Introduction
Psoriasis is a chronic inflammatory skin disease
with genetic predisposition (1–4),
characterized by the hyperproliferation and abnormal
differentiation of epidermal keratinocytes (5). Inhibition of the excessive
proliferation of keratinocytes is the main treatment method of
psoriasis (6). HaCaTs are
immortalized cell lines derived from keratinocytes in normal adult
skin and the excessive proliferation of these keratinocytes results
in psoriatic lesions (7).
Therefore, HaCaT cells have been widely used as an in vitro
model for the study of anti-psoriasis agents (8,9).
Metformin is an insulin sensitizer, it is the
first-line treatment method for type II diabetes and was
recommended by the American Diabetes Association in 2012 for its
hypoglycemic effects and ability to reduce cardiovascular morbidity
and mortality. In addition, metformin rarely causes lactic acidosis
(10,11). Previous studies have shown that
metformin inhibits cell growth and proliferation of a number of
types of cancer, including liver, colon and prostate cancer
(12–14). However, whether metformin inhibits
the proliferation of HaCaT cells has, to the best of our knowledge,
not been studied.
The mitogen-activated protein kinase (MAPK)
signaling pathway is important for the proliferation of HaCaT
cells. It has been reported that metformin activates adenosine
monophosphate-activated protein kinase (AMPK) in breast cancer
MCF-7 cells, inhibits the mTOR signaling pathway to reduce protein
translation initiation and decreases cell proliferation (15). Following metformin treatment in
ovarian cancer cells, the AMPK signaling pathway is activated and
phosphorylated (p)-AMPK protein expression is increased. This
results in the inhibition of proliferation-associated protein
molecule synthesis and thus suppression of ovarian cancer cell
proliferation (16). Metformin
also activates AMPK to reduce proliferative signaling in tumor
cells and provide direct anti-tumor effects (17).
In the present study, the effects of metformin on
HaCaT cell proliferation and the regulatory protein expression were
evaluated and the molecular mechanisms of action of metformin in
the treatment of psoriasis were investigated.
Materials and methods
Cells and reagents
The HaCaT cell line was purchased from the American
Type Culture Collection (Manassas, VA, USA). Metformin
hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO,
USA). The cell proliferation and cytotoxicity assay kit (Cell
Counting Kit-8; CCK-8) was purchased from Dojindo (Kunamoto,
Japan). p-AMPK α1 and p-MAPK 1/2 antibodies were purchased from
Abcam (Cambridge, MA, USA). The quantitative automatic microplate
reader (model no., 2010) was purchased from Anthos Labtec Co., Ltd.
(Salzburg Austria). The study was approved by the ethics committee
of Shandong University (Jinan, China).
Metformin treatment
HaCaT cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) with 10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in a 5%
CO2 humidified and sterile environment. HaCaT cells
during the logarithmic growth phase were collected and inoculated
in 96-well (1×104 cells/well) or 6-well plates
(3×105 cells/well). Two groups, specifically, control
(without metformin) and metformin (50 mM metformin) were
established. After 24 h of inoculation, the culture medium in the
metformin group was replaced with DMEM containing metformin to
maintain the metformin concentration at 50 mM. An equal volume of
phosphate-buffered saline (PBS) was added to the control group.
Cell morphology observation
Following 24 h of 50 mM metformin treatment, the
morphology of HaCaT cells was observed under an inverted microscope
(Olympus BX-51; Olympus optical Co., Ltd., Tokyo, Japan).
CCK-8 assay
Following 3–5 stable passages, HaCaT cells in the
logarithmic phase were inoculated in 96-well plates. Cell culture
medium (100 μl DMEM; Invitrogen, Carlsbad, CA, USA) and 100 μl
metformin were added to the center of 60 wells. Following cross
mixing, cells were cultured at 37°C in 5% CO2 for 24, 48
and 72 h. CCK-8 solution was added and the optical density (OD)
values were detected at 450 nm using a quantitative automatic
microplate reader (model no. 2010; Anthos Labtec Co., Ltd.). Cell
survival rates at the various treatment times were calculated and
the cell survival curve was drawn. The cell survival rate (%) was
calculated using the following formula: (ODmetformin -
ODcontrol)/(ODcontrol -
ODmetformin) × 100.
