Introduction
Hepatocellular carcinoma (HCC) is one of the ten
most common malignant tumors worldwide, and its morbidity and
mortality is increasing annually (1,2).
There is a high incidence rate of HCC in China, with 292,966 new
cases and 266,830 fatalities in 2008, which accounted for 40% of
the total global incidence. Therefore, prevention and treatment
methods for patients with HCC are required.
Treatment with radiopharmaceuticals, including
131I-lipiodol and microspheres, may be conducted by
injection into the hepatic artery. The selective retention of the
radionuclide in the liver tumor spares the normal tissues, which
receive below the tolerated dose of internal radiation therapy
(IRT) (3). Using HCC-associated
antigens as a target, the specific binding of antigens and
antibodies has been used to target the radionuclide to tumor
lesions. This type of radioimmunotherapy (RAIT) has been
increasingly studied for the treatment of cancer (4,5).
The therapeutic effects of Licartin
(131I-lipiodol metuximab injection) are mainly
implemented through the biological effects resulting from the
ionizing radiation of 131I β-rays and the inhibition of
signal transduction caused by the bound antibody fragments and
targeting antigens (6,7). Specific and highly selective
metuximab combines with liver cancer cell antigens (HAb18G/CD147)
to result in the radionuclide 131I being targeted to and
retained at the HCC tissues, while the vital tissues and organs
have a low uptake. The high-dose concentrations of the radionuclide
that are achieved irradiate the HCC and cause tumor cell death. The
radiation doses in the whole body remain low and do not cause
unrecoverable damage to other tissues and organs (8). HAb18G/CD147 is a newly discovered
type of liver cancer-associated antigen. Studies have demonstrated
that it is closely associated with metastasis and invasion
(9–11); therefore, it may be used as an
effective indicator for the early diagnosis of liver cancer and as
an independent indicator of prognosis (12,13).
Metuximab binds to the HAb18G/CD147 antigen on the surface of
hepatoma cells and blocks HAb18G/CD147 antigen-induced signal
transduction pathways, thereby inhibiting the metastasis and
invasion of hepatoma cells and reducing liver cancer metastasis and
recurrence. In pre-clinical studies, the control of tumor
progression, extension of patient survival and improvement of
quality of life were demonstrated (14).
In clinical studies, Licartin is locally
administered via the hepatic artery and combined with transcatheter
arterial chemoembolization (TACE). Due to efficient targeting,
peripheral intravenous bolus administration of Licartin may be
safer and more effective and convenient for radioimmunotherapy.
However, no studies concerning the effects of peripheral
intravenous bolus administration of Licartin exist in the
literature. The present study analyzed a total of 33 patients (38
cases) with advanced liver cancer who attended the Tianjin Medical
University Cancer Institute and Hospital (Tianjin, China) from
October 2010 to July 2012 and received molecular imaging and
Licartin radioimmunotherapy at the Department of Molecular Imaging
and Nuclear Medicine. This study aimed to investigate the safety,
efficacy and clinical applications of Licartin for the treatment of
patients with HCC.
Materials and methods
General information
The study comprised 33 patients (38 cases) with
advanced HCC who received Licartin (Chengdu Hoist Hitech Co. Ltd.,
Chengdu, China and the Fourth Military Medical University, Xi’an,
China) in the Tianjin Medical University Cancer Institute and
Hospital between October 2010 and July 2012. The study was
conducted in accordance with the Declaration of Helsinki and with
approval from the ethics committee of Tianjin Medical University
Cancer Institute and Hospital. Written informed consent was
obtained from all participants. Peripheral intravenous bolus
injection was adopted for Licartin administration. The 38 cases
comprised 29 who received Licartin treatment once, three who
received Licartin twice and one who received Licartin three times.
