Introduction
Patients with type 2 diabetes mellitus (T2DM) have a
higher risk of cardiovascular disease (CVD), including coronary
artery disease (CAD), the main cause of premature morbidity and
mortality in these patients (1,2).
Inflammation has been indicated to be an important contributor to
the development of T2DM and CAD, not only by promoting
atherogenesis, but also by inducing insulin resistance (IR) and
β-cell impairment.
Peroxisome proliferator-activated receptors (PPARs)
belong to the nuclear receptor superfamily and are ligand-activated
transcription factors. There are three isoforms, α, β and γ
(3). PPARs play an significant
role in the regulation of energy homeostasis by regulating the
expression of a variety of genes involved in lipid and carbohydrate
metabolism (4). Data from murine
models also suggest that PPAR agonists have independent
anti-atherosclerotic actions, including the suppression of vascular
inflammation, oxidative stress and activation of the
renin-angiotensin system (RAS) (5). PPAR agonists reduce the production of
tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β
(6,7). PPARα activation indirectly modulates
inflammatory components in high-density lipoprotein (HDL),
including apolipoprotein A1 (apoA1), serum amyloid A and
paraoxonase-1 (8). PPARγ
activators inhibit the expression of matrix metalloproteinase
(MMP)-9 in human vascular smooth muscle cells and macrophages
(9). PPARγ activators also inhibit
the production of TNF-α, IL-6 and IL-1β by activated monocytes
(10). A number of these effects
are mediated by transrepression of the nuclear factor-κB (NF-κB)
and activator protein-1 (AP-1) dependent pathways (7,11).
The dual PPARα/γ agonists were developed due to the
apparent efficacy of PPARα and PPARγ agonists individually on
metabolic control, providing the possibility of optimizing the
metabolic and anti-atherosclerotic actions through activation of
the two receptors. These dual agonists, including ragaglitazar and
muraglitazar, demonstrate a higher affinity for PPARγ than
conventional thiazolidinediones and are highly effective at
improving metabolic parameters (12,13).
However, the effects of these agents on atherogenesis are not
clearly understood. Treatment with a dual PPARα/γ agonist, compound
3q, notably increased atherosclerosis in control apoE knockout mice
(14), while PPARγ and α agonists
used alone in this model were protective (15,16).
Furthermore, treatment with a dual PPAR agonist in mice resulted in
plaque accumulation accompanied by an increase in aortic gene
expression of the pro-inflammatory molecules, P-selectin, CD36,
vascular cell adhesion molecule 1 (VCAM-1) and monocyte
chemoattractant protein-1 (MCP-1) and increased macrophage
infiltration, an effect not observed with the single PPAR agonists,
rosiglitazone (RSG) or gemfibrozil (14). One study suggested that dual
PPARα/γ agonists are also associated with an increased risk of
adverse cardiovascular events when used by individuals with
diabetes (17). Therefore, there
is a long distance from the bench to the clinic for the dual
PPARα/γ agonists and a new strategy that may benefit T2DM patients
with CAD should be explored.
RSG, a PPARγ agonist typically used to treat T2DM
patients (18,19), significantly reduces
homocysteine-induced reactive oxygen species and the secretion of
MCP-1 and IL-8 in human monocytes. It also significantly decreases
plasma C-reactive protein (CRP) and MCP-1 levels in T2DM patients
with CAD (20). Bezafibrate (BEZ),
a PPARα agonist and an effective drug in the treatment of
dyslipidemia, has been shown to be effective in the primary
prevention of cardiovascular events (21). Therefore, we combined these PPARα
and PPARγ agonists in the treatment of T2DM patients with CAD, with
the expectation of greater efficacy and other advantages.
Materials and methods
Subjects
Eighty T2DM patients with CAD were enrolled from the
Department of Cardiovascular, The Fifth Affiliated Hospital of
Guangxi Medical University, China, from January 2006 to December
2008. T2DM was defined as the level of glucose, including fasting
blood glucose (FBG) ≥7.0 mmol/l, casual plasma glucose ≥11.1 mmol/l
or plasma glucose ≥11.1 mmol/l, 2 h after an oral glucose tolerance
test (OGTT).
T2DM patients with CAD, including angina pectoris
and myocardial infarction, were diagnosed according to the criteria
set out in the American College of Cardiology/American Heart
Association (ACC/AHA) guidelines (22). Coronary angiography confirmed at
least one stenosis >50%. The exclusion criteria were:
complications with infectious or inflammatory diseases or impaired
liver or kidney function; coexisting heart failure [New York Heart
Association (NYHA) class III–IV]; presence of a malignant tumor;
hematological diseases; or treatment with protamine zinc insulin or
isophane insulin.
