Introduction
Previous large-scale studies have demonstrated the
beneficial effects of statin loading prior to elective and early
percutaneous coronary intervention (PCI) for the prevention of
major adverse cardiac events (MACEs), including angina pectoris,
mortality, nonfatal myocardial infarction (MI) and target vessel
revascularization, as well as stable angina pectoris (SAP),
unstable angina pectoris (UAP) and non-ST-segment elevation
myocardial infarction (NSTEMI) (1–10).
The ‘pleiotropic effects’ of statins include the modulation of
endothelial function, inhibition of inflammation and attenuation of
thrombosis, all of which can provide clinical benefits for elective
and early PCI via reductions in the postprocedural incidence of MI
and MACEs.
However, little is known with regard to the effect
of statin loading prior to primary PCI in patients with acute
ST-segment elevation myocardial infarction (STEMI). Previous
observational studies on patients with STEMI have suggested that
chronic previous statin use may improve coronary blood flow, and is
associated with reduced short-term (30-day) mortality (11–14).
However, the beneficial effects of chronic statin pretreatment have
limitations in their applicability due to the unexpected nature of
the onset of acute STEMI, whereas the acute effects of high-dose
statins may be more clinically relevant in the emergent setting in
STEMI. In a retrospective cohort study, statin therapy at the time
of primary PCI for STEMI and cardiogenic shock was associated with
a significant mortality advantage at early follow-up (15). The STATIN STEMI trial (16) was a randomized, prospective study,
which demonstrated that high-dose atorvastatin pretreatment prior
to primary PCI did not lead to a significant reduction in MACEs
compared with low-dose atorvastatin. However, the study showed
improved immediate coronary flow following primary PCI. Another
randomized controlled study demonstrated that pretreatment with
high-dose atorvastatin, followed by further treatment for five
days, did not reduce infarct size, measured by single-photon
emission computed tomography, in patients undergoing primary PCI
(17). By contrast, a recent study
showed that preprocedural high-dose atorvastatin prevented
contrast-induced nephropathy (CIN) and protected renal function in
patients with acute STEMI undergoing primary PCI (18). However, to date, the efficacy of
atorvastatin loading in patients with STEMI undergoing primary PCI
has not been demonstrated. In addition, it has not yet been
elucidated whether the ‘pleiotropic effects’ of statins can explain
the possible mechanism(s) behind the action of statins.
Therefore, the aim of this prospective randomized
trial was to examine the efficacy of high-dose atorvastatin
immediately prior to primary PCI on coronary endothelial function
and inflammation in patients with STEMI.
Patients and methods
Patient selection
This study was a randomized, prospective clinical
trial and was approved by the Ethics Review Boards of Peking
University Third Hospital (Beijing, China). All patients provided
consent for a sample of their blood to be used for scientific
purposes.
The inclusion criteria were as follows: STEMI
diagnosed according to the 2004 American College of
Cardiology/American Heart Association guidelines; patients
receiving primary PCI within 12 h from symptom onset; and
Thrombolysis In Myocardial Infarction (TIMI) flow grade ≥2 at the
end of the procedure. The exclusion criteria comprised: Patients
aged >80 or <18 years; cardiogenic shock or severe heart
failure on admission; patients receiving electric defibrillation, a
temporary pacemaker or intra-aortic balloon pump (IABP) during the
PCI; TIMI flow grade <2 at the end of the procedure; previous
history of MI or PCI; previous (within three months) or current
treatment with statins; known allergy to statins; and chronic
inflammatory, significant kidney or hepatic diseases, tumor,
myositis or myopathy.
A total of 80 consecutive patients with STEMI
admitted to the Department of Cardiology, Peking University Third
Hospital, between October 2010 and June 2011, were included in the
study. Of the 80 patients, 6 patients were excluded due to previous
(within three months) or current treatment with statins, 5 patients
received a temporary pacemaker and/or IABP during PCI, 4 patients
had a previous history of MI or PCI, 2 patients underwent a
percutaneous transluminal coronary angioplasty instead of PCI, 3
patients received electric defibrillation and 1 patient was >80
years. The eligible patients (n=60) were randomized into three
groups: Loading dose (80 mg atorvastatin prior to PCI; n=20),
regular dose (20 mg atorvastatin prior to PCI; n=20) and control
(without atorvastatin prior to PCI; n=20).