Western blot analyses
Total protein was extracted from each sample and
antibody incubation was performed according to the instructions of
the manufacturer of the one-step rapid WB kit (rabbit; Shanghai
Biological Engineering Co., Ltd., Shanghai, China) using antibodies
against p-AMPK (1:200) and p-extracellular signal-regulated kinase
(ERK1/2; 1:250). The ultra-sensitive enhanced chemiluminescence kit
(Biyuntian Biotechnology Institute, Beijing, China) was used for
color development. The membrane was incubated with the ECL Plus A
and Plus B reagents for 2 min at room temperature. The membrane was
developed in the dark. The developed films were scanned using the
AlphaImager gel imaging systems (AlphaImager, Santa Clara, CA,
USA). The western blot images were then analyzed using Quantity One
software (Bio-Rad Laboratories, Hercules, CA, USA). β-actin was
used as an internal control. The relative absorbance ratios of
p-AMPK to β-actin and p-ERK1/2 to β-actin were defined as the
relative values of p-AMPK and p-ERK1/2, respectively.
Statistical analyses
All experimental data are presented as the mean ±
standard deviation. SPSS statistical software (v13.0; SPSS, Inc.,
Chicago, IL, USA) was used for analysis. One-way analysis of
variance was used for mean comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of metformin on HaCaT cells
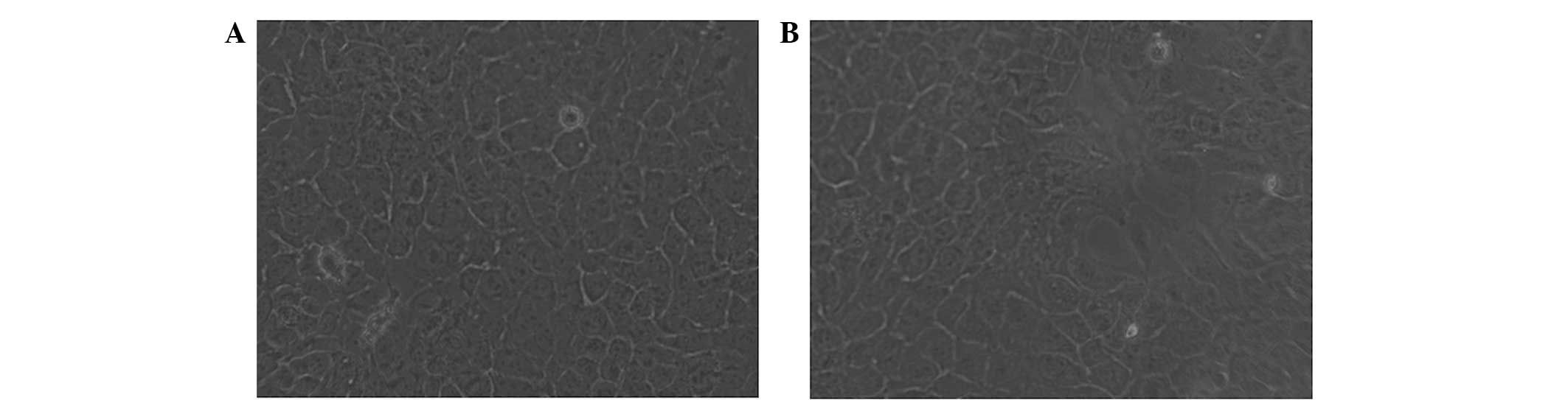
To determine the effects of metformin treatment on
the morphology of HaCaT cells, cells were observed under an
inverted microscope after 24 h of treatment. The untreated HaCaT
cells were in adherent growth and arranged in cobblestone and
mosaic shapes. Cells were flat and polygonal with a clear boundary,
abundant cytoplasm, and a round or oval nucleus (Fig. 1A). The cells in the metformin group
were treated with 50 mM metformin for 24 h. The sizes of treated
cells were slightly smaller compared with those of the untreated
cells (Fig. 1B). The sizes of cell
granules and vacuoles were increased and a number of cells had been
killed by the treatment (Fig.
1B).