Study design
The patients were randomly assigned to an RSG group
(4 mg/day, n=20), a BEZ group (400 mg/day, n=20), a combination
group (RSG plus BEZ, n=20) or a control group (conventional
therapy, n=20). All patients received conventional therapy
(including oral hypoglycemic agents or subcutaneous insulin
injection and low dose statins) for DM and CAD for 12 weeks. RSG
was purchased from GlaxoSmithKline (Tianjing, China) and BEZ was
purchased from Tianlishidiyi, Ltd. (Huaian, China). Inflammatory
and metabolic factors were tested before treatment and after 12
weeks in fasting venous blood. This study was approved by the
ethics committee of the Fifth Affiliated Hospital of Guangxi
Medical University. All subjects gave their written informed
consent.
Blood parameter analysis
Before treatment and at 12 weeks after treatment,
venous blood samples were collected and centrifuged immediately at
3,000 rpm for 20 min. The supernatant was collected and stored at
−70°C. Plasma CRP and MCP-1 were determined by enzyme-linked
immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions. FBG was measured by
the standard oxidase method and lipids were measured by the
standard dry chemistry method. Hemoglobin A1c (HbA1c) was measured
by high performance liquid chromatography (HPLC). Insulin was
determined by chemiluminescence. The estimate of IR by homeostasis
model assessment (HOMA-IR) was as follows: IR = [fasting insulin
(Fins; IU/ml) − fasting glucose (mmol/l)] /22.5 (23).
Responsiveness of monocytes to
lipopolysaccharide
Twelve weeks after treatment, venous blood samples
were obtained from fasting subjects to evaluate the effect of RSG
and BEZ on MCP-1 production induced by low-dose lipopolysaccharide
(LPS) in isolated monocytes. Whole blood was separated into
peripheral blood mononuclear cells (PBMCs) and neutrophils using
NycoPrep™ 1.077 (Life Technologies Co., Carlsbad, CA, USA) and then
monocytes were isolated by their adherence to the flasks. Adherent
cells were then detached and resuspended in RPMI-1640 medium
containing 5% autologous serum. Then, monocytes (5×105)
were incubated at 37°C with or without LPS (0.01 μg/ml) for
24 h. The supernatant was harvested and stored at −70°C for further
MCP-1 examination.
Statistical analysis
CRP and MCP-1 were expressed as the median and range
and were analyzed by the Wilcoxon matched-pairs signed ranks test.
The difference between groups was analyzed by the Mann-Whitney
test. Age, body mass index, metabolic factors, including
cholesterol, triglycerides (TGs) and glucose were expressed as the
mean ± standard deviation. A paired Student’s t-test was used to
compare values pre- and post-treatment. One-way analysis of
variance (ANOVA) was performed for multiple comparisons followed by
Dunnett’s test. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
Baseline demographic characteristics
Clinical and metabolic parameters of the patients
are presented in Table I. Age,
gender, smoking status, hypertension, FBG, Fins, HOMA-IR, HbA1c,
lipid levels and MCP-1 did not demonstrate significant differences
among the four groups (P>0.05). The CRP levels in the BEZ and
combination groups were higher than those in the RSG and control
groups; however, the differences were not statistically significant
(P>0.05).
 | Table I.Clinical and metabolic characteristics
before treatment. |
Table I.
Clinical and metabolic characteristics
before treatment.
| Variables | RSG group | BEZ group | Combination
group | Control group | P-value |
|---|
| Male/female (n) | 14/6 | 13/7 | 12/8 | 14/6 | 0.535 |
| Age (years) | 60.6±9.61 | 62.8±9.41 | 62.9±9.00 | 60.4±8.46 | 0.715 |
| Smoking (n) | 5 | 6 | 5 | 6 | 0.275 |
| Hypertension (n) | 5 | 6 | 5 | 6 | 0.230 |
| BMI
(kg/m2) | 25.19±4.01 | 24.79±3.50 | 24.97±3.03 | 24.37±2.80 | 0.407 |
| FPG (mmol/l) | 7.27±1.69 | 7.77±4.08 | 8.57±4.42 | 7.45±2.86 | 0.925 |
| Fins (mU/l) | 11.48±4.65 | 13.07±4.89 | 12.28±4.61 | 12.07±4.94 | 0.868 |
| HOMA-IR | 3.69±1.61 | 4.07±2.05 | 4.77±3.29 | 3.74±1.56 | 0.423 |
| HbA1c (%) | 7.84±1.58 | 7.58±2.01 | 8.05±2.10 | 7.49±1.83 | 0.582 |
| TC (mmol/l) | 4.73±0.92 | 4.43±0.81 | 4.49±0.77 | 4.52±1.07 | 0.541 |
| TG (mmol/l) | 1.92±0.98 | 2.06±1.25 | 2.07±1.70 | 2.06±1.10 | 0.714 |
| HDL (mmol/l) | 1.04±0.18 | 1.04±0.21 | 0.94±0.28 | 1.05±0.24 | 0.267 |
| LDL (mmol/l) | 2.85±0.82 | 2.55±0.78 | 2.65±0.75 | 2.73±0.96 | 0.674 |
| CRP (mg/l) | 8.87
(5.47–15.91) | 18.11
(7.71–34.44) | 13.32
(4.22–21.06) | 10.05
(4.73–18.64) | 0.922 |
| MCP-1 (pg/ml) | 174.8
(83.2–223.3) | 199.2
(70.3–297.3) | 188.6
(84.1–253.2) | 179.5
(65.9–241.6) | 0.476 |
FBG, Fins, HOMA-IR and HbA1c levels
The FBG levels in the RSG and combination groups
were significantly decreased compared with those before treatment;
however, no significant reduction was noted in the BEZ and control
groups (Fig. 1A). Fins levels
decreased after 12 weeks of treatment with RSG, BEZ or the
combination of RSG and BEZ (Fig.
