Introduction
Euphorbia hirta is otherwise known as the
Australian asthma herb, fei yang tsao in China, dudhi
in India, gelang susu in Malaysia, patikan kerbau in
Indonesia and tawa-tawa or gatas-gatas in the
Philippines, and is included in traditional remedies in numerous
cultures (1–3). Euphorbia hirta belongs to the
Euphorbiaceae family. Visually, the plant displays purple
flowers and produces white, creamy latex when its stem is split
(4,5). Its leaves grow in opposition to each
other and are short and oblong shaped. The edges of the leaves are
serrated and they exhibit a purple splotch down the middle
(2). However, regional differences
in the appearance of the plant have been noted.
Analyses of Euphorbia hirta extracts have
revealed the presence of chemicals, including tannins, phenols,
flavanoids, butanol and alkaloids (2,6–8).
These components have been hypothesised to be responsible for the
properties shown by the plant.
Traditionally the plant has been applied as a
poultice to wounds and cuts (5,9),
while skin ailments, such as warts, boils and abscesses, have also
been treated using Euphorbia hirta (5,9).
Furthermore, Euphorbia hirta has been used to treat
dysentery, gonorrhoea and conjunctivitis (10) and more severe illnesses, such as
malaria, have apparently shown improvement with consumption of the
herb (11). In addition, male
sexual dysfunction is remedied by Nigerian traditional healers
using Euphorbia hirta (12), while Swahili and Sukuma individuals
treat hypertension and oedema using this herb (13).
Euphorbia hirta has been shown to possess
potent antimicrobial, anti-inflammatory and antihelminthic
activities. Its anti-inflammatory properties have demonstrated
efficacy against rheumatoid arthritis in Ncf1DA
arthritis-prone rats, superseding that of etanercept, which is a
tumour necrosis factor α (TNF-α) inhibitor currently used as a
frontline disease-modifying anti-rheumatic drug (14). Ethanolic and aqueous leaf extracts
of Euphorbia hirta have shown diuretic effects similar to
those of acetazolamide when tested on rats (13). Furthermore, the blood glucose
levels of albino mice with streptozocin-induced diabetes have also
demonstrated improvements following treatment using aqueous
extracts of the Euphorbia hirta (15) and the herb has been shown to reduce
the gastrointestinal motility of rats with castor oil-induced
diarrhoea (16).
There has been comparatively little investigation
into the potential side-effects of Euphorbia hirta
consumption. Adedapo et al (17,18)
described an increase in serum biomarkers for liver [aspartate
aminotransferase (AST) and alanine aminotransferase (ALT)] and
renal (creatinine and urea) function, as well as leucocytosis and
uraemia in rats that were force-fed with Euphorbia hirta
extracts. Furthermore, Chee (19)
demonstrated an increase in the contractility of isolated thoracic
rat aortae in vitro with respect to phenylephrine.
The aim of this study was to examine the effects of
aqueous extracts of Euphorbia hirta on the ultrastructure of
the murine liver, kidney and aorta.
Material and methods
Materials
Plant material
Raw Euphorbia hirta was procured from Taiwan
and authenticated by Ron Vickery, Department of Botany, Natural
History Museum of London (UK).
Animals
Thirty-two adult male Sprague-Dawley rats, weighing
300–500 g, were used in this study. All rats were purchased from
Universiti Putra Malaysia (UPM; Selangor, Malaysia). The rats were
housed in the Animal Holding Facility of the International Medical
University (IMU; Kuala Lumpur, Malaysia) which was maintained at a
constant room temperature of 24°C with a 12-h dark/light cycle.
Reverse osmosis water and standard rat feed (Perniagaan Usaha
Cahaya, Serdang, Malaysia) were provided ad libitum. Prior
to commencing the feeding of the rats with Euphorbia hirta
extracts every alternate day for 50 days, a one-week adjustment
period was allotted. Experimental procedures were reviewed,
monitored and approved by the Research and Ethics Committee of the
IMU.
Methods
Preparation of extract
Powdered Euphorbia hirta (30 g) was
sequentially extracted with 300 ml of each n-hexane, chloroform,
methanol and water. A total of 90 g powdered Euphorbia hirta
was used in the preparation of the extract.