Treatment and procedures
All patients were pretreated with a loading dose of
aspirin (300 mg) and clopidogrel (300–600 mg) at the emergency
department prior to intervention. The patients were administered
with weight-adjusted intravenous heparin at 100 U/kg in the absence
of glycoprotein IIb/IIIa inhibitor therapy and 70 U/kg with
glycoprotein IIb/IIIa inhibitor. Glycoprotein IIb/IIIa inhibitors
and thrombus aspiration were used during the procedure at the
discretion of the surgeon. The PCI procedure was performed
according to standard technique (19). Following PCI, the patients were
administered with standard therapy, including aspirin (100 mg/day)
indefinitely, clopidogrel (75 mg/day) for ≥1 year, atorvastatin (20
mg/day), β-blockers and angiotensin-converting enzyme (ACE)
inhibitors if there were no contraindications, irrespective of the
initial randomization assignment.
Laboratory assays
Venous blood samples were collected from all
patients prior to, and immediately, 6 and 24 h after PCI. All
samples were collected into vacuum blood collection tubes with EDTA
and were immediately placed in refrigerators at 4°C. Within 30 min
after collection, the samples were centrifuged at 1,000 × g for 10
min at 4°C, divided into aliquots and stored at −80°C. Repeated
freeze-thaw cycles were avoided.
Plasma concentrations of endothelial nitric oxide
synthase (eNOS), nitric oxide (NO), interleukin-6 (IL-6), tumor
necrosis factor-α (TNF-α) and intercellular adhesion molecule-1
(ICAM-1) were measured using ELISA, in accordance with the
manufacturer’s instructions (ELISA kit; R&D Systems,
Minneapolis, MN, USA). The upper and lower detection limits, were
100 and 1.56 U/ml for eNOS and NO, 300 and 4.7 pg/ml for IL-6,
1,000 and 15.6 pg/ml for TNF-α, and 1.0×104 and 156
pg/ml for ICAM-1, respectively. The sensitivities were <0.3 U/ml
for eNOS and NO, <0.3 pg/ml for IL-6, <1.5 pg/ml for TNF-α
and <15 pg/ml for ICAM-1. The assays were performed by an
investigator who was blinded to the source of the samples.
In all patients, the blood samples were collected
whenever it was possible prior to and 2, 6, 12, 24, 48, 72 h
post-PCI to measure the serum creatine kinase-myocardial band
(CK-MB) isoenzyme and troponin T elevation; other measurements were
performed in cases of post-procedural symptoms suggestive of
myocardial ischemia. Normal limits of CK-MB and troponin T were
defined as ≤24 U/l and ≤0.1 ng/ml, respectively. Liver function and
the levels of high-sensitivity C-reactive protein (hs-CRP) and
amino terminal-pro brain natriuretic peptide (NT-proBNP) were
evaluated 12–24 h after primary PCI.
Coronarography, electrocardiographic
analysis and echocardiography
The TIMI flow grade and Gensini Score were analyzed
by two experienced scientists (20,21).
The electrocardiograms (ECGs) were read prior to and 60 min
post-primary PCI by one physician. ST-segment resolution was
calculated as the maximum ST-segment elevation on the initial ECG
minus the ST-segment elevation of the same lead on the ECG at 60
min post-PCI, divided by the maximum ST-segment elevation on the
initial ECG, expressed as a percentage (22). The echocardiography evaluation was
performed by specialist physicians.
Clinical follow-up
A six-month clinical follow-up was completed for all
patients to evaluate the incidence of MACEs and the safety of
atorvastatin loading.
Statistical analysis
All analyses were performed with SPSS 19.0
statistical software (SPSS Inc, Chicago, IL, USA). The
Kolmogorov-Smirnov test was used to assess the normal distribution
of continuous variables. Normally distributed homogeneous data were
compared using one-way analysis of variance for >2 groups,
otherwise a rank sum test was performed. Proportions were compared
using the χ2 test. P<0.05 (two-tailed) was considered
to indicate a statistically significant difference. The data are
expressed numerically (as a percentage), as the mean ± standard
deviation or as the median (minimum; maximum), as appropriate.
