 | ns |
| LVEF | −0.649 | <0.0001 | −0.579 | <0.001 | - | - |
Correlation and concordance between RT3DE
and TDI
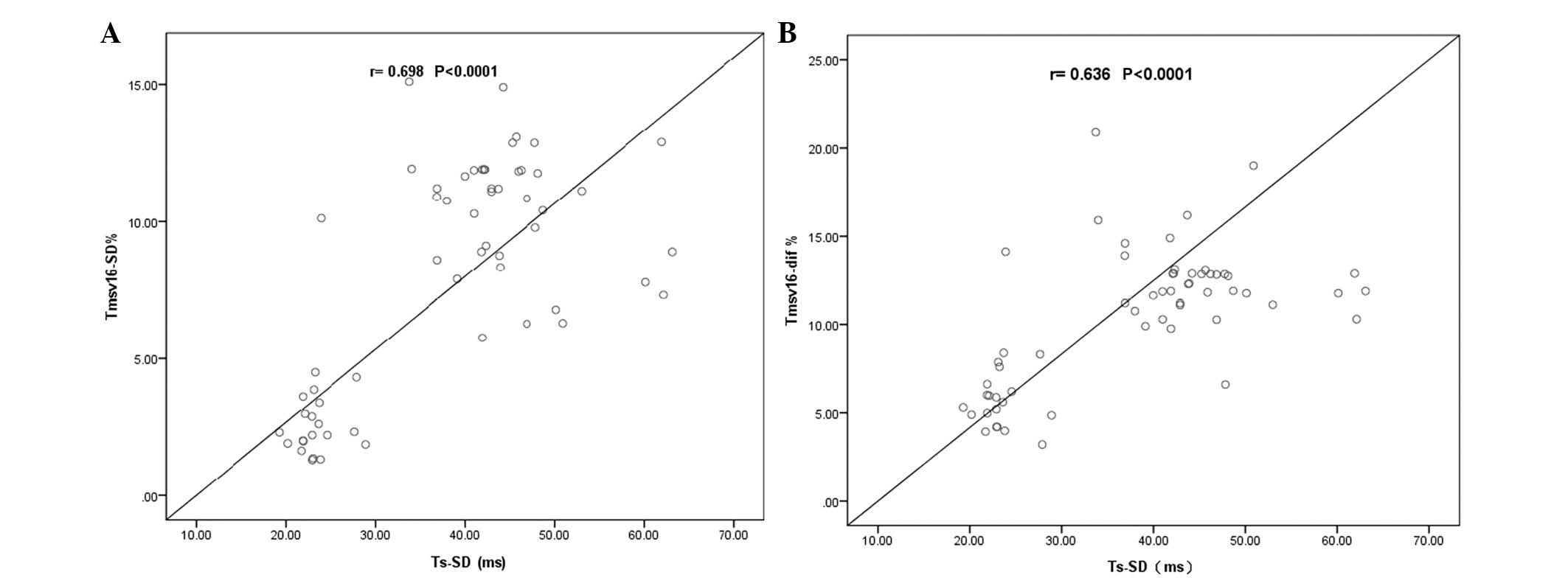
The correlations between the RT3DE and TDI-derived
LV dyssynchrony indices are shown in Table III. As shown in Fig. 3A, the correlation between
Tmsv16-SD% and Ts-SD was 0.698 (P<0.0001), while the
correlation between Tmsv16-Dif% and Ts-SD was 0.636
(P<0.0001, Fig. 3B). Moderate
correlations were also observed between the other RT3DE and
TDI-derived LV dyssynchrony indices (r, 0.509–0.639; P<0.05).
Tmsv16-SD% and Ts-SD showed a concordance rate of 76%
for detecting LVMD, with two variables being above the normal
cutoff values in 34 cases, and two variables being below the normal
cut-off values in 12 cases. In the remaining 14 cases, eight had
only a Tmsv16-SD% above the normal cut-off value, while
six had only a Ts-SD above the normal cut-off value.
Discussion
Numerous studies have demonstrated that the use of
DDD and VVI modes in the setting of right ventricular apical (RVA)
pacing may lead to abnormal electrical activation and LV
dyssynchrony (10–13). Cardiac pacing in various modes may
induce AV and inter- and intraventricular dyssynchrony accordingly.
Previous investigations have also shown LVMD to be correlated with
LV remodeling, LV enlargement, LV dysfunction, poor hemodynamic
outcome and the occurrence of major cardiac events (14,15).