1B). Although a slight decrease was also observed in the
control group, no significant difference was detected. Patients in
the RSG, BEZ and combination groups demonstrated improved HOMA-IR
after 12 weeks of treatment; however, the amelioration was not
significant in the control group (Fig.
1C). HOMA-IR in the RSG and combination groups was lower than
that in the BEZ and control groups at 12 weeks and no significant
difference was observed between the RSG group and the BEZ group.
All patient HbA1c was not well-controlled before treatment
(Fig. 1D). With RSG and combined
treatment for 12 weeks, HbA1c markedly decreased compared with that
before treatment; however, the decreases were not significant in
the BEZ and control groups. HbA1c in the combination group was
almost reduced to a normal level (HbA1c <7%).
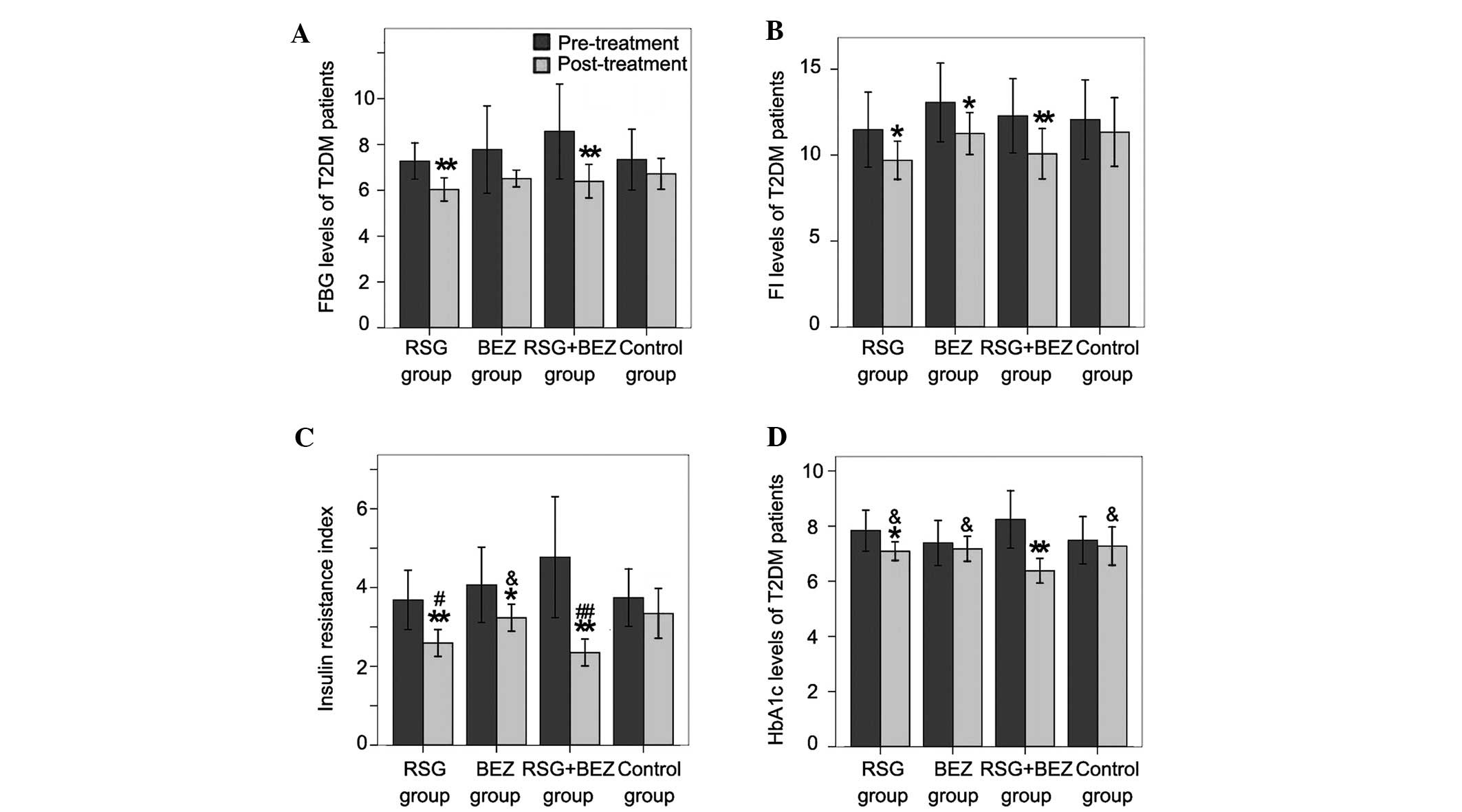 | Figure 1.Blood glucose related parameters. (A)
FBG, (B) Fins, (C) HOMA-IR and (D) HbA1c levels were determined
before and after treatment. Each bar represents the mean ± standard
deviation (SD; n=20). *P<0.05,
**P<0.01, compared with pre-treatment in the same
group; &P<0.05, compared with the combination
group; #P<0.05, ##P<0.01, compared with
the control group. FBG, fasting blood glucose; T2DM, type 2
diabetes mellitus; RSG, rosiglitazone; BEZ, bezafibrate; FI,
fasting insulin; HbA1c, hemoglobin A1c; HOMA-IR, insulin resistance
by homeostasis model assessment. |
Changes in lipid parameters