This study included 26 males and seven females (age range, 35–80
years; mean age, 46 years) with four, 15 and 14 cases in tumor node
metastasis (TNM) stage II, III and IV, respectively. Patient
information is shown in Table
I.
 | Table IPatient information (n=33). |
Table I
Patient information (n=33).
| Variable | Value | Percentage (%) |
|---|
| Age (years) |
| Median | 46 | |
| Range | 35–80 | |
| Gender (n) |
| Male | 26 | 78.79 |
| Female | 7 | 21.21 |
| KPS ≥90 (n) | 33 | 100.00 |
| Child-Pugh stage
(n) |
| A | 31 | 93.94 |
| B | 2 | 6.06 |
| Hepatitis positive
(n) |
| B | 32 | 96.97 |
| C | 1 | 3.03 |
| TNM stage (n) |
| II | 4 | 12.12 |
| III | 15 | 45.45 |
| IV | 14 | 42.43 |
| Tumor emboli (n) |
| Yes | 14 | 42.42 |
| No | 19 | 57.58 |
| Abnormal rise of AFP
(n) |
| Yes | 9 | 27.27 |
| No | 24 | 72.73 |
| History of treatment
(n) |
| With radical
surgery | 24 | 72.73 |
| Without radical
surgery | 9 | 27.27 |
Pre-treatment preparation
All patients underwent a skin test of human
anti-mouse antibody (HAMA) response, using one bottle of Metuximab
injection (Chengdu Hoist Hitech Co. Ltd., Chengdu, China) dissolved
in 1 ml saline. Dissolved solution (0.1 ml) was intradermally
injected into the forearm. After 15 min the results were analyzed.
If the flush diameter at injection point was >0.5 cm or the
surrounding pseudopodia were observed, the skin test results were
positive, and they were repeated until a negative result was
achieved before treatment began.
To block and protect the thyroid gland, 0.5 ml
compound iodine solution (Lugol’s solution; Department of
Pharmaceutical Preparation in Tianjin Medical University General
Hospital, Tianjin, China) was administered orally, three times a
day for 10 days (from 3 days before administration of the bolus to
7 days afterwards). This was to avoid unnecessary radiation injury,
which may be caused by the uptake of off-target radioactive
131I-lipiodol in the thyroid tissue.
Drug delivery
Following the establishment of intravenous access or
through peripheral veins, specified doses of 131I
metuximab [27.75 MBq(0.75 mCi)/kg; maximum dose, ≤50 mCi] were
injected slowly within 5–10 min. Tubes were immediately rinsed with
10 ml 0.9% normal saline to ensure full drug administration, while
hepatoprotective symptomatic treatments were provided as
appropriate.
Treatment outcomes
The incidence and severity of nausea, vomiting,
fever, pain and various adverse reactions were observed in all
patients following treatment. Routine blood examinations and liver,
kidney and thyroid function tests were performed 1 week prior to
treatment and 1 and 3 months following treatment, and patients were
regularly followed up. The preliminary assessment standard of the
treatment effects were changes in α-fetoprotein (AFP) expression
and from the imaging studies.
Statistical analysis
Data are expressed as values or percentages. SPSS
software, version 19.0 (SPSS, Inc., Chicago, IL, USA) was applied
for statistical analysis and toxicity was tested by Wilcoxon
rank-sum test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HAMA response
All patients underwent a skin test prior to
metuximab injection; 15 min following injection, if the point flush
diameter was >0.5 cm or pseudopods and blisters were observed,
then this was regarded as a HAMA-positive reaction. Three months
following the first Licartin treatment, four of the nine patients
who were due to receive a secondary treatment did not receive
further treatment owing to their HAMA reaction.
Adverse reactions during treatment
Numerous patients experienced adverse reactions
following the peripheral intravenous bolus administration of
Licartin, including three cases (7.89%) with a non-infectious
fever, four (10.53%) with burning sensation accompanied by liver
area pain, two (5.26%) with nausea and one (2.63%) with vomiting
(Table II). Compared with similar
studies regarding the combination of Licartin administration and
TACE, there was a lower incidence, fewer types and a reduced extent
of adverse reactions in this group (specific classification is
shown in Table II). In the
present study, the majority of patients who tolerated the treatment
demonstrated spontaneous remissions within 1 month.
 | Table IIClassification of adverse reactions in
patients who received a peripheral intravenous bolus of
Licartin. |
Table II
Classification of adverse reactions in
patients who received a peripheral intravenous bolus of
Licartin.