Results
General characteristics
The clinical characteristics are summarized in
Table I. There were no significant
differences in any of the clinical parameters with medications,
such as aspirin, clopidogrel, β-blockers or ACE inhibitors.
 | Table IBaseline clinical characteristics. |
Table I
Baseline clinical characteristics.
| Parameter | Loading dose group
(n=20) | Regular dose group
(n=20) | Control group
(n=20) | P-value |
|---|
| Age, years | 54.5±12.7 | 61.5±11.7 | 55.6±7.8 | 0.119 |
| Male, % | 90.0 | 75.0 | 80.0 | 0.437 |
| Abdominal girth,
cm | 95.8±13.7 | 92.1±9.1 | 93.5±8.0 | 0.550 |
| Diabetes mellitus,
% | 20.0 | 10.0 | 25.0 | 0.437 |
| Hyperlipidemia,
% | 40.0 | 25.0 | 15.0 | 0.198 |
| Hypertension, % | 60.0 | 60.0 | 55.0 | 0.934 |
| Stroke, % | 15.0 | 25.0 | 10.0 | 0.436 |
| Current smoker,
% | 75.0 | 70.0 | 85.0 | 0.508 |
| CHD family history,
% | 20.0 | 10.0 | 20.0 | 0.597 |
| Anterior MI, % | 55.0 | 45.0 | 60.0 | 0.626 |
| Killip classification
>I, % | 10.0 | 5.0 | 5.0 | 1.000 |
| Time from symptom
onset to PCI, h | 4.2 (2.3;12.0) | 4.0 (1.0;12.5) | 3.9 (1.5;31.5) | 0.998 |
The coronary-angiography characteristics are
summarized in Table II. There
were no significant differences in the studied coronarography
parameters. There were also no significant differences in the
laboratory results, including glucose, glycosylated hemoglobin,
triglyceride, total cholesterol, low-density lipoprotein
cholesterol, creatinine and complete blood count, with the
exception of the high-density lipoprotein cholesterol levels (data
not shown).
 | Table IICoronarography characteristics. |
Table II
Coronarography characteristics.
| Parameter | Loading dose group
(n=20) | Regular dose group
(n=20) | Control group
(n=20) | P-value |
|---|
| Counts |
| Single vessel,
% | 35.0 | 30.0 | 20.0 | 0.396 |
| Double vessel,
% | 40.0 | 20.0 | 40.0 | 0.396 |
| Triple vessel,
% | 25.0 | 50.0 | 40.0 | 0.396 |
| Culprit vessel |
| LAD, % | 60.0 | 45.0 | 60.0 | 0.471 |
| LCX, % | 10.0 | 10.0 | 20.0 | 0.471 |
| RCA, % | 30.0 | 45.0 | 20.0 | 0.471 |
| Gensini score | 47.3
(24.0;106.0) | 58.3
(20.0;98.5) | 53.0
(14.0;92.0) | 0.720 |
| TIMI prior to PCI,
% | 70.0 | 75.0 | 80.0 | 0.766 |
| Stent=1, % | 85.0 | 90.0 | 95.0 | 0.561 |
| Collateral
formation, % | 10.0 | 20.0 | 30.0 | 0.273 |
| Periprocedural
arrhythmia, % | 45.0 | 25.0 | 20.0 | 0.189 |
| Glycoprotein
IIb/IIIa inhibitor therapy, % | 30.0 | 45.0 | 60.0 | 0.162 |
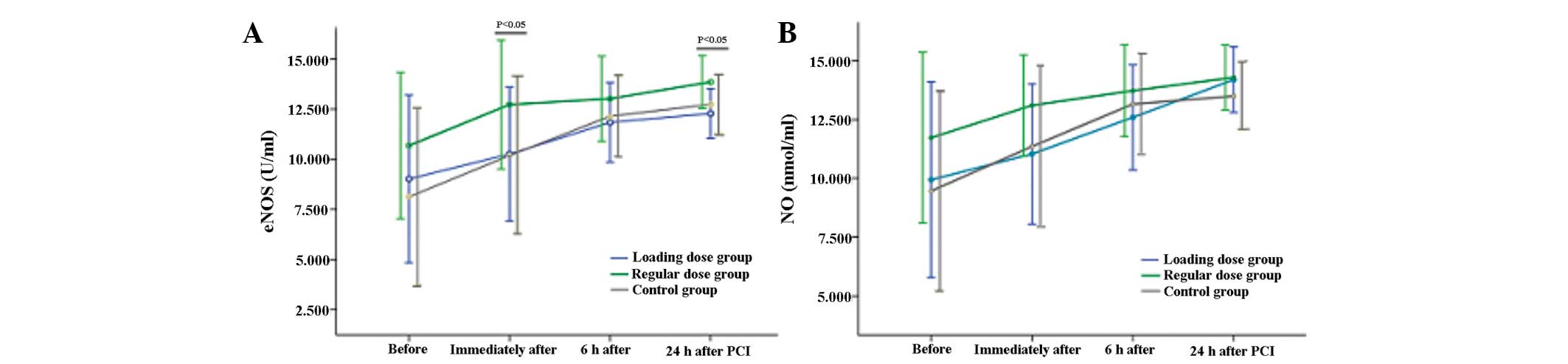
Plasma eNOS and NO
Plasma eNOS and NO levels from the three groups at
four time-points are shown in Fig.