After 12 weeks treatment, total cholesterol (TC)
levels in the four groups all decreased; however, no significant
differences were observed compared with those before treatment
(Fig. 2A). TG levels in the RSG
and control groups were slightly increased without significant
differences (Fig. 2B), while in
the BEZ and combination groups, TG was markedly decreased. The
low-density lipoprotein (LDL) level in the combination group was
markedly decreased (Fig. 2C);
however, the reduction of HDL levels in the other groups was not
significant. After 12 weeks of treatment, the HDL level in the
combination group was markedly increased, while the HDL levels in
the other groups were not significantly elevated (Fig. 2D).
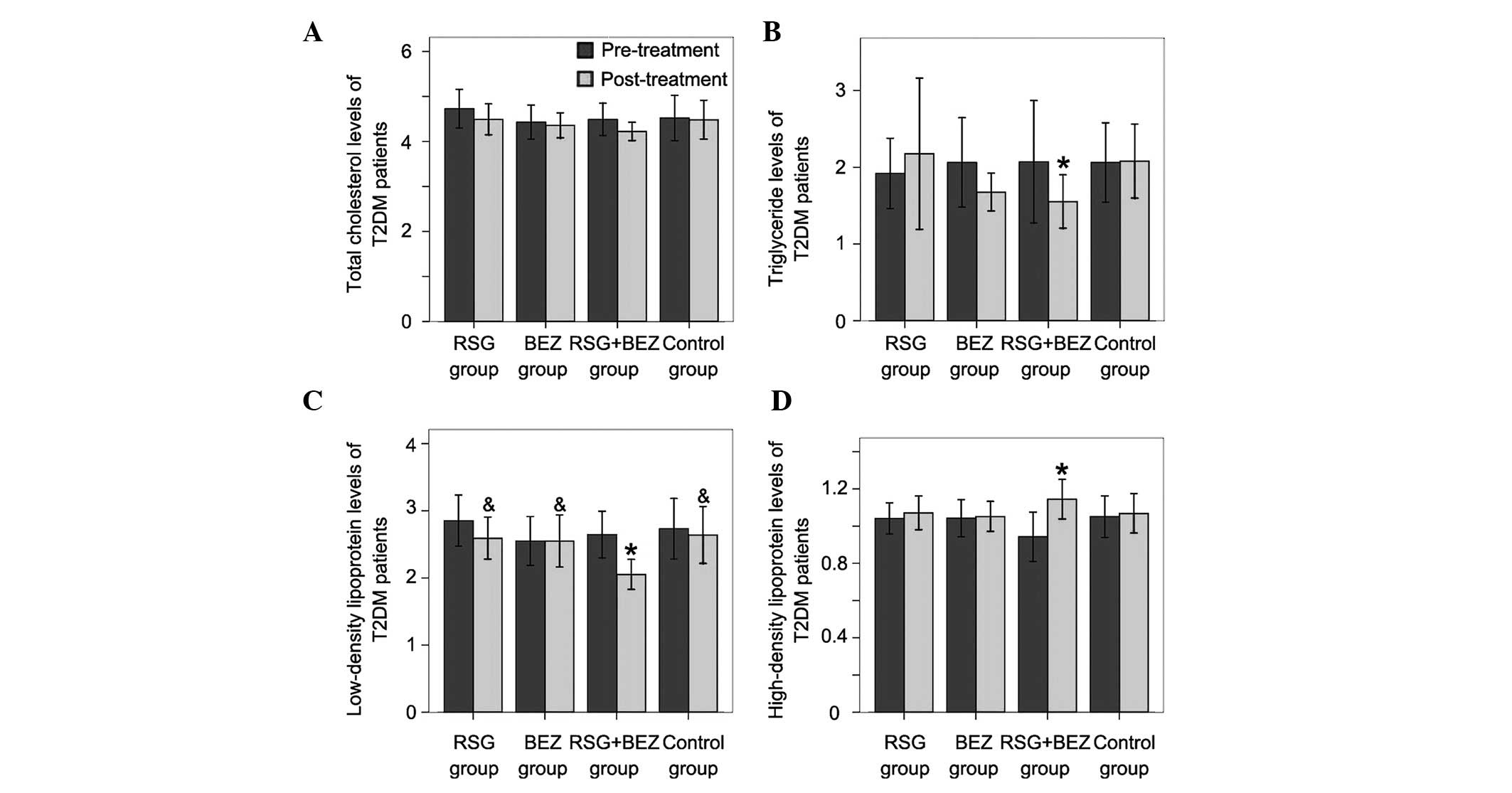 | Figure 2.Changes in lipid parameters. (A) TC,
(B) TG, (C) LDL and (D) HDL levels were determined before and after
treatment. Each bar represents the mean ± standard deviation (SD;
n=20). *P<0.05, compared with pre-treatment in the
same group; &P<0.05, compared with the
combination group. T2DM, type 2 diabetes mellitus; RSG,
rosiglitazone; BEZ, bezafibrate; TC, total cholesterol; TG,
triglyceride; LDL, low-density lipoprotein; HDL, high-density
lipoprotein. |
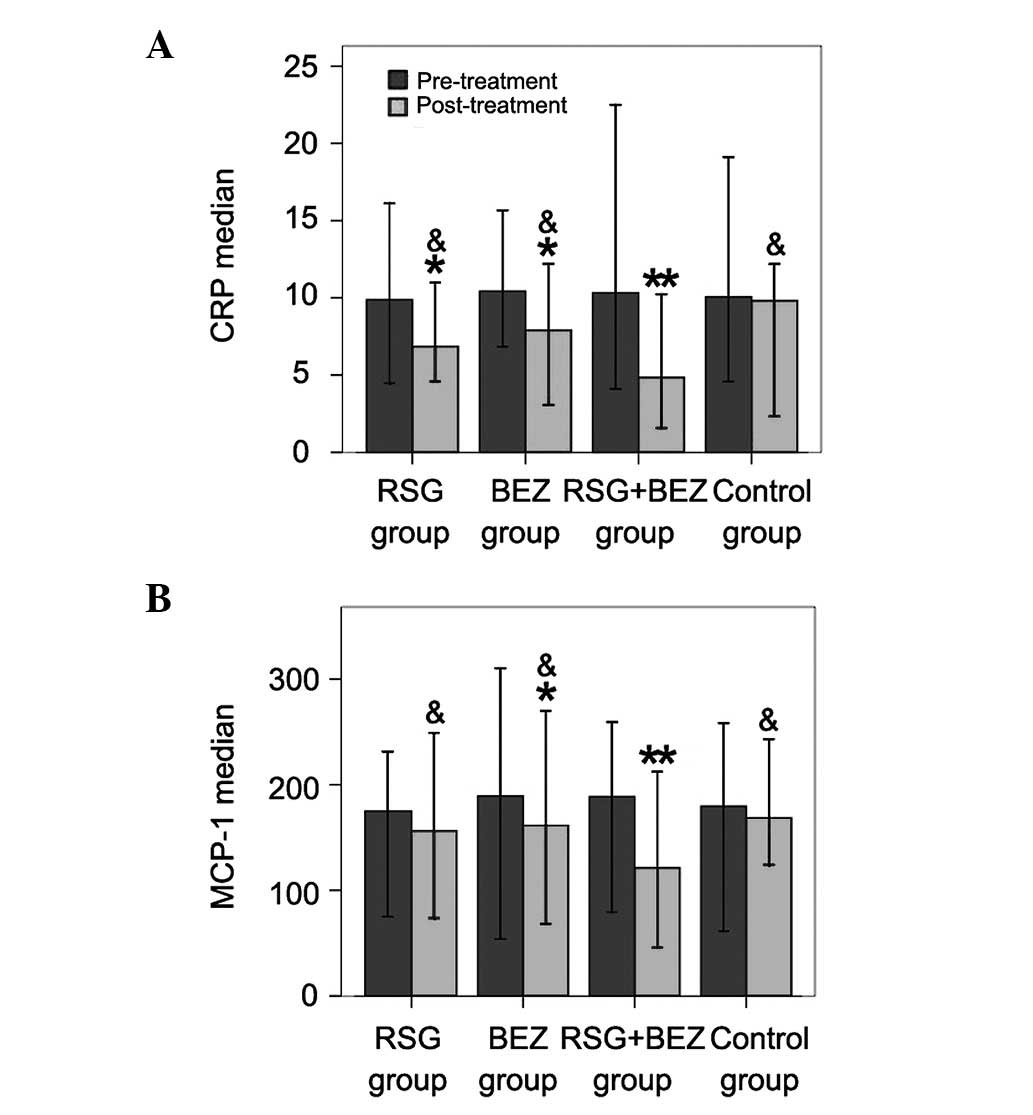
Plasma CRP and MCP-1 levels
After 12 weeks of treatment, the CRP level in each
of the four groups decreased (Fig.
3A). Significant differences in CRP and MCP-1 levels before and
after treatment were observed in the RSG, BEZ and combination