| WHO acute and
subacute toxicity grading of drugs, n (%) |
|---|
|
|
|---|
| Adverse
reactions | 0 | I | II | III | IV |
|---|
| Non-infectious
fever | 35 (92.11) | 1 (2.63) | 2 (5.26) | 0 | 0 |
| Liver area pain | 34 (89.47) | 4 (10.53) | 0 | 0 | 0 |
| Nausea | 36 (94.74) | 1 (2.63) | 1 (2.63) | 0 | 0 |
| Vomiting | 37 (97.37) | 1 (2.63) | 0 | 0 | 0 |
Electrocardiogram (ECG) results
An ECG was recorded 1 week before treatment and 1
and 3 months following treatment. No significant differences were
identified from the ECG results following the treatment.
Vital signs
Patient vital signs were observed 1 week before
treatment and 1 and 3 months following treatment. There were no
significant differences in all indicators before and after the
treatment.
Hematologic toxicity
Following treatment, 15 out of 38 cases (39.47%)
experienced adverse reactions to Licartin including 11 cases
(28.95%) with reduced numbers of white blood cells (WBCs), seven
(18.42%) with decreased platelet (PLT) counts and seven (18.42%)
with increased alanine aminotransferase (ALT) levels, six (15.79%)
with increased aspartate aminotransferase (AST) levels, five
(13.16%) with increased direct bilirubin (SDB) levels, four
(10.53%) with reduced hemoglobin (Hgb) levels, four (10.53%) with
neutral neutropenia, three (7.89%) with increased total bilirubin
(STB) levels, one (2.63%) with increased creatinine (Cr) levels and
one (2.63%) with increased blood urea nitrogen (BUN) levels.
Changes in hematological indices are shown in
Table III. Following treatment,
the levels of WBCs and PLTs decreased, while those of ALT and AST
increased. These results were compared with data from phase II
clinical trials for Licartin (15), and the probability of reductions of
WBC and neutrophil levels was higher than that with local
administration. However, the probabilities of reductions in PLT and
Hgb levels and increases in ATL, AST, SDB, STB, CR and BUN levels
was lower than that for local administration. Two independent
sample t-tests indicated no significant difference in the
probability of adverse reactions caused between the peripheral
intravenous bolus administration of Licartin and local
administration (P>0.05).
 | Table IIIClassification of blood count, liver
and renal function changes before and after treatment. |
Table III
Classification of blood count, liver
and renal function changes before and after treatment.
| 1 week before
treatment | 1 month after
treatment | 3 months after
treatment |
|---|
|
|
|
|
|---|
| Indicators | 0 | I | II | III/IV | 0 | I | II | III/IV | 0 | I | II | III/IV |
|---|
| WBC | 29 | 8 | 1 | 0 | 18 | 15 | 4 | 0 | 23 | 8 | 5 | 0 |
| PLT | 28 | 6 | 4 | 0 | 21 | 8 | 6 | 2 | 23 | 3 | 7 | 3 |
| N | 33 | 4 | 1 | 0 | 32 | 4 | 1 | 0 | 26 | 8 | 2 | 0 |
| Hgb | 33 | 3 | 2 | 0 | 33 | 4 | 2 | 0 | 30 | 6 | 0 | 0 |
| ALT | 30 | 8 | 0 | 0 | 30 | 6 | 1 | 0 | 28 | 6 | 1 | 1 |
| AST | 28 | 8 | 1 | 1 | 25 | 10 | 2 | 0 | 21 | 10 | 5 | 0 |
| STB | 32 | 4 | 2 | 0 | 33 | 3 | 0 | 1 | 27 | 8 | 0 | 1 |
| SDB | 33 | 4 | 1 | 0 | 32 | 4 | 0 | 1 | 34 | 2 | 0 | 0 |
| Cr | 31 | 3 | 0 | 0 | 28 | 4 | 0 | 0 | 35 | 1 | 0 | 0 |
| BUN | 36 | 2 | 0 | 0 | 31 | 5 | 1 | 0 | 31 | 5 | 0 | 0 |
Impact on thyroid function
Thyroid follicular cells have a strong ability to
uptake and concentrate iodine, resulting in a concentration of
iodide in the thyroid that is ≥20–25-fold greater than that in the
plasma. Therefore, all patients in this study, before and following
radioimmunotherapy, were orally treated with Lugol’s solution (0.5
ml three times a day for 10 days, 3 days before and 7 days after
treatment) to block the thyroid tissue and avoid uptake of
off-target radioactive 131I-lipiodol, which would result
in unnecessary radiation injury.