At present, a number of different types of
echocardiographic technologies are used as major methods for the
evaluation of LVMD; however, there is no gold standard. In our
prospective study, we attempted to quantify LV dyssynchrony and
cardiac function in patients with AAI, DDD and VVI pacing modes
using RT3DE, and compared the results from the RT3DE with the
different dyssynchrony indices derived from TDI for the same
patient. Previous studies have indicated that measuring the
standard deviation and the maximal difference in time from the QRS
onset to the peak systolic longitudinal velocity for 12 LV
myocardial segments using TDI is useful for quantifying systolic
dyssynchrony (9,16–20).
However, the physics of TDI present certain limitations, such as
the fact that TDI is not able to reflect the dyssynchrony of the
entire left ventricle. This is due to TDI being unable to obtain
information from the apical region, as a result of the angle
dependency of TDI. In addition, tethering from adjacent ventricular
segments may affect TDI measurements, and TDI is restricted to the
analysis of the longitudinal wall motion of relatively few cardiac
segments. By contrast, RT3DE presents a number of advantages over
the TDI technique for assessing LVMD. It enables the observation of
the overall left ventricle as an entire structure (16 or 17-segment
model) in the same heart beat, leading to VTCs derived from this
analysis. Furthermore, while RT3DE has been demonstrated to assess
LVMD, it additionally appears to evaluate LV remodeling and
function. Thus, LV remodeling (i.e. LV volumes) and synchronicity
may be assessed in a single analysis.
In the present study, the RT3DE and TDI-derived
dyssynchrony indices in the AAI mode were significantly lower than
those in the DDD and VVI modes (P<0.05); however, there was no
significant difference between the DDD and VVI modes (P>0.05).
These results showed that LV synchronization in the AAI mode was
significantly superior to that in the DDD and VVI modes. In the AAI
pacing mode, normal AV, left-right inter- and intraventricular
conduction sequences were maintained, which was consistent with
physiological ventricular activation; therefore, the
synchronization of the AAI pacing mode was normal. In the DDD
pacing mode, while the AV conduction sequence was maintained, the
normal inter- and intraventricular conduction sequences were lost
due to RVA pacing, which induced systolic and diastolic mechanical
dyssynchrony. With regard to the VVI pacing mode, normal inter- and
intraventricular conduction sequences, in addition to the AV
conduction sequence, were lost. Therefore, the dyssynchrony in the
VVI mode was worse than that in the DDD mode, although our study
revealed no significant differences between the two modes
(P>0.05).
In addition to inducing LVMD, cardiac pacing may
also cause LV dysfunction. By measuring LVEF, LVEDV and LVESV,
RT3DE was used to estimate the changes in cardiac systolic
function. In this study, the LVEF decreased in the DDD and VVI
modes following pacing for 24 h; however, there were no significant
differences among the AAI, DDD and VVI modes (P>0.05). Previous
studies have demonstrated an acute reduction in the LVEF of 6–13%
following the initiation of DDD and VVI pacing (21,22).
In addition, we observed that the RT3DE and TDI-derived
dyssynchrony indices increased significantly with the severity of
the LV systolic dysfunction. The Tmsv16-SD% and Ts-SD
were inversely correlated with LVEF (r, −0.651; P<0.001 and r,
−0.649; P<0.0001), which is consistent with previous studies
(23,24). These results all indicate that the
degree of LV dyssynchrony adversely affected global LV systolic
function.
In the current investigation, a moderate correlation
between RT3DE and TDI-derived dyssynchrony indices was identified
(r, 0.509–0.698; P<0.05). A possible explanation is that it is
not possible to perform examinations in certain segments, such as
the apex, with TDI whereas RT3DE enabled 16 segments of the whole
left ventricle to be analyzed. Furthermore, TDI only studied
longitudinal cardiac motion, while RT3DE was able to analyze the
longitudinal, circumferential and radial cardiac mechanical
motions. This result was controversial, taking previous studies
into consideration. A number of studies have revealed contradictory
results concerning RT3DE and TDI indices, ranging from poor (r,
0.11; P=not significant) (25,26)
to good (r, 0.80; P<0.01) correlation (6,23,24,27–32).