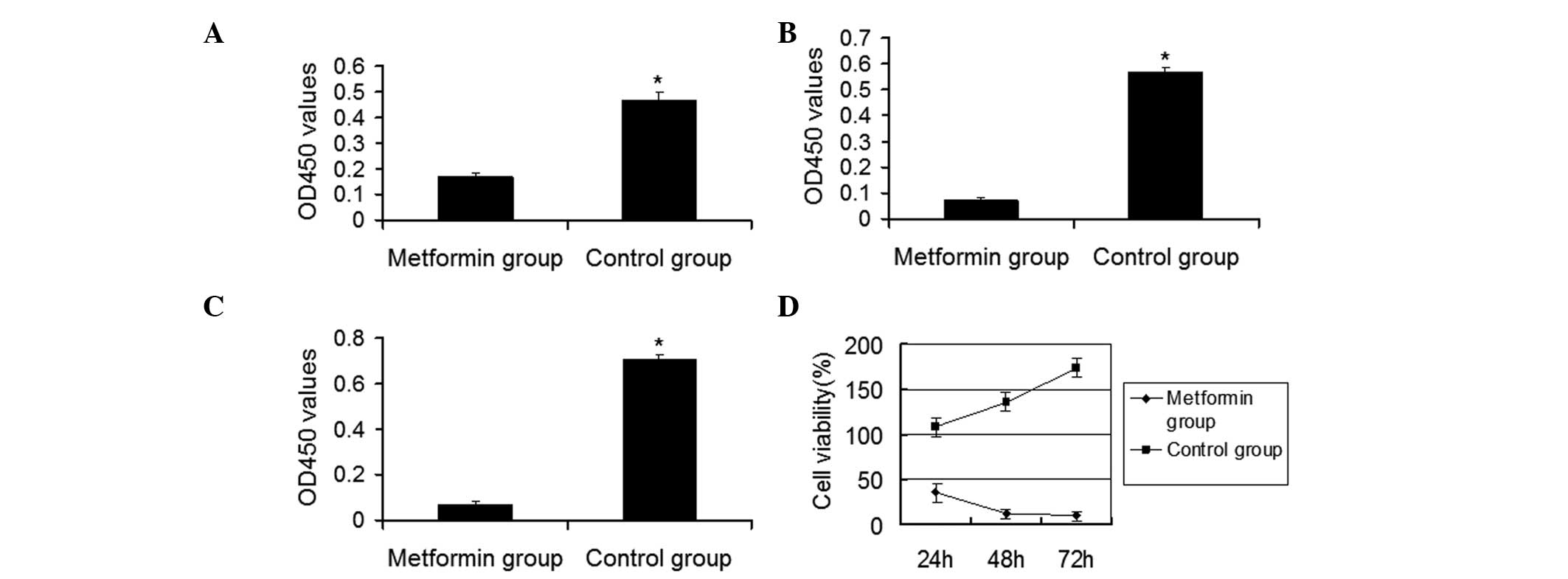
To investigate cell proliferation following
metformin treatment, the CCK-8 cell viability assay was performed.
As shown in Fig. 2, the survival
fractions of HaCaT cells treated with metformin were 36.18, 12.70
and 10.12% at 24, 48 and 72 h, respectively, and were significantly
lower compared with those of the untreated control (P<0.05).
With extended metformin treatment time, HaCaT cell survival rates
gradually decreased.
Collectively, these results suggest that metformin
induces changes in HaCaT cell morphology and inhibits HaCaT cell
proliferation in vitro.
Effect of metformin on AMPK and ERK1/2
protein expression in HaCaT cells
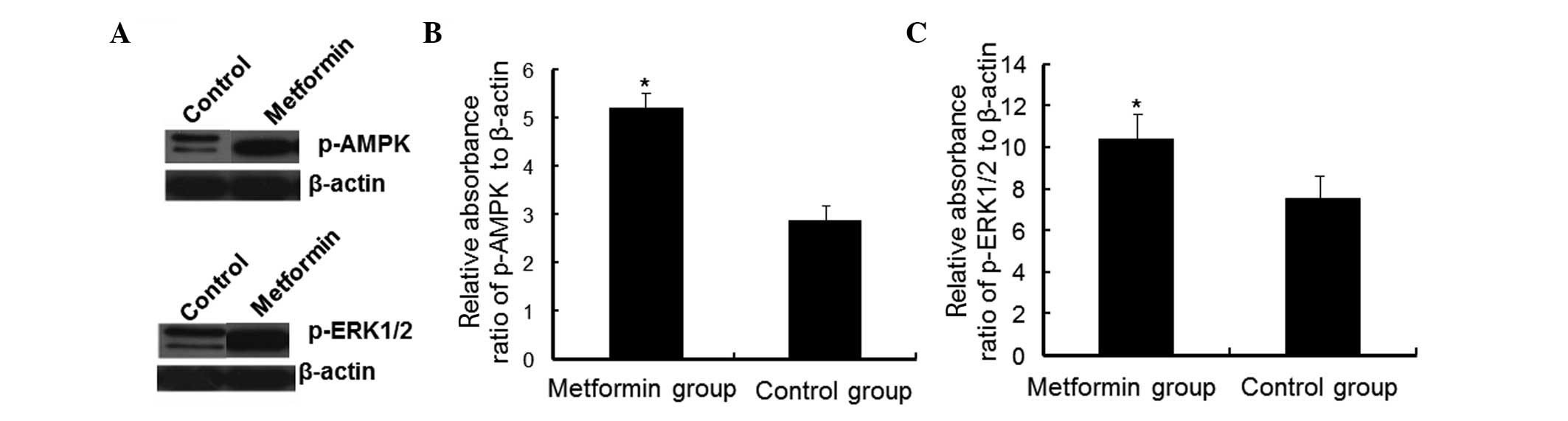
To determine whether the MAPK signaling pathway was
activated by metformin, the levels of p-AMPK and p-ERK1/2 were
detected by western blot analysis. As shown in Fig. 3A and B, in the cells treated with
PBS, the mean absorbance ratio of p-AMPK relative to β-actin in
HaCaT cells was 2.856±0.323. However the mean absorbance ratio of
the cells treated with metformin was 5.198±0.625. The expression
levels of p-AMPK between these two groups were identified to be
significantly different (P<0.05; Fig. 3).
As shown in Fig. 3A and
C, the mean absorbance ratio of p-ERK1/2 relative to β-actin in
the untreated HaCaT cells was 7.550±1.087, but the ratio in the
cells treated with metformin was 10.430±1.217, with a significant
difference between the two groups (P<0.05). These results
indicated that the above proteins were activated following
metformin treatment for 24 h. After 24 h of metformin treatment,
the expression levels of p-AMPK and p-ERK1/2 markedly increased.
This suggests that AMPK and ERK1/2 proteins were phosphorylated due
to metformin treatment. Therefore, metformin inhibited HaCaT cell
proliferation, possibly via a mechanism associated with the
activation of the MAPK signaling pathway.
Discussion
In the present study, HaCaT cells were used as an
in vitro model of psoriatic keratinocyte proliferation to
analyze the effects of metformin on HaCaT cell proliferation and
investigate the possible mechanisms of its treatment for psoriasis.
The CCK-8 assay showed that metformin significantly inhibited the