The solvents in the extracts were removed via rotary
evaporation under reduced pressure. The aqueous extract was further
freeze dried and stored at 4°C. The yield of the aqueous extract
was 9%, based on the dry weight of the plant.
Animal feeding
The male Sprague-Dawley rats were divided into four
groups, with eight rats assigned to each group. Every cage housed
four rats, which were tagged on their tails. Group 1 was fed
phosphate-buffered saline (PBS) as a control, while Groups 2-4 were
fed with increasing concentrations of aqueous extracts of
Euphorbia hirta, at 1, 10 and 50 mg/kg, respectively.
All animals were weighed daily. The rats were fed
the extracts using oral gavage and the standard volume of feed was
1 ml. The rats received the extracts or PBS every alternate day for
a period of 50 days prior to the animals being sacrificed via
cardiac puncture.
Organ harvesting and processing for
storage
The thoracic aorta, defined as the section of the
aorta stretching from the end of the arch of the aorta to the
diaphragm, was used in this study. The perivascular adipose tissue
(PVAT) was completely stripped off. Similarly, the livers and
kidneys were harvested and the surrounding fatty tissue was
removed.
A single aortic ring was apportioned out and sizable
portions of the liver and kidneys were removed; all were placed in
2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. The aortic
ring was then sectioned into smaller rings, with a maximum width of
1 mm3, and the liver and kidney were manually sectioned
into cubes, also measuring ≤1 mm3. All sectioning was
performed whilst the organs were soaked in 2.5% glutaraldehyde in
0.1 M sodium cacodylate buffer.
Saline (9 g NaCl per 1 litre H2O) was used to rinse
the sectioned tissues, prior to the tissues being placed in 2.5%
glutaraldehyde in 0.1 M sodium cacodylate buffer and stored at 4°C.
A minimum ratio of 1:17 (tissue to buffer volume) was maintained to
ensure hydration.
Tissue processing for electron
microscopy
Sample processing was conducted in the Electron
Microscopy Unit of the Institute of Medical Research (Kuala Lumpur,
Malaysia). Tissue samples were embedded in epoxy resin using a set
protocol from the University of Bristol (20). The embedded samples were then
sectioned into ultrathin slices using glass knives at a thickness
of 90 nm.
Results
Electron microscopy was performed to view the
changes that may otherwise not have been visible with light