1. The plasma eNOS levels immediately (12.73±3.22 vs.
10.26±3.35 vs. 10.19±3.93 for regular dose group, loading dose
group and control group, respectively; P=0.026) and 24 h
(13.86±1.33 vs. 12.28±1.24 vs. 12.74±1.46 for regular dose group,
loading dose group and control group, respectively; P=0.002)
post-PCI were significantly higher in the regular dose group
compared with the other two groups. However, there were no
significant differences in the plasma eNOS concentrations prior to
and 6 h post-PCI, or in the plasma NO concentration at any of the
time-points among the three groups (P>0.05).
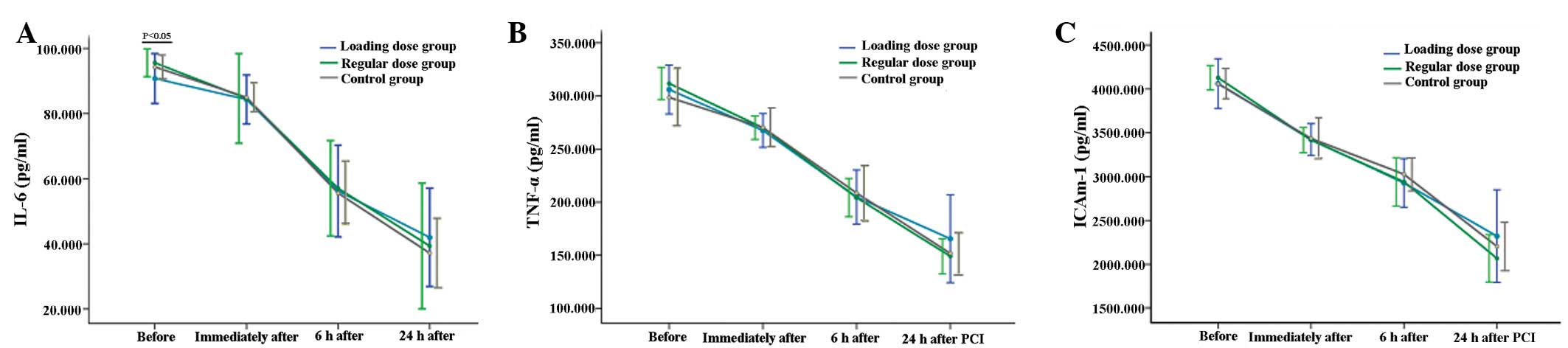
Plasma IL-6, TNF-α and ICAM-1
Plasma IL-6, TNF-α and ICAM-1 levels of the three
groups at four time-points are shown in Fig. 2. The plasma IL-6 levels prior to
PCI were significantly lower in the loading dose group compared
with the other two groups (90.77±7.65 vs. 95.59±4.27 vs. 94.32±3.69
for loading dose group, regular dose group and control group,
respectively; P=0.023). However, there were no significant
differences in the plasma IL-6 concentration following PCI or in
the plasma TNF-α and ICAM-1 concentrations at any of the
time-points among the three groups (P>0.05).
Clinical efficacy index
The clinical efficacy indices are shown in Table III. Peak CK-MB, hs-CRP,
NT-proBNP, electrocardiograph ST-segment resolution at 60 min and
echocardiography did not show any significant differences among the
three groups (P>0.05). MACEs occurred in 2 patients (10.0%) in
the loading dose group, 2 patients (10.0%) in the regular dose
group and 3 patients (15.0%) in the control group, respectively
(P>0.05).
 | Table IIIClinical efficacy index in various
groups. |
Table III
Clinical efficacy index in various
groups.