groups; however, this was not observed in the control group
(Fig. 3A and B). Moreover, the
levels of CRP and MCP-1 in the combination group decreased more
than those in the other treatment groups.
Chemokine secretion from isolated
monocytes in response to LPS
To test whether monocytes isolated from T2DM
patients with CAD demonstrate an enhanced inflammatory response,
PBMCs were incubated with LPS (0.01–0.1 μg/ml) for 24 h. LPS
at 0.01 lg/ml induced greater MCP-1 secretion by PBMCs from T2DM
patients with CAD than from control subjects (Fig. 4). RSG, BEZ and combined RSG and BEZ
treatment for 12 weeks significantly decreased low-dose LPS-induced
MCP-1 secretion. Combined treatment with RSG and BEZ inhibited the
LPS-induced MCP-1 secretion more efficiently than RSG or BEZ used
alone.
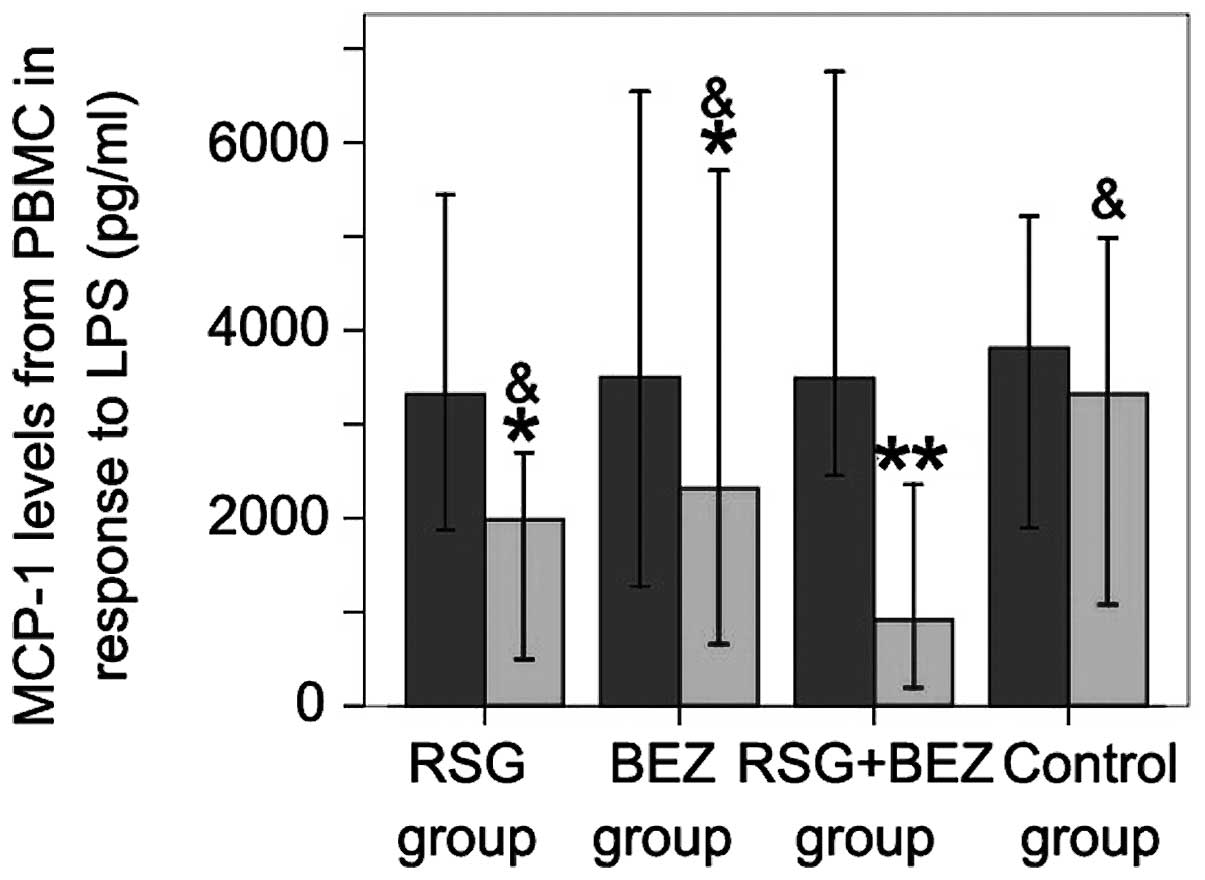 | Figure 4.Effect of combined RSG and BEZ on
MCP-1 levels secreted by PBMCs in response to LPS. PBMCs in T2DM
patients with CAD were incubated with LPS for 24 h. Then, the
supernatant was harvested and MCP-1 levels were determined by
ELISA. Each bar represents the mean ± standard deviation (SD).
*P<0.05, **P<0.01, compared with
pre-treatment in the same group; &P<0.05,
compared with the combination group. MCP-1, monocyte
chemoattractant protein 1; PBMC, peripheral blood mononuclear cell;
LPS, lipopolysaccharide; RSG, rosiglitazone; BEZ, bezafibrate;
T2DM, type 2 diabetes mellitus; CAD, coronary artery disease;