Furthermore, the concordance rate between Tmsv16-SD% and
Ts-SD was 76% in the current study. A previous study showed that
Ts-SD and Tmsv16-SD% had a concordance rate of 56.5% and
when there was no agreement between the two indices, it was
observed that Ts-SD was abnormal in 38% of patients and
Tmsv16-SD% was abnormal in 8% (6). Another study showed the concordance
rate to be 79% (27). However, it
is difficult to compare the results from the various studies, since
the studies used different selection criteria.
In conclusion, this study demonstrated that RT3DE
and TDI were able to objectively and accurately evaluate LV
function and LVMD in patients with various pacing modes and that LV
systolic synchronicity in the AAI mode was superior to that in the
DDD and VVI modes. The RT3DE and TDI-derived LV dyssynchrony
indices increased with worsening LVEF. In addition, the
RT3DE-derived dyssynchrony index Tmsv16-SD% had a high
concordance rate with the well-established TDI-derived dyssynchrony
index Ts-SD. In the same patient, there was variability in the
incidence of LVMD depending on the echocardiographic method used.
Due to the lack of gold standard for the evaluation of LVMD, two
different echocardiographic dyssynchrony indices appear to provide
complementary, rather than opposing, information regarding the
presence of LVMD. In clinical applications, if the conditions
permit, it is preferable for all pacemakers to be programmed in the
AAI mode, in order to reduce LV ventricular dyssynchrony, heart
dysfunction and complications and to improve the survival
rates.
The present study had certain limitations. In
particular, the study was a self-contrasted and was a single center
study with a small sample size. A gold standard for the validation
of the various dyssynchrony indices does not exist to date. It is
necessary to note that the relatively low temporal and spatial
resolution of RT3DE imaging may make the feasibility of this
technique in the overall population limited. The current study only
analyzed the changes in the different pacemaker modes in the acute
phase; therefore, the acquisition of data from long-term follow-up
studies is necessary. Furthermore, compared with the results from
the RT3DE, only longitudinal cardiac motion was studied with TDI;
it is possible that a stronger correlation may have been identified
with other methods, such as circumferential or radial strain
analysis using speckle tracking. In this study, the right
ventricular leads were placed in the right ventricular apex;
further discussion regarding the LVMD in patients with different
pacing modes may be appropriate when the right ventricular leads
are placed in the right ventricular outflow tract.
References
|
1.
|
Nielsen JC, Kristensen L, Andersen HR,
Mortensen PT, Pedersen OL and Pedersen AK: A randomized comparison
of atrial and dual-chamber pacing in 177 consecutive patients with
sick sinus syndrome: echocardiographic and clinical outcome. J Am
Coll Cardiol. 42:614–623. 2003. View Article : Google Scholar
|
|
2.
|
Sweeney MO, Hellkamp AS, Ellenbogen KA,
Greenspon AJ, Freedman RA, Lee KL and Lamas GA; MOde Selection
Trial (MOST) Investigators: Adverse effect of ventricular pacing on
heart failure and atrial fibrillation among patients with normal
baseline QRS duration in a clinical trial of pacemaker therapy for
sinus node dysfunction. Circulation. 107:2932–2937. 2003.
View Article : Google Scholar
|
|
3.
|
Wilkoff BL, Cook JR, Epstein AE, Greene
HL, Hallstrom AP, Hsia H, Kutalek SP and Sharma A; Dual Chamber and
VVI Implantable Defibrillator Trial Investigators: Dual-chamber
pacing or ventricular backup pacing in patients with an implantable
defibrillator: the Dual Chamber and VVI implantable Defibrillator
(DAVID) Trial. JAMA. 288:3115–3123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Cerqueira MD, Weissman NJ, Dilsizian V,
Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and
Verani MS; American Heart Association Writing Group on myocardial
Segmentation and Registration for Cardiac Imaging: Standardized
myocardial segmentation and nomenclature for tomographic imaging of
the heart. A statement for healthcare professionals from the
Cardiac Imaging Committee of the Council on Clinical Cardiology of
the American Heart Association. Circulation. 105:539–542. 2002.
View Article : Google Scholar
|
|
5.
|
Horstman JA, Monaghan MJ and Gill EA:
Intraventricular dyssynchrony assessment by real-time
three-dimensional echocardiography. Cardiol Clin. 25:253–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kapetanakis S, Kearney MT, Siva A, Gall N,
Cooklin M and Monaghan MJ: Real-time three-dimensional
echocardiography: a novel technique to quantify global left
ventricular mechanical dyssynchrony. Circulation. 112:992–1000.