In this study, 21.05% of patients indicated varying
degrees of abnormal thyroid function. However, following treatment,
a number of patients showed recovery of the abnormal thyroid, while
certain patients who were euthyroid before treatment experienced
thyroid dysfunction. The thyroid function changes before and after
treatment are shown in Table IV.
The vast majority of patients, before and following the peripheral
intravenous bolus administration of Licartin, indicated no
significant changes in thyroid function and the thyroid was
successfully blocked and protected. One patient developed
hypothyroidism ~3 months following the treatment. Analysis of the
patient’s thyroid function showed thyroid stimulating hormone (TSH)
levels were >100 mIU/l, and oral levothyroxine sodium alleviated
the symptoms. According to the analysis, this patient demonstrated
abnormal thyroid function before treatment (TSH=11.03 mIU/l) and
did not receive a normal dose of Lugol’s solution, which may
explain the ineffective blocking and protection of the thyroid.
 | Table IVChanges in thyroid function before
and after treatment. |
Table IV
Changes in thyroid function before
and after treatment.
|
Thyroidfunction | 1 week before
treatment, n (%) | 1 month after
treatment, n (%) | 3 months after
treatment, n (%) |
|---|
|
|
|
|---|
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal |
|---|
| T3 | 36 (94.74) | 2 (5.26) | 37 (100.00) | 0 | 36 (10.00) | 0 |
| T4 | 37 (97.37) | 1 (2.63) | 37 (100.00) | 0 | 34 (94.44) | 2 (5.56) |
| TSH | 33 (86.84) | 5 (13.16) | 34 (91.89) | 3 (8.11) | 32 (88.89) | 4 (11.11) |