ELISA, enzyme-linked immunosorbent assay. |
Discussion
T2DM is an independent risk factor of CAD.
Epidemiological investigation revealed that patients with T2DM had
a 2- to 4-fold higher risk than normal individuals of suffering
from CAD (24). In the present
study, we demonstrated that the combination of PPARγ and PPARα
agonists downregulates the blood glucose and lipid levels more
efficiently in T2DM patients with CAD than the PPARγ or PPARα
agonist alone. The combination of PPARγ and PPARα agonists also
inhibited inflammatory cytokine secretion in the T2DM patients with
CAD and attenuated the LPS-induced MCP-1 secretion by PBMCs in the
T2DM patients. These results suggest that the combination of RSG
and BEZ is more effective than RSG or BEZ alone for T2DM patients
with CAD, and acts by inhibiting the secretion of inflammatory
cytokines from monocytes.
RSG, a well-recognized oral anti-diabetic drug,
belongs to the thiazolidinedione class of drugs. RSG increases the
expression of glucose transporter-1 and facilitates the uptake of
glucose by muscle and fat tissue through its receptors. Thereby, it
reduces the output of liver glucose and blood glucose and improves
hyperinsulinemia (25,26). RSG also acts as an insulin
sensitizer. It inhibits glucagon production and thus increases
insulin sensitivity. In the present study, we demonstrated that RSG
is capable of alleviating IR and decreasing FBG and HbA1c in T2DM
patients. Similar results were observed in the BEZ group. However,