| Parameter | Loading dose group
(n=20) | Regular dose group
(n=20) | Control group
(n=20) | P-value |
|---|
| Peak CK, U/l | 1877
(632;8927) | 1600
(820;6229) | 1607
(275;5221) | 0.502 |
| Peak CK-MB,
U/l | 240 (91;720) | 209 (113;900) | 246 (33;741) | 0.558 |
| hs-CRP, mg/l | 5.98
(0.68;29.74) | 7.21
(1.17;50.36) | 6.22
(1.10;117.44) | 0.651 |
| NT-proBNP,
pg/ml | 1005 (24;7699) | 1047 (83;3705) | 1049
(102;13839) | 0.994 |
| ST-segment
resolution, % | 63±37 | 65±31 | 52±35 | 0.464 |
| LVESD, mm | 36.6±7.0 | 35.9±7.4 | 35.2±3.5 | 0.729 |
| LVEDD, mm | 49.8±5.4 | 47.7±9.9 | 49.9±3.2 | 0.507 |
| LVEF, % | 51±7 | 51±8 | 53±7 | 0.501 |
| Left atrial area,
mm2 | 19.2±2.9 | 20.1±3.3 | 20.1±4.1 | 0.618 |
| Left atrial
diameter, mm | 37.1±3.2 | 35.2±4.1 | 36.5±4.4 | 0.326 |
| LAP, mmHg | 12±3 | 12±4 | 14±6 | 0.399 |
| MACEs, % | 10 | 10 | 15 | 0.855 |
| Angina pectoris,
% | 10 | 10 | 15 | |
| Nonfatal MI, % | 0 | 0 | 0 | |
| Mortality, % | 0 | 0 | 0 | |
| Target vessel
revascularization, % | 0 | 0 | 0 | |
Safety of atorvastatin loading
Patient liver function prior to being discharged did
not show any significant differences among the three groups
(P>0.05). None of the patients suffered from myalgia during the
study.
Discussion
Endothelium-derived NO can mediate vascular smooth
muscle relaxation (23), inhibit
platelet activation (24) and the
proliferation of vascular smooth muscle cells (25), and protect against
leukocyte-endothelium interactions (26,27);
therefore, it exhibits anti-atherosclerotic effects. Statin
treatment improves endothelium-dependent coronary vasomotion within
24 h in the absence of significant cholesterol reduction (28–31).
Furthermore, statins upregulate eNOS expression (32) and increase the production of
endothelium-derived NO. To the best of our knowledge, the present
study is the first to demonstrate that atorvastatin loading in
patients with STEMI undergoing primary PCI may not exert protective
effects on endothelial function. This may be attributed to heavily
damaged endothelial function in patients with STEMI and the damage
may be too severe for a single dose of atorvastatin in a short time
(~1.2 h) to elicit any improvement. However, vascular endothelial
function was only assessed via the measurements of plasma eNOS and
NO levels, rather than by the direct observation of the relaxation
and contraction of the coronary artery.
Previous studies have demonstrated that a number of
inflammatory factors are involved in the course of coronary heart
disease, such as IL, TNF-α and ICAM-1. An animal experiment
revealed that arterial injury in human CRP-transgenic mice resulted
in an expedited and higher rate of thrombotic occlusion (33). Another study showed that there were
significant differences in the TNF-α IL-6 and CRP levels of
patients who were troponin T-positive versus patients who were
troponin T-negative following selective PCI (34). The extent of inflammation may
affect the prognosis of patients post-PCI. Patients with a higher
degree of inflammatory cell activation are more likely to suffer
from coronary artery restenosis. However, the inflammatory reaction
triggered by PCI is not limited to the position of stents and may
spread to surrounding tissue, including the myocardial layer.
Furthermore, a higher level of inflammatory reaction is also
capable of exacerbating the lesions of the arteries (35). The Atorvastatin for Reduction of
Myocardial Damage during Angioplasty (ARMYDA) (6), ARMYDA-acute coronary syndromes
(8), ARMYDA-RECAPTURE (10) and Novel Approaches for Preventing
or Limiting Events II (9) studies
demonstrated that pretreatment with atorvastatin significantly
reduced procedural CRP levels of patients with SAP, UAP and NSTEMI
in elective coronary intervention. The ARMYDA during
Angioplasty-Cell Adhesion Molecules (ARMYDA-CAMS) study (36) revealed that, in patients undergoing
PCI, a reduction in procedural myocardial injury following a
seven-day pretreatment regimen with atorvastatin was paralleled by
a concomitant attenuation of post-procedural increases in ICAM-1
and E-selectin levels. Thus, it was suggested that a reduction in
the endothelial inflammatory response may explain the protective
effect of statins (36). Another
study indicated that the administration of a single dose of
cerivastatin to patients with UAP or NSTEMI at the time of
admission was capable of decreasing the serum level of CRP and IL-6
24 h later (37). In contrast with
these previous studies, the present study is the first to
demonstrate that atorvastatin loading in patients with STEMI
undergoing primary PCI may not decrease the inflammatory response.