microscopy or the naked eye. Minute changes conferred reasonable
confidence into the pathological processes that may have taken
place.
Aorta
The ultrastructure of the aorta showed no change in
the endothelium, smooth muscle and elastin of the treated groups
when compared with those in the control group. Photomicrographs of
the aorta are shown in Fig. 1A and
B.
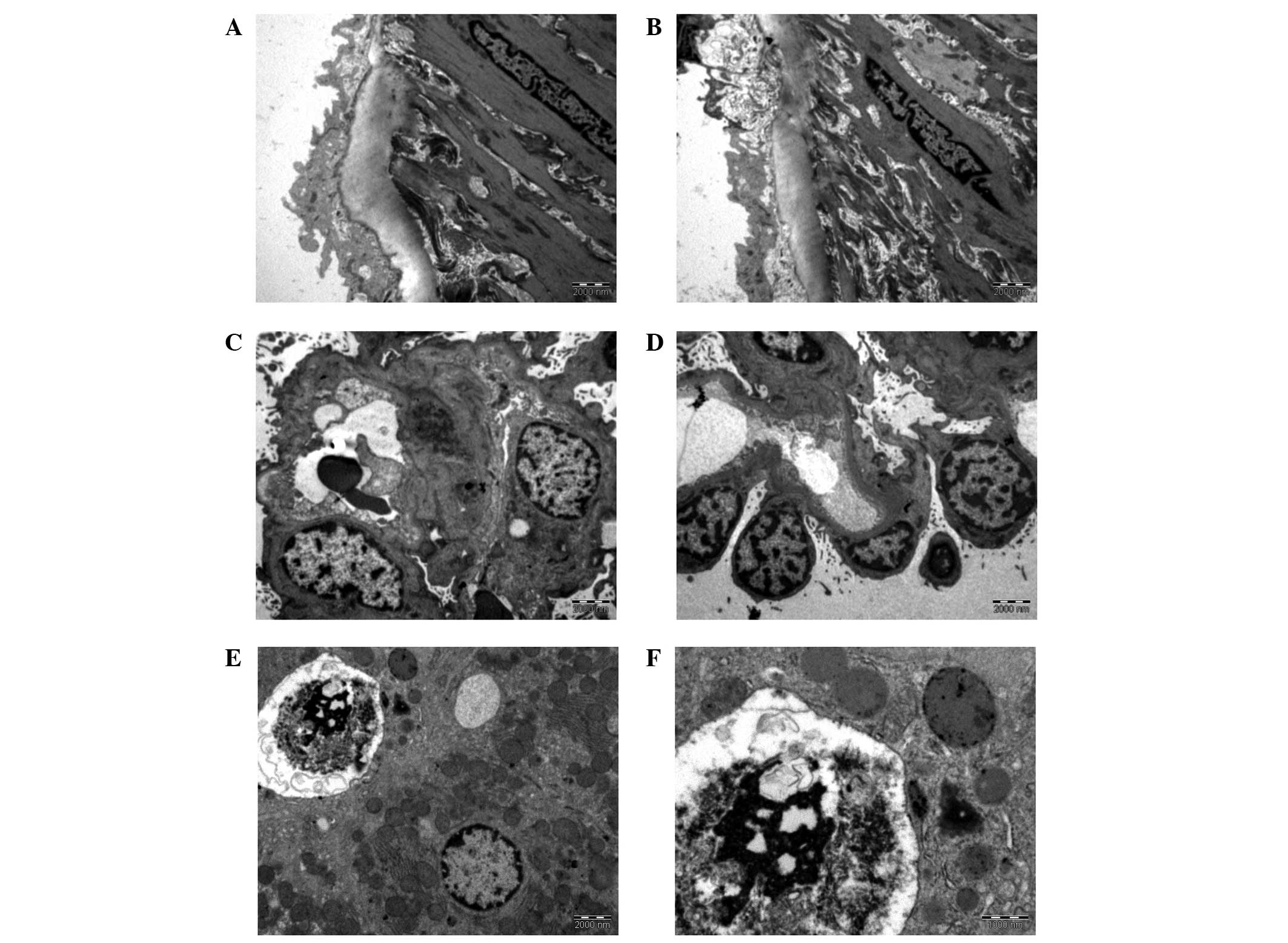 | Figure 1.Tunica intima of an aorta from (A) the
control group (magnification, ×13,500) and (B) group 2
(magnification, ×13,500). (C) Glomerulus with thickened basement
membrane and near-normal podocytic nuclei, which are irregular with
appreciable euchromatin (group 2; magnification, ×4,400). (D) The
few podocytes here show much euchromatin clumping together,
probably demonstrating early stage pyknosis. The basement membrane
is also thickened (group 2; magnification, ×4,400). (E) The top
left hand corner shows a degenerated hepatocyte from group 4. The
nuclear material has completely condensed. A vacuole may be
observed next to the degenerated hepatocyte, as well as a healthy
hepatocyte at the bottom right (magnification, ×4,400). (F) Close
up view of a degenerated nucleus from group 4 (magnification,
×11,000). Group 1, control group, fed with phosphate-buffered
saline; groups 2 and 4, treatment groups, fed with 1 and 50 mg/kg
Euphorbia hirta, respectively. |
Kidneys
Glomeruli of the control group displayed features
typical to those of a normal glomerulus. The basement membranes
were intact without any signs of damage, while the podocytes and
mesangial cells were observed to be healthy. All treated groups
displayed signs of damage to the basement membrane. Podocytes and
mesangial cells showed changes to their nuclei, such as pyknosis
and karyolysis, with the damage appearing to be dose-dependent.
These photomicrographs are shown in Fig. 1C and D.
Liver
All liver samples viewed under the electron
microscope showed the clear presence of hepatocytes. The control
group hepatocytes possessed the typical features of a normal
hepatocyte. By contrast, the hepatocytes in the treated groups were
in various stages of degeneration. These included a marked
reduction in the size of the nucleus, chromatin condensation
(pyknosis) and nuclear fragmentation (karyorrhexis). The changes
appeared to be dose-dependent, consistent with the changes in the
kidney. Photomicrographs of the liver are shown in Fig. 1E and F.