when treated with combined RSG and BEZ for 12 weeks, the FBG,
HbA1c, insulin and HOMA-IR all reached satisfactory levels. The
results indicate that the combination of PPARα/γ agonists improves
glucose metabolism more effectively than the PPARγ or PPARα agonist
alone.
T2DM patients usually have one or more lipid
abnormalities. The main characteristics of dyslipidemia in T2DM
patients are an elevated TG level, increased LDL level and
decreased HDL level. LDL cholesterol is a major risk factor for CVD
in general and in particular in T2DM patients. Statins are
extremely effective drugs for reducing the levels of LDL
cholesterol. Numerous clinical trials have shown that treatment
with statins lowers the rates of heart attack and cardiovascular
mortality. Moreover, fibrates reduce TGs and increase the blood
levels of HDL cholesterol, which is considered as ‘good’
cholesterol. However, there has been no definitive evidence from
previous clinical trials that have used combined fibrate and statin
to control blood lipid levels and thereby reduce the risk of heart
attack and stroke in T2DM patients. In the present study, we
identified that after 12 weeks treatment with combined RSG and BEZ,
the TC, TG, LDL-C and HDL-C levels significantly improved. However,
no significant changes in the lipid parameters, were observed in
the other groups suggesting that the combination of the two drugs
produces an improved outcome compared with RSG or BEZ alone.