This may be associated with the more severe inflammatory reaction
in patients with STEMI, which was thus not capable of being
alleviated by a single dose of atorvastatin in a short time (~1.2
h). In addition, only three inflammatory factors (IL-6, TNF-α and
ICAM-1) were observed in the study. There are numerous other
inflammatory factors involved in the inflammatory response in
coronary heart disease that were not studied in the present
investigation, such as IL-1 and E-selectin. In the STATIN STEMI
trial, 171 patients were randomized to two groups receiving
pretreatment with 80 mg atorvastatin (n=86) or 10 mg atorvastatin
(n=85) prior to PCI. There was no difference in the CRP levels at
24 h post-PCI between the two groups, which was consistent with the
results of the present study.
The efficacy of atorvastatin loading in patients
with STEMI undergoing primary PCI has not been confirmed. If it
does exhibit clinical benefit, the mechanism underlying the
effects, and whether the ‘pleiotropic effects’ of statins are
capable of explaining the possible mechanism(s) have yet to be
elucidated. The present study examined the potential effects of
atorvastatin loading prior to primary PCI on coronary endothelial
function and inflammatory factors in patients with STEMI. According
to present protocol, patients are administered with 300 mg asprin
(100 mg/per pill) and 300–600 mg clopidogrel (75 mg/per pill)
pretreatment prior to primary PCI. As a result, patients are
required to take 7–11 pills. If high-dose atorvastatin pretreatment
prior to PCI does not lead to a significant reduction in MACEs, the
atorvastatin pretreatment is unnecessary. This may result in a
reduction in doses, cost and side effects, for example
gastrointestinal discomfort.
To the best of our knowledge, the present study is
the first randomized trial to examine the potential effects of
atorvastatin loading prior to primary PCI on coronary endothelial
function and inflammatory factors in patients with STEMI. However,
there were certain limitations to the study which require further
investigation. The study sample size was not large enough to
evaluate the efficacy of high-dose atorvastatin loading (80 mg)
prior to primary PCI in STEMI. Furthermore, the effects of 80-mg
atorvastatin pretreatment on other ‘pleiotropic effects’, including
antithrombosis, antiarrhythmia and the prevention of CIN, and the
efficacy of pretreatment with other statins prior to primary PCI,
were not investigated.
In conclusion, atorvastatin loading in patients with
STEMI undergoing primary PCI may not have protective effects on
endothelial function, inflammation, cardiac perfusion, heart
function or MACEs; however, it did not result in damage to the
liver or muscles.
Acknowledgements
This work was supported by the National Natural
Sciences Grant of China (81070260, 31070948) and Beijing Natural
Sciences Grant (7102099, 7122200).
References
|
1
|
Schwartz GG, Olsson AG, Ezekowitz MD, Ganz
P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S and Stern
T: Effects of atorvastatin on early recurrent ischemic events in
acute coronary syndromes: the MIRACL study: a randomized controlled
trial. JAMA. 285:1711–1718. 2001. View Article : Google Scholar
|
|
2
|
Briguori C, Colombo A, Airoldi F, et al:
Statin administration before percutaneous coronary intervention:
impact on periprocedural myocardial infarction. Eur Heart J.
25:1822–1828. 2004. View Article : Google Scholar
|
|
3
|
Cannon CP, Braunwald E, McCabe CH, Rader
DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA and Skene
AM: Intensive versus moderate lipid lowering with statins after
acute coronary syndromes. N Engl J Med. 350:1495–1504. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang SM, Yazbek N and Lakkis NM: Use of
statins prior to percutaneous coronary intervention reduces
myonecrosis and improves clinical outcome. Catheter Cardiovasc
Interv. 62:193–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Lemos JA, Blazing MA, Wiviott SD, et
al: Early intensive vs a delayed conservative simvastatin strategy
in patients with acute coronary syndromes: phase Z of the A to Z
trial. JAMA. 292:1307–1316. 2004.
|
|
6
|
Pasceri V, Patti G, Nusca A, Pristipino C,
Richichi G and Di SG; ARMYDA Investigators. Randomized trial of
atorvastatin for reduction of myocardial damage during coronary
intervention: results from the ARMYDA (Atorvastatin for Reduction
of MYocardial Damage during Angioplasty) study. Circulation.