2005. View Article : Google Scholar
|
|
7.
|
Schiller NB, Shah PM, Crawford M, DeMaria
A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D,
Schnittger I, et al American Society of Echocardiography Committee
on Standards, Subcommittee on Quantitation of Two-Dimensional
Echocardiograms: Recommendations for quantitation of the left
ventricle by two-dimensional echocardiography. J Am Soc
Echocardiogr. 2:358–367. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yu CM, Fung WH, Lin H, Zhang Q, Sanderson
JE and Lau CP: Predictors of left ventricular reverse remodeling
after cardiac resynchronization therapy for heart failure secondary
to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol.
91:684–688. 2003. View Article : Google Scholar
|
|
9.
|
Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS,
Lin H, Kum LC, Kong SL, Zhang Y and Sanderson JE: Tissue Doppler
imaging is superior to strain rate imaging and postsystolic
shortening on the prediction of reverse remodeling in both ischemic
and nonischemic heart failure after cardiac resynchronization
therapy. Circulation. 110:66–73. 2004. View Article : Google Scholar
|
|
10.
|
van Oosterhout MF, Prinzen FW, Arts T,
Schreuder JJ, Vanagt WY, Cleutjens JP and Reneman RS: Asynchronous
electrical activation induces asymmetrical hypertrophy of the left
ventricular wall. Circulation. 98:588–595. 1998.PubMed/NCBI
|
|
11.
|
Prinzen FW, Hunter WC, Wyman BT and
McVeigh ER: Mapping of regional myocardial strain and work during
ventricular pacing: experimental study using magnetic resonance
imaging tagging. J Am Coll Cardiol. 33:1735–1742. 1999. View Article : Google Scholar
|
|
12.
|
Wyman BT, Hunter WC, Prinzen FW, Faris OP
and McVeigh ER: Effects of single- and biventricular pacing on
temporal and spatial dynamics of ventricular contraction. Am J
Physiol Heart Circ Physiol. 282:H372–H379. 2002.PubMed/NCBI
|
|
13.
|
Wyman BT, Hunter WC, Prinzen FW and
McVeigh ER: Mapping propagation of mechanical activation in the
paced heart with MRI tagging. Am J Physiol Heart Circ Physiol.
276:H881–H891. 1999.PubMed/NCBI
|
|
14.
|
Bader H, Garrigue S, Lafitte S, Reuter S,
Jaïs P, Haïssaguerre M, Bonnet J, Clementy J and Roudaut R:
Intra-left ventricular electromechanical asynchrony. A new
independent predictor of severe cardiac events in heart failure
patients. J Am Coll Cardiol. 43:248–256. 2004.
|
|
15.
|
Fauchier L, Marie O, Casset-Senon D,
Babuty D, Cosnay P and Fauchier JP: Interventricular and
intraventricular dyssynchrony in idiopathic dilated cardiomyopathy:
a prognostic study with fourier phase analysis of radionuclide
angioscintigraphy. J Am Coll Cardiol. 40:2022–2030. 2002.
View Article : Google Scholar
|
|
16.
|
Yu C, Chau E, Sanderson JE, Fan K, Tang
MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR and Lau CP: Tissue
Doppler echocardiographic evidence of reverse remodeling and
improved synchronicity by simultaneously delaying regional
contraction after biventricular pacing therapy in heart failure.
Circulation. 105:438–445. 2002. View Article : Google Scholar
|
|
17.
|
Bax JJ, Bleeker GB, Marwick TH, Molhoek
SG, Boersma E, Steendijk P, van der Wall EE and Schalij MJ: Left
ventricular dyssynchrony predicts response and prognosis after
cardiac resynchronization therapy. J Am Coll Cardiol. 44:1834–1840.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Søgaard P, Egeblad H, Kim WY, Jensen HK,
Pedersen AK, Kristensen BØ and Mortensen PT: Tissue Doppler imaging
predicts improved systolic performance and reversed left
ventricular remodeling during long-term cardiac resynchronization
therapy. J Am Coll Cardiol. 40:723–730. 2002.
|
|
19.
|
Bax JJ, Marwick TH, Molhoek SG, Bleeker
GB, van Erven L, Boersma E, Steendijk P, van der Wall EE and
Schalij MJ: Left ventricular dyssynchrony predicts benefit of
cardiac resynchronization therapy in patients with end-stage heart
failure before pacemaker implantation. Am J Cardiol. 92:1238–1240.