Discussion
To the best of the authors’ knowledge, there have
not been any studies, to date, in which similar methods have been
used to identify the side-effects of Euphorbia hirta.
Therefore, further studies concerning this issue are warranted.
The results obtained from the present study
demonstrated a dose-dependent effect of Euphorbia hirta on
the murine liver and kidneys. Consolidation of these data and the
various other properties of the herb described in previous studies
appear to suggest that Euphorbia hirta exhibits
pharmacological activities similar to those of quinolones, a group
of broad-spectrum antibiotics. A number of examples are described
below.
Oxolinic acid, a first generation quinolone, has
been shown to exert stimulant effects on mice, due to the
properties that classify it as a dopamine reuptake inhibitor
(21). This stimulant effect is
consistent with the findings of induced stress reduction studies
using Euphorbia hirta (22–24).
A common interaction site, the γ-aminobutyric acid A
(GABAA) receptor Cl− complex, has been
identified (25,26). Moxifloxacin, a fourth generation
synthetic fluoroquinolone, has been contra-indicated in patients
suffering from myasthenia gravis, which is in accordance with the
apparent ability of Euphorbia hirta to restore
acetylcholinesterase activity (23,27).
Interestingly, the quinolones are associated with the quinoline
family, as are mefloquine and lariam, a synthetic analogue of
quinine (11,28). This correlates with the
antimalarial activity of Euphorbia hirta. Tosufloxacin, a
fluoroquinolone, has been indicated to cause severe nephritis
(29), while there have been
numerous case reports revealing renal toxicity issues associated
with norfloxacin, such as nephritic syndrome, renal failure and
acute interstitial nephritis (30–33).
Furthermore, there were sufficient postmarketing complaints
concerning renal dysfunction and hepatotoxicity following the use
of temafloxacin for it to be withdrawn from the market (29,34).
Electron microscopy isolates minute portions of
sample organs, which may not be reflective of the global condition
of the organ. Whilst in the current study, isolated focal areas of
damage were isolated, this does not imply that organ integrity and
function were compromised. This study is not able to confirm or
deny the presence of compromised organ function. It is merely an
indication of the consequence of regular Euphorbia hirta
consumption.
Current medical interpretation techniques focus on
histopathology and biochemistry, as well as the correlation with
clinical signs and symptoms. While our results demonstrated damage
to the murine organs with Euphorbia hirta use, the use of
enzyme biomarkers for the respective organs, i.e., AST and ALT for
the liver and urea and creatinine for the kidneys, is likely to
validate our results further.
There are relatively few articles that discuss the
negative effects of any herbal remedy while at the same time
utilising modern pathological techniques. This is probably not due
to disinterest, rather to the immense difficulty of obtaining
verified data on the usage and consumption of herbal remedies
across communities. To complicate matters, the majority of remedies
are passed on by word of mouth. As a result, there are not many
studies with which to compare our results.
Despite the limitations faced, our results indicate
that Euphorbia hirta has a certain ill effects on the end
consumer. The proposed association between quinolones and
Euphorbia hirta is premature and may prove to be incidental.
In conclusion, this study is indicative of the effects of
Euphorbia hirta; however, it should not be used to predict
the effects of the herb. To date, the authors have not uncovered
any study using similar methods to identify the side-effects of
Euphorbia hirta. Therefore, further studies are
required.
The results revealed in the present study, which
indicate that the consumption of Euphorbia hirta may be
associated with side-effects, warrant further investigations.
Moreover, a further detailed analysis of the herb’s biochemistry is
required.
Acknowledgements
This study was funded by a grant from
the International Medical University (Kuala Lumpur, Malaysia). The
authors would like to thank the Director General of Health,
Malaysia for his permission to publish this paper. Similarly, the
authors extend their gratitude to the Director of the Institute of
Medical Research, Malaysia for her support for this project.