110:674–678. 2004. View Article : Google Scholar
|
|
7
|
Mood GR, Bavry AA, Roukoz H and Bhatt DL:
Meta-analysis of the role of statin therapy in reducing myocardial
infarction following elective percutaneous coronary intervention.
Am J Cardiol. 100:919–923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patti G, Pasceri V, Colonna G, Miglionico
M, Fischetti D, Sardella G, Montinaro A and Di SG: Atorvastatin
pretreatment improves outcomes in patients with acute coronary
syndromes undergoing early percutaneous coronary intervention:
results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol.
49:1272–1278. 2007. View Article : Google Scholar
|
|
9
|
Briguori C, Visconti G, Focaccio A, Golia
B, Chieffo A, Castelli A, Mussardo M, Montorfano M, Ricciardelli B
and Colombo A: Novel approaches for preventing or limiting events
(Naples) II trial: impact of a single high loading dose of
atorvastatin on periprocedural myocardial infarction. J Am Coll
Cardiol. 54:2157–2163. 2009. View Article : Google Scholar
|
|
10
|
Di Sciascio G, Patti G, Pasceri V,
Gaspardone A, Colonna G and Montinaro A: Efficacy of atorvastatin
reload in patients on chronic statin therapy undergoing
percutaneous coronary intervention: results of the ARMYDA-RECAPTURE
(Atorvastatin for Reduction of Myocardial Damage During
Angioplasty) Randomized Trial. J Am Coll Cardiol. 54:558–565.
2009.
|
|
11
|
Celik T, Kursaklioglu H, Iyisoy A, Kose S,
Kilic S, Amasyali B, Kardesoglu E and Isik E: The effects of prior
use of atorvastatin on coronary blood flow after primary
percutaneous coronary intervention in patients presenting with
acute myocardial infarction. Coron Artery Dis. 16:321–326. 2005.
View Article : Google Scholar
|
|
12
|
Ishii H, Ichimiya S, Kanashiro M, Aoyama
T, Ogawa Y, Murakami R, Amano T, Naruse K, Matsubara T and Murohara
T: Effects of receipt of chronic statin therapy before the onset of
acute myocardial infarction: a retrospective study in patients
undergoing primary percutaneous coronary intervention. Clin Ther.
28:1812–1819. 2006. View Article : Google Scholar
|
|
13
|
Iwakura K, Ito H, Kawano S, Okamura A,
Kurotobi T, Date M, Inoue K and Fujii K: Chronic pre-treatment of
statins is associated with the reduction of the no-reflow
phenomenon in the patients with reperfused acute myocardial
infarction. Eur Heart J. 27:534–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lev EI, Kornowski R, Vaknin-Assa H,
Ben-Dor I, Brosh D, Teplitsky I, Fuchs S, Battler A and Assali A:
Effect of previous treatment with statins on outcome of patients
with ST-segment elevation myocardial infarction treated with
primary percutaneous coronary intervention. Am J Cardiol.
103:165–169. 2009. View Article : Google Scholar
|
|
15
|
Garot P, Bendaoud N, Lefevre T and Morice
MC: Favourable effect of statin therapy on early survival benefit
at the time of percutaneous coronary intervention for ST-elevation
myocardial infarction and shock. EuroIntervention. 6:350–355. 2010.
View Article : Google Scholar
|
|
16
|
Kim JS, Kim J, Choi D, et al: Efficacy of
high-dose atorvastatin loading before primary percutaneous coronary
intervention in ST-segment elevation myocardial infarction: the
STATIN STEMI trial. JACC Cardiovasc Interv. 3:332–339. 2010.
View Article : Google Scholar
|
|
17
|
Hahn JY, Kim HJ, Choi YJ, et al: Effects
of atorvastatin pretreatment on infarct size in patients with
ST-segment elevation myocardial infarction undergoing primary
percutaneous coronary intervention. Am Heart J. 162:1026–1033.
2011. View Article : Google Scholar
|
|
18
|
Li W, Fu X, Wang Y, et al: Beneficial
effects of high-dose atorvastatin pretreatment on renal function in
patients with acute ST-segment elevation myocardial infarction
undergoing emergency percutaneous coronary intervention.
Cardiology. 122:195–202. 2012. View Article : Google Scholar
|
|
19
|
Task Force on Myocardial Revascularization
of the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS); European
Association for Percutaneous Cardiovascular Interventions (EAPCI).
Wijns W, Kolh P, Danchin N, et al: Guidelines on myocardial
revascularization. Eur J Cardiothorac Surg. 38(Suppl): S1–S52.