2003. View Article : Google Scholar
|
|
20.
|
Ansalone G, Giannantoni P, Ricci R,
Trambaiolo P, Laurenti A, Fedele F and Santini M: Doppler
myocardial imaging in patients with heart failure receiving
biventricular pacing treatment. Am Heart J. 142:881–896. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nahlawi M, Waligora M, Spies SM, Bonow RO,
Kadish AH and Goldberger JJ: Left ventricular function during and
after right ventricular pacing. J Am Coll Cardiol. 44:1883–1888.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Rosenqvist M, Isaaz K, Botvinick EH, Dae
MW, Cockrell J, Abbott JA, Schiller NB and Griffin JC: Relative
importance of activation sequence compared to atrioventricular
synchrony in left ventricular function. Am J Cardiol. 67:148–156.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhang Q, Yu C, Fung J, Zhang Y, Chan Y,
Chan H, Yip GW and Sanderson JE: Assessment of the effect of
cardiac resynchronization therapy on intraventricular mechanical
synchronicity by regional volumetric changes. Am J Cardiol.
95:126–129. 2005.
|
|
24.
|
Park SM, Kim KC, Jeon MJ, Lee CK, Kim DH,
Park KS, Lee WH and Kwan J: Assessment of left ventricular
asynchrony using volume-time curves of 16 segments by real-time 3
dimensional echocardiography: Comparison with tissue Doppler
imaging. Eur J Heart Fail. 9:62–67. 2007.
|
|
25.
|
Burgess MI, Jenkins C, Chan J and Marwick
TH: Measurement of left ventricular dyssynchrony in patients with
ischaemic cardiomyopathy: a comparison of real-time
three-dimensional and tissue Doppler echocardiography. Heart.
93:1191–1196. 2007. View Article : Google Scholar
|
|
26.
|
Samir R, Tawfik M, EI Missiri AM, EI
Shahid G, Maaty MA and EI Sayed M: Assessment of left ventricular
mechanical dyssynchrony using real-time three-dimensional
echocardiography: a comparative study to Doppler tissue imaging.
Echocardiography. 29:173–181. 2012. View Article : Google Scholar
|
|
27.
|
Takeuchi M, Jacobs A, Sugeng L, Nishikage
T, Nakai H, Weinert L, et al: Assessment of left ventricular
dyssynchrony with real-time 3-dimensional echocardiography:
comparison with Doppler tissue imaging. J Am Soc Echocardiogr.
20:1321–1329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Soliman OI, van Dalen BM, Nemes A, Zwaan
HB, Vletter WB, ten Cate FJ, Theuns DA, Jordaens LJ and Geleijnse
ML: Quantification of left ventricular systolic dyssynchrony by
real-time three-dimensional echocardiography. J Am Soc
Echocardiogr. 22:232–239. 2009. View Article : Google Scholar
|
|
29.
|
Marsan NA, Bleeker GB, Ypenburg C, Ghio S,
van de Veire NR, Holman ER, van der Wall EE, Tavazzi L, Schalij MJ
and Bax JJ: Real-time three-dimensional echocardiography permits
quantification of left ventricular mechanical dyssynchrony and
predicts acute response to cardiac resynchronization therapy. J
Cardiovasc Electrophysiol. 19:392–399. 2008. View Article : Google Scholar
|
|
30.
|
Faletra FF, Conca C, Klersy C, Klimusina
J, Regoli F, Mantovani A, Pasotti E, Pedrazzini GB, De Castro S,
Moccetti T, Auricchio A, et al: Comparison of eight
echocardiographic methods for determining the prevalence of
mechanical dyssynchrony and site of latest mechanical contraction
in patients scheduled for cardiac resynchronization therapy. Am J
Cardiol. 103:1746–1752. 2009. View Article : Google Scholar
|
|
31.
|
van Dijk J, Dijkmans PA, Gotte MJ,
Spreeuwenberg MD, Visser CA and Kamp O: Evaluation of global left
ventricular function and mechanical dyssynchrony in patients with
an asymptomatic left bundle branch block: a real-time 3D
echocardiography study. Eur J Echocardiogr. 9:40–46. 2008.
|
|
32.
|
Raedle-Hurst TM, Mueller M, Rentzsch A,
Schaefers HJ, Herrmann E and Abdul-Khaliq H: Assessment of left
ventricular dyssynchrony and function using real-time 3-dimensional
echocardiography in patients with congenital right heart disease.
Am Heart J. 157:791–798. 2009. View Article : Google Scholar : PubMed/NCBI
|