References
|
1.
|
Prajapathi ND, Purohit SS, Sharma AK and
Kumar T: A Handbook of Medicinal Plants. 1st edition. Agrobios
India; Jodhpur, India: 2003
|
|
2.
|
British Herbal Pharmacopoeia. 5th edition.
British Herbal Medicine Association; Exeter: 1995
|
|
3.
|
Chevallier A: Encyclopedia of Herbal
Medicine. DK Publishing; New York: 2000
|
|
4.
|
Kew Royal Botanic Gardens: World checklist
of selected plant families. http://apps.kew.org/wcsp/synonomy.do;jsessionid=C2E72FE08A14CAE1130BB94B19007663?name_id=80144uri.
Accessed Apr 5, 2011.
|
|
5.
|
Wiart C: Medicinal Plants of the Asia
Pacific: Drugs for the Future? 1st edition. World Scientific
Publishing Co. Pte. Ltd.; Singapore: 2006
|
|
6.
|
Ogunlesi M, Okiei W, Ofor E and Osibote
AE: Analysis of the essential oil from the dried leaves of
Euphorbia hirta Linn. (Euphorbiaceae), a potential
medication for asthma. African J Biotech. 8:7042–7050. 2009.
|
|
7.
|
Kandalkar AM, Manjunath K, Sholapur HP,
Patel AM and Darade SS: Phytochemical and pharmacognostic
evaluation of Euphorbia hirta Linn. Leaves J Pharm Res.
2:349–352. 2009.
|
|
8.
|
Mahajan R and Badgujar S: Phytochemical
investigations of some laticiferous plants belonging to Khandesh
region of Maharashtra. Ethnobotanical Leaflets. 12:1145–1152.
2008.
|
|
9.
|
Rahmatullah M, Mollik MAH, Azam A, Islam
MR, Chowdhury MAM, Jahan R, et al: Ethnobotanical survey of the
Santal tribe residing in Thakurgaon District, Bangladesh. Am
Eurasian J Sustain Agric. 3:889–898. 2009.
|
|
10.
|
Idu M, Obaruyi G and Erhabor J:
Ethnobotanical uses of plants among the binis in the treatment of
ophthalmic and ENT (ear, nose and throat) ailments. Ethnobotanical
Leaflets. 13:480–496. 2008.
|
|
11.
|
Tona L, Cimanga RK, Mesia K, Musuamba CT,
De Bruyne T, Apers S, et al: In vitro antiplasmodial activity of
extracts and fractions from seven medicinal plants used in the
Democratic Republic of Congo. J Ethnopharmacol. 93:27–32. 2004.
View Article : Google Scholar
|
|
12.
|
Yakubu MT, Akanji MA and Oladiji AT: Male
sexual dysfunction and methods used in assessing medicinal plants
with aphrodisiac potentials. Phcog Rev. 1:49–56. 2007.
|
|
13.
|
Johnson PB, Abdurahman EM, Tiam EA,
Abdu-Aguye I and Hussaini IM: Euphorbia hirta leaf extracts
increase urine output and electrolytes in rats. J Ethnopharmacol.
65:63–69. 1999. View Article : Google Scholar
|
|
14.
|
Hultqvist M, Olofsson P, Gelderman KA,
Holmberg J and Holmdahl R: A new arthritis therapy with oxidative
burst inducers. PLoS Med. 3:e3482006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kumar S, Rashmi and Kumar D: Evaluation of
antidiabetic activity of Euphorbia hirta Linn. in
streptozotocin induced diabetic mice. Indian J Nat Prod Resour.
1:200–203. 2010.
|
|
16.
|
Hore SK, Ahuja V, Mehta G, Kumar P, Pandey
SK and Ahmad AH: Effect of aqueous Euphorbia hirta leaf
extract on gastrointestinal motility. Fitoterapia. 77:35–38.
2006.
|
|
17.
|
Adedapo AA, Abatan MO, Idowu SO and
Olorunsogo OO: Effects of chromatographic fractions of Euphorbia
hirta on the rat serum biochemistry. Afr J Biomed Res.
8:185–189. 2005.
|
|
18.
|
Adedapo AA, Abatan MO and Olorunsogo OO:
Toxic effects of some plants in the genus Euphorbia on
haematological and biochemical parameters of rats. Vet Arh.