Clinical efficacy
In July 2012 (follow-up period, ≥3 months), in 33
patients, the clinical remission rate was 9.09% (three cases), the
clinical efficiency was 21.21% (seven cases) and the clinical
response (CR) rate was 60.60% (20 cases). The following are two
specific example cases of the clinical efficacy of peripheral
intravenous bolus administration.
Case one is a male patient (age, 42 years) with HCC
who had undergone liver transplantation, and the pathological
analysis indicated portal vein tumor thrombus; therefore, Licartin
preventative treatment was administered. The patient received 30
mCi Licartin by intravenous injection on April 12, 2011. Eight days
after treatment, an electrical capacitance tomography scan showed
visible radioactivity in the surrounding liver area and bladder;
however, the thyroid indicated no abnormal polyradioactivity,
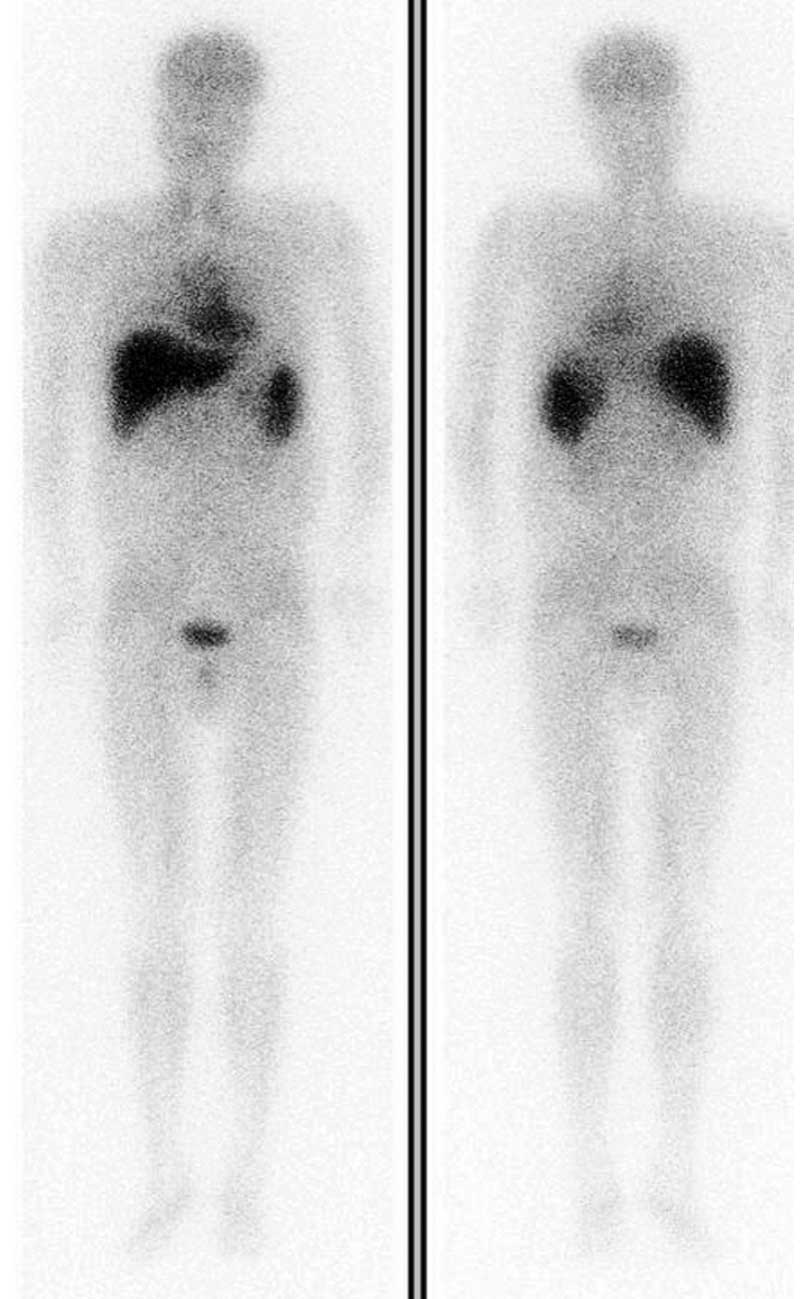
suggesting selective retention of the radionuclide (Fig. 1).
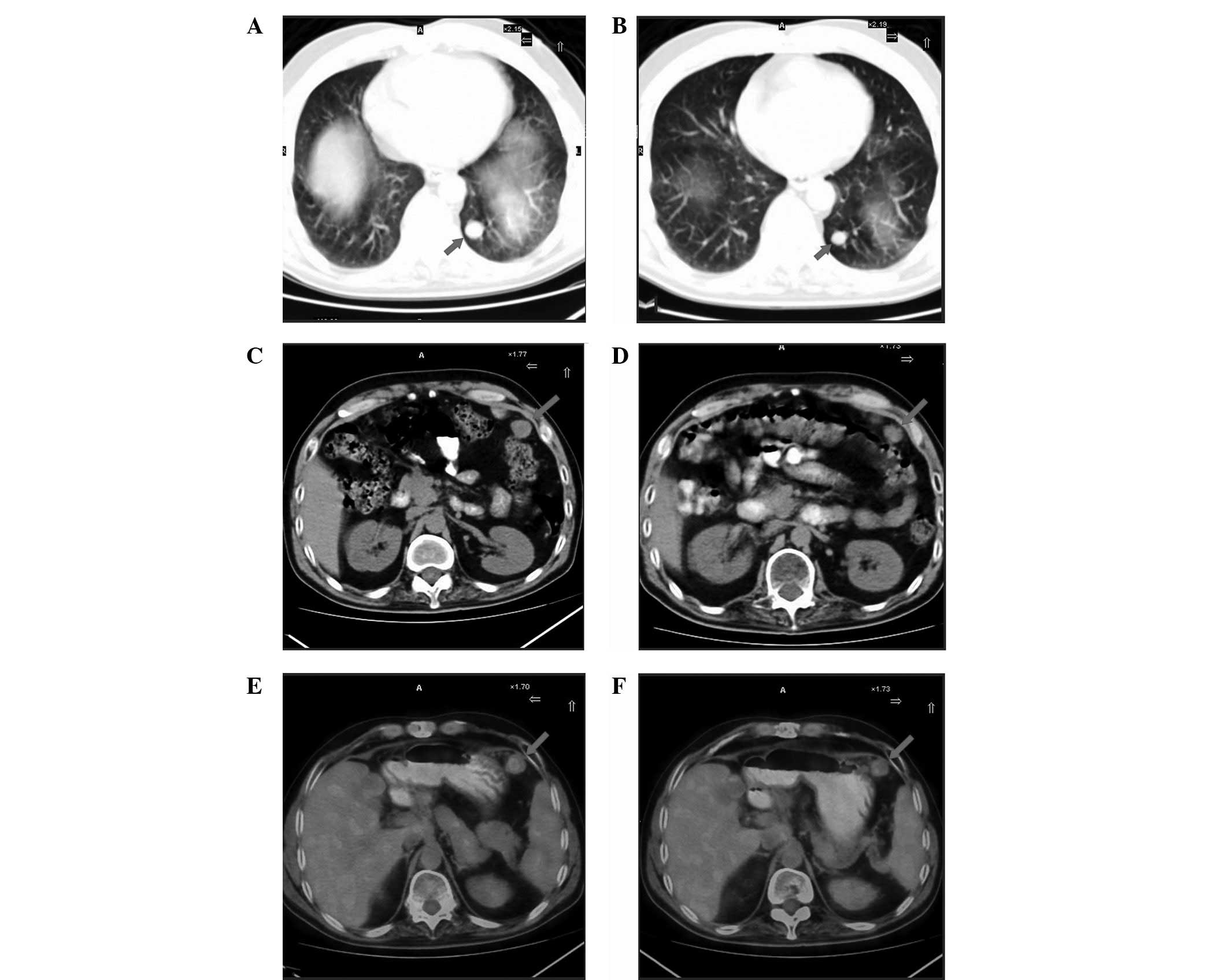
Case two was a male patient (age, 48 years) with TNM
stage II and III who had undergone liver segment resection.
Postoperative pathology of the left hepatic lobe demonstrated
moderate and differentiated HCC. In the first year postoperative
review, positron emission tomography-computed tomography (PET-CT)
scanning identified a high recurrence probability of HCC in the
left hepatic lobe, and basilar segment metastasis of the left lower
lobe, peritoneal mesenteric multiple metastases and remnant liver
metastasis near gallbladder fossa were observed. Following a