2010. View Article : Google Scholar
|
|
20
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
TIMI Study Group. The Thrombolysis in
Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med.
312:932–936. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van ‘t HAW, Liem A, de Boer MJ and
Zijlstra F: Clinical value of 12-lead electrocardiogram after
successful reperfusion therapy for acute myocardial infarction.
Zwolle Myocardial infarction Study Group. Lancet. 350:615–619.
1997.
|
|
23
|
Ignarro LJ, Buga GM, Wood KS, Byrns RE and
Chaudhuri G: Endothelium-derived relaxing factor produced and
released from artery and vein is nitric oxide. Proc Natl Acad Sci
USA. 84:9265–9269. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radomski MW, Rees DD, Dutra A and Moncada
S: S-nitroso-glutathione inhibits platelet activation in vitro and
in vivo. Br J Pharmacol. 107:745–749. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garg UC and Hassid A: Nitric
oxide-generating vasodilators and 8-bromo-cyclic guanosine
monophosphate inhibit mitogenesis and proliferation of cultured rat
vascular smooth muscle cells. J Clin Invest. 83:1774–1777. 1989.
View Article : Google Scholar
|
|
26
|
Kubes P, Suzuki M and Granger DN: Nitric
oxide: an endogenous modulator of leukocyte adhesion. Proc Natl
Acad Sci USA. 88:4651–4655. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gauthier TW, Scalia R, Murohara T, Guo JP
and Lefer AM: Nitric oxide protects against leukocyte-endothelium
interactions in the early stages of hypercholesterolemia.
Arterioscler Thromb Vasc Biol. 15:1652–1659. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson TJ, Meredith IT, Yeung AC, Frei
B, Selwyn AP and Ganz P: The effect of cholesterol-lowering and
antioxidant therapy on endothelium-dependent coronary vasomotion. N
Engl J Med. 332:488–493. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Treasure CB, Klein JL, Weintraub WS,
Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ,
Cedarholm JC and Alexander RW: Beneficial effects of
cholesterol-lowering therapy on the coronary endothelium in
patients with coronary artery disease. N Engl J Med. 332:481–487.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lefer AM, Campbell B, Shin YK, Scalia R,
Hayward R and Lefer DJ: Simvastatin preserves the
ischemic-reperfused myocardium in normocholesterolemic rat hearts.
Circulation. 100:178–184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wassmann S, Faul A, Hennen B, Scheller B,
Bohm M and Nickenig G: Rapid effect of 3-hydroxy-3-methylglutaryl
coenzyme a reductase inhibition on coronary endothelial function.
Circ Res. 93:e98–e103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laufs U, La Fata V, Plutzky J and Liao JK:
Upregulation of endothelial nitric oxide synthase by HMG CoA
reductase inhibitors. Circulation. 97:1129–1135. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danenberg HD, Szalai AJ, Swaminathan RV,
Peng L, Chen Z, Seifert P, Fay WP, Simon DI and Edelman ER:
Increased thrombosis after arterial injury in human C-reactive
protein-transgenic mice. Circulation. 108:512–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonz AW, Lengenfelder B, Jacobs M,
Strotmann J, Held S, Ertl G and Voelker W: Cytokine response after
percutaneous coronary intervention in stable angina: effect of
selective glycoprotein IIb/IIIa receptor antagonism. Am Heart J.
145:693–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gomes WJ and Buffolo E: Coronary stenting
and inflammation: implications for further surgical and medical
treatment. Ann Thorac Surg. 81:1918–1925. 2006.PubMed/NCBI
|
|
36
|
Patti G, Chello M, Pasceri V, Colonna D,
Nusca A, Miglionico M, D’Ambrosio A, Covino E and Di SG: Protection
from procedural myocardial injury by atorvastatin is associated
with lower levels of adhesion molecules after percutaneous coronary
intervention: results from the ARMYDA-CAMs (Atorvastatin for
Reduction of MYocardial Damage during Angioplasty-Cell Adhesion
Molecules) substudy. J Am Coll Cardiol. 48:1560–1566. 2006.
|
|
37
|
Ostadal P, Alan D, Hajek P, Horak D,
Vejvoda J, Trefanec J, Mates M and Vojacek J: The effect of early
treatment by cerivastatin on the serum level of C-reactive protein,
interleukin-6, and interleukin-8 in the patients with unstable
angina and non-Q-wave myocardial infarction. Mol Cell Biochem.
246:45–50. 2003. View Article : Google Scholar : PubMed/NCBI
|