proliferation of HaCaT cells in vitro. Western blot analysis
results suggested that metformin stimulated AMPK and ERK1/2
phosphorylation in HaCaT cells. These observations indicate that
metformin activates AMPK in the ERK1/2 signaling pathway and
regulates its downstream gene expression to inhibit cell
proliferation. In addition, following metformin treatment, the
levels of p-AMPK protein were significantly increased.
ERK1/2 is an important signaling pathway in the MAPK
family that regulates cell growth and differentiation. The ERK1/2
signaling pathway is closely associated with psoriasis, but whether
there is phosphorylation of ERK1/2 in psoriasis lesions remains
controversial. ERK1/2 activation is dependent upon stimulus
intensity, thus the intensity of ERK activation is likely to affect
the response (18). It has been
previously reported that the ERK1/2 signaling pathway exhibits dual
effects in promoting HaCaT cell proliferation through epidermal
growth factors whose effects on HaCaT cell proliferation and
activation are associated with ERK signal intensity (19). However, overactivation of the ERK
signal inhibits cell proliferation. For example, Pumiglia et
al(20) reported that
activation of ERK inhibits CDK activity and induces cell cycle
arrest through induction of the CDK inhibitor
p21Cip1/WAF1. Wang et al (21) also observed that persistent
activation of ERK lead to cell cycle arrest. Tang et
al(22) showed that ERK
activation partially contributed to p21Cip1/WAF1 induction. In the
present study, we found that after metformin treatment, HaCaT cell
proliferation was inhibited while p-ERK was upregulated. Therefore
we suggest that the inhibition of metformin on HaCaT cells is
mediated by ERK activation.
In conclusion, the MAPK signal transduction pathway
is important in mammalian cells. In the present study, metformin
enhanced the expression of p-ERK1/2, suggesting that the mechanism
by which metformin inhibits cell proliferation may be associated
with activation of the MAPK signaling pathway. However, the
mechanisms associated with the transduction of stimulus signals via
the ERK1/2 signaling pathway and whether other signaling pathways
are involved, requires further study.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81071291).