clinical consultation, the patient received radioimmunotherapy with
a 50 mCi intravenous bolus of Licartin on April 28, 2012. After 1
month, there were no evident symptoms and results of the routine
blood test and liver and kidney function tests were normal.
Additionally, the PET-CT scan showed that the HCC had improved
(Fig. 2).
Discussion
131I-labeled monoclonal antibodies are a
biological treatment for cancer. 131I is targeted to the
tumor site by an action-oriented antibody, while
131I-β-rays generate the biological effects. As the
carrier, radionuclide-labeled antibodies concentrate in the tumor
tissue and kill the tumor cells without destroying normal tissue.
Compared with chemotherapy or radiotherapy, the toxicity of RAIT is
low.
Pre-clinical studies have demonstrated that adverse
reactions to Licartin are mainly transient blood toxicity and liver
injury. In a phase II clinical study of Licartin (15) in 103 patients, there were 37 cases
with drug-related adverse reactions (35.92%). The main adverse
reactions were reductions in PLT (25.24%), WBC (18.45%) and Hgb
(13.59%) levels, and an increases in ALT (21.36%), AST (21.36%),
SDB (14.56%) and STB (8.74%) levels and proteinuria (8.74%). Wu
et al(7) studied 110
patients with advanced liver cancer who received combined
treatments of transhepatic arterial Licartin and TACE. The main
adverse reactions identified were reductions in WBC and PLT counts;
compared with the TACE treatment group, the incidence rates were
significantly decreased (50.9 versus 21.2 and 42.7 versus 9.0%) and
mainly stayed at I–II degree.