74:53–62. 2004.
|
|
19.
|
Chee YC: Exploring the effects and
mechanisms of action of Euphorbia hirta extracts on isolated
rat aorta (unpublished Bachelor thesis). International Medical
University,. 2009
|
|
20.
|
University of Bristol: Bristol Veterinary
School: Specimen preparation for electron microscopy. http://www.bristol.ac.uk/vetscience/pathology/labprot/emtechs.htmluri.
Accessed April 24, 2011.
|
|
21.
|
Garcia de Mateos-Verchere J, Vaugeois JM,
Naudin B and Costentin J: Behavioural and neurochemical evidence
that the antimicrobial agent oxolinic acid is a dopamine uptake
inhibitor. Eur Neuropsychopharmacol. 8:255–259. 1998.
|
|
22.
|
Anuradha H, Srikumar BN, Shankaranarayana
Rao BS and Lakshmana M: Euphorbia hirta reverses chronic
stress-induced anxiety and mediates its action through the
GABAA receptor benzodiazepine receptor-Cl−
channel complex. J Neural Transm. 115:35–42. 2008. View Article : Google Scholar
|
|
23.
|
Anuradha H, Srikumar B and Deepti N:
Restoration of acetylcholinesterase activity by Euphorbia
hirta in discrete brain regions of chronically stressed rats.
Pharm Biol. 48:499–503. 2010.PubMed/NCBI
|
|
24.
|
Lanhers MC, Fleurentin J, Mortier F,
Misslin R and Cabalin P: Behavioural and neurotropic effects of an
aqueous extract of Euphorbia hirta L. (Euphorbiaceae). Actes
du Colloque Europeen d’Ethnopharmacologie et de la 11th Conférence
internationale d’Ethnomedecine; Heidelberg. 24–27 March 1993;
|
|
25.
|
Akaike N, Shirasaki T and Yakushiji T:
Quinolones and fenbufen interact with GABAA receptor in
dissociated hippocampal cells of rat. J Neurophysiol. 66:497–504.
1991.
|
|
26.
|
Unseld E, Ziegler G, Gemeinhardt A,
Janssen U and Klotz U: Possible interaction of fluoroquinolones
with the benzodiazepine-GABAA-receptor complex. Br J
Clin Pharmacol. 30:63–70. 1990. View Article : Google Scholar
|
|
27.
|
US Food Drug Administration: Risk of
fluoroquinolone-associated myasthenia gravis exacerbation -
February 2011 label changes for fluoroquinolones. http://www.fda.gov/safety/medwatch/safetyinformation/ucm247115.htmuri.
Accessed October 20, 2011.
|
|
28.
|
Titanji VPK, Zofou D and Ngemenya MN: The
antimalarial potential of medicinal plants used for the treatment
of malaria in Cameroonian folk medicine. Afr J Tradit Complement
Altern Med. 5:302–321. 2008.PubMed/NCBI
|
|
29.
|
Rubinstein E: History of quinolones and
their side effects. Chemotherapy. 47(Suppl 3): 3–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Boelaert J, de Jaegere PP, Daneels R,
Schurgers M, Gordts B and Van Landuyt HW: Case report of renal
failure during norfloxacin therapy. Clin Nephrol.
25:2721986.PubMed/NCBI
|
|
31.
|
Hanson B, D’Hondt A, Depierreux M and
Lustman F: Nephrotic syndrome after norfloxacin. Nephron.
74:4461996. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Hestin D, Hanesse B, Frimat L, Renaudin
JM, Netter P and Kessler M: Norfloxacin-induced nephrotic syndrome.
Lancet. 345:732–733. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Nakamura M, Ohishi A, Aosaki N and
Hamaguchi K: Norfloxacin-induced acute interstitial nephritis.
Nephron. 86:204–205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Goldstein EJ: Possible role for the new
fluoroquinolones (levofloxacin, grepafloxacin, trovafloxacin,
clinafloxacin, sparfloxacin, and DU-6859a) in the treatment of
anaerobic infections: review of current information on efficacy and
safety. Clin Infect Dis. 23(Suppl 1): S25–30. 1996. View Article : Google Scholar
|