References
|
1
|
Vestergaard C, Deleuran M, Gesser B and
Grønhøj Larsen C: Expression of the T-helper 2-specific chemokine
receptor CCR4 on CCR10-positive lymphocytes in atopic dermatitis
skin but not in psoriasis skin. Br J Dermato1. 149:457–463. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bowcock AM and Krueger JG: Getting under
the skin: the immunogenetics of psoriasis. Nat Rev Immunol.
5:699–711. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhalerao J and Bowcock AM: The genetics of
psoriasis: a complex disorder of the skin and immune system. Hum
Mol Genet. 7:1537–1545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bowcock AM and Cookson WO: The genetics of
psoriasis, psoriatic arthritis and atopic dermatitis. Hum Mol
Genet. 13:R43–R55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdou AG and Hanout HM: Evaluation of
survivin and NF-kappaB in psoriasis, an immunohistochemical study.
J Cutan Pathol. 35:445–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman M, Alam K, Ahmad MZ, et al:
Classical to current approach for treatment of psoriasis: a review.
Endocr Metab Immune Disord Drug Targets. 12:287–302. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fusenig NE and Boukamp P: Multiple stages
and genetic alterations in immortalization, malignant
transformation, and tumor progression of human skin keratinocytes.
Mol Carcinog. 23:144–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stein M, Bernd A, Ramirez-Bosca A,
Kippenberger S and Holzmann H: Measurement of anti-inflammatory
effects of glucocorticoids on human keratinocytes in vitro.
Comparison of normal human keratinocytes with the keratinocyte cell
line HaCaT. Arzneimittelforschung. 47:1266–1270. 1997.PubMed/NCBI
|
|
9
|
Müller K and Prinz H: Antipsoriatic
anthrones with modulated redox properties. 4. Synthesis and
biological activity of novel 9,
10-dihydro-1,8-dihydroxy-9-oxo-2-anthracenecarboxylic and
-hydroxamic acids. J Med Chem. 40:2780–2787. 1997.PubMed/NCBI
|
|
10
|
Kahn BB, Alquier T, Caning D and Hardie
DG: AMP-activated protein kinase: ancient energy gauge provides
clues to modern understanding of metabolism. Cell Metab. 1:15–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witters LA: The blooming of the French
lilac. J Clin Invest. 108:1105–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q
and Kip KE: Metformin for liver cancer prevention in patients with
type 2 diabetes: a systematic review and meta-analysis. J Clin
Endocrinol Metab. 97:2347–2353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buzzai M, Jones RG, Amaravadi RK, Lum JJ,
DeBerardinis RJ, Zhao F, Viollet B and Thompson CB: Systemic
treatment with the antidiabetic drug metformin selectively impairs
p53-deficient tumor cell growth. Cancer Res. 67:6745–6752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ben Sahra I, Laurent K, Loubat A, et al:
The antidiabetic drug metformin exerts an antitumoral effect in
vitro and in vivo through a decrease of cyclin D1 level. Oncogene.
27:3576–3586. 2008.PubMed/NCBI
|
|
15
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rattan R, Giri S, Hattmann L and Shridhar
V: Metformin attenuates ovarian cancer cell growth in an AMP-kinase
dispensable manner. J Cell Mol Med. 15:166–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bosch M, Gil J, Bachs O and Agell N:
Calmodulin inhibitor W13 induces sustained activation of ERK2 and
expression of p21(cip1). J Biol Chem. 273:22145–22150. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Zeng Y, Ji Y and Xing F: Two-sided
effect of ERK signal pathway on HaCaT cell proliferation induced by
EGF. Basic & Clinical Medicine. 26:471–475. 2006.(In
Chinese).
|
|
20
|
Pumiglia KM and Decker SJ: Cell cycle
arrest mediated by the MEK/mitogen-activated protein kinase
pathway. Proc Natl Acad Sci USA. 94:448–452. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Zhang B, Wang M and Carr BI:
Persistent ERK phosphorylation negatively regulates cAMP response
element-binding (CREB) activity via recruitment of CREB-binding
protein to pp90RSK. J Biol Chem. 278:11138–11144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang D, Wu D, Hirao A, et al: ERK
activation mediates cell cycle arrest and apoptosis after DNA
damage independently of p53. J Biol Chem. 277:12710–12717. 2002.
View Article : Google Scholar : PubMed/NCBI
|