Owing to ischemia and hypoxia of the targeted liver
tissue, as well as reperfusion injury and the side-effects of
chemotherapy, varying degrees of liver dysfunction and
hematological toxicity (16,17)
have been observed with TACE treatment. In addition,
131I radiation injury accompanied by metuximab may
mutually superimpose the two side-effects and aggravate the adverse
reactions and liver damage from combination therapy. Therefore,
studies have been conducted regarding combination therapy with TACE
(18,19). Metuximab has effective targeting
properties; it binds to HAb18G/CD147 on the surface of hepatoma
cells to achieve maximum protection and avoid unnecessary liver
damage and blood toxicity. In the present study, all patients
received radioimmunotherapy intravenously by bolus administration
of Licartin to assess the early adverse reactions and safety of the
treatment for advanced HCC, and to explore its clinical value.
In the present study of 33 patients (38 cases) with
liver cancer, 15 cases (39.47%) experienced possible drug-related
adverse reactions, including 11 cases with a reduction in the
number of WBCs (28.95%), seven with a reduction in the number of
PLTs (18.42%), seven with increased ALT levels (18.42%), six with
increased AST levels (15.79%), five with increased SDB levels
(13.16%), four with a reduction in Hgb (10.53%) levels, four with
neutropenia (10.53%), three with increased STB levels (7.89%), one
with an increased CR (2.63%) and one with increased BUN levels
(2.63%). Compared with intervention treatment, patients treated
intravenously with Licartin indicated a reduced loss of liver
function.
Due to strong iodine uptake and concentrating
ability of thyroid follicular cells, a ≥20–25-fold greater
concentration of iodide was observed in the thyroid compared with
that in plasma; therefore, the changes in patient thyroid function
were analyzed. Three months following the treatment, one patient
experienced hypothyroidism; thyroid function tests showed that TSH
levels were >100 mIU/l. However, following oral administration
of levothyroxine sodium, this symptom alleviated. This may have
been due to the insufficient blocking and protection of the
thyroid, which were the result of abnormal thyroid function
(TSH=11.03 mIU/l) and non-standard administration of Lugol’s
solution before the treatment. This indicated that oral
administration of Lugol’s solution before and after treatment may
avoid unnecessary radiation injury and effectively protect thyroid
function. Therefore, the volume of Lugol’s solution administered to
patients should be regulated, supervised and patients must be
reminded of the administration time.
The results of this study support the hypothesis
that intravenous injection effectively reduces liver damage, which
is conducive to the protection of liver function in patients with
advanced HCC, increases the survival rate and significantly
improves patient quality of life.
Short-term follow-ups were carried out for patients
who received Licartin intravenous treatment. In July 2012
(follow-up, ≥3 months), the clinical remission rate of the 33
patients was 9.09% (three cases), the clinical efficiency was
21.21% (seven cases) and the CR rate was 60.60% (20 cases).
According to the short-term follow-up results, Licartin
demonstrated excellent targeting and inhibition of tumor
progression, particularly in remote metastases, showing a unique
advantage of Licartin radioimmunotherapy. The radionuclide
131I internal radiation effects occurred via binding to
metastatic lesions, including those of the lungs, mesentery and
stomach, by metuximab. Therefore, Licartin is a novel treatment for
patients with HCC.
In conclusion, compared with intervention methods,
intravenous bolus administration of Licartin demonstrated clear
efficiency and mild adverse reactions. It effectively reduced liver
injury and protected liver function in patients with advanced HCC,
subsequently promoting patient survival. However, due to the
relatively small number of cases in this study and the short-term
follow-up of patients, a large-sample, multicenter clinical study
is required for further analysis of the long-term effects of
Licartin treatment.
Acknowledgements
This study was supported by the Tianjin Natural
Science Foundation of China (grant no. 08JCZDJC23700), the Tianjin
Education Topic (grant no. 20080133) and the Tianjin City High
School Science & Technology Fund Planning Project (grant no.
20120109).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
3
|
Lambert B and Van de Wiele C: Treatment of
hepatocellular carcinoma by means of radiopharmaceuticals. Eur J
Nucl Med Mol Imaging. 32:980–989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharkey RM and Goldenberg DM: Perspectives
on cancer therapy with radiolabeled monoclonal antibodies. J Nucl
Med. 46(Suppl 1): 115S–127S. 2005.PubMed/NCBI
|
|
5
|
Goldenberg DM and Sharkey RM: Advances in
cancer therapy with radiolabeled monoclonal antibodies. Q J Nucl
Med Mol Imaging. 50:248–264. 2006.PubMed/NCBI
|
|
6
|
Xu J, Xu HY, Zhang Q, et al: HAb18G/CD147
functions in invasion and metastasis of hepatocellular carcinoma.
Mol Cancer Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu L, Yang YF, Ge NJ, et al: Hepatic
arterial iodine-131-labeled metuximab injection combined with
chemoembolization for unresectable hepatocellular carcinoma:
interim safety and survival data from 110 patients. Cancer Biother
Radiopharm. 25:657–663. 2010. View Article : Google Scholar
|
|
8
|
Zhu H, Yang B, Yang X, et al: A novel
antibody fragment targeting HAb18G/CD147 with cytotoxicity and
decreased immunogenicity. Cancer Biol Ther. 8:1035–1044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai JY, Dou KF, Wang CH, et al: The
interaction of HAb18G/CD147 with integrin α6β1 and its implications
for the invasion potential of human hepatoma cells. BMC Cancer.
9:3372009.
|
|
10
|
Ke X, Li L, Dong HL and Chen ZN:
Acquisition of anoikis resistance through CD147 upregulation: A new
mechanism underlying metastasis of hepatocellular carcinoma cells.
Oncol Lett. 3:1249–1254. 2012.PubMed/NCBI
|
|
11
|
Zhao P, Zhang W, Wang SJ, et al:
HAb18G/CD147 promotes cell motility by regulating annexin
II-activated RhoA and Rac1 signaling pathways in hepatocellular
carcinoma cells. Hepatology. 54:2012–2024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mamori S, Nagatsuma K, Matsuura T, et al:
Useful detection of CD147 (EMMPRIN) for pathological diagnosis of
early hepatocellular carcinoma in needle biopsy samples. World J
Gastroenterol. 13:2913–2917. 2007.PubMed/NCBI
|
|
13
|
Zhang Q, Zhou J, Ku XM, et al: Expression
of CD147 as a significantly unfavorable prognostic factor in
hepatocellular carcinoma. Eur J Cancer Prev. 16:196–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Shen ZY, Chen XG, et al: A
randomized controlled trial of Licartin for preventing hepatoma
recurrence after liver transplantation. Hepatology. 45:269–276.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZN, Mi L, Xu J, et al: Targeting
radioimmunotherapy of hepatocellular carcinoma with iodine
(131I) metuximab injection: clinical phase I/II trials.
Int J Radiat Oncol Biol Phys. 65:435–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao W, Li J, Hu C, et al: Symptom clusters
and symptom interference of HCC patients undergoing TACE: a
cross-sectional study in China. Support Care Cancer. 21:475–483.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sergio A, Cristofori C, Cardin R, et al:
Transcatheter arterial chemoembolization (TACE) in hepatocellular
carcinoma (HCC): the role of angiogenesis and invasiveness. Am J
Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seinstra BA, Defreyne L, Lambert B, et al:
Transarterial radioembolization versus chemoembolization for the
treatment of hepatocellular carcinoma (TRACE): study protocol for a
randomized controlled trial. Trials. 13:1442012. View Article : Google Scholar
|
|
19
|
Wu L, Yang YF, Ge NJ, et al: Hepatic
artery injection of 131I-labelled metuximab combined
with chemoembolization for intermediate hepatocellular carcinoma: a
prospective nonrandomized study. Eur J Nucl Med Mol Imaging.
39:1306–1315. 2012.
|