Chronic inflammation has been shown to be a common
precursor of T2DM and CAD, while hyperglycemia and IR may
contribute to inflammation. Therefore, inflammation may act as a
bridge between T2DM and coronary atherosclerosis (27). The process of PBMC migration into
the vascular intima and transformation to foam cells, constitutes
the early event of atherosclerosis and is regulated by plasma
inflammatory cytokines, including MCP-1 and CRP. MCP-1 is secreted
mainly by endothelial cells, vascular smooth muscle cells,
monocytes and macrophages. Elevation of the MCP-1 level in CAD
patients often indicates the increase and activation of
inflammatory cells in the plaque. The increase of MMPs degrades
structural matrices in the fibrous cap, which causes plaque rupture
and vessel injury. Therefore, MCP-1 plays an important role in the
acute coronary syndrome caused by atherosclerosis and plaque
rupture (28). PPARs negatively
regulate NF-κB and stimulate protein-1 activity, thereby inhibiting
inflammatory gene transcription (29). Studies in animal models verified
that PPARα and PPARγ are able to exert anti-atherosclerotic effects
in vivo. Clinical studies suggested that PPARα or PPARγ
alone are capable of lowering plasma inflammatory cytokine
secretion in T2DM patients with CAD (30–33).
Our study revealed that RSG or BEZ alone was able to lower plasma
CRP and MCP-1 levels in T2DM patients with CAD. However, the effect
was clearer with combined RSG and BEZ, indicating that a
combination of RSG and BEZ alleviates the inflammatory reaction
more effectively. The mechanisms underlying the effect of RSG or
BEZ on CRP and MCP-1 levels may include the modulation of NF-κB,
AP-1 and signal transducer and activator of transcription (STAT)
signal pathways. The present study demonstrated that a combination
of RSG and BEZ exerts a more efficient anti-inflammatory effect
than RSG or BEZ alone, indicating that a combination of PPARα/γ
agonists is capable of inhibiting the expression of MCP-1, thus
delaying the process of atherosclerosis.
In conclusion, our study demonstrated that a
combination of RSG and BEZ is capable of reducing the level of
blood glucose, alleviating IR and improving lipid modulation. A
combination of RSG and BEZ is also able to lower plasma CRP and
MCP-1 levels and decrease low-dose LPS-induced MCP-1 secretion by
PBMCs in T2DM patients. The effects of combined RSG and BEZ are
greater than those of RSG or BEZ used alone. The results of the
present study suggest that the combination of PPARα/γ agonists may
be more effective for the treatment of T2DM patients with CAD.
References
|
1.
|
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K
and Laakso M: Mortality from coronary heart disease in subjects
with type 2 diabetes and in nondiabetic subjects with and without
prior myocardial infarction. N Engl J Med. 339:229–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shirani J and Dilsizian V: Screening
asymptomatic patients with type 2 diabetes mellitus for coronary
artery disease: does it improve patient outcome? Curr Cardiol Rep.
12:140–146. 2010. View Article : Google Scholar
|
|
3.
|
Desvergne B and Wahli W: Peroxisome
proliferator-activated receptors: nuclear control of metabolism.
Endocr Rev. 20:649–688. 1999.PubMed/NCBI
|
|
4.
|
Becker J, Delayre-Orthez C, Frossard N and
Pons F: Regulation of inflammation by PPARs: a future approach to
treat lung inflammatory diseases? Fundam Clin Pharmacol.
20:429–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Calkin AC and Thomas MC: PPAR agonists and
cardiovascular disease in diabetes. PPAR Res. 2008:2454102008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Inoue I, Goto S, Mizotani K, et al:
Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory
effect: reduction of mRNA levels for interleukin-1beta,
interleukin-6, cyclooxygenase-2, and p22phox by regulation of
peroxisome proliferator-activated receptor alpha (PPARalpha) in
primary endothelial cells. Life Sci. 67:863–876. 2000.
|
|
7.
|
Staels B, Koenig W, Habib A, et al:
Activation of human aortic smooth-muscle cells is inhibited by
PPARalpha but not by PPARgamma activators. Nature. 393:790–793.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Turay J, Grniaková V and Valka J: Changes
in paraoxonase and apolipoprotein A-I, B, C-III and E in subjects
with combined familiar hyperlipoproteinemia treated with
ciprofibrate. Drugs Exp Clin Res. 26:83–88. 2000.PubMed/NCBI
|
|
9.
|
Marx N, Sukhova G, Murphy C, Libby P and
Plutzky J: Macrophages in human atheroma contain PPARgamma:
differentiation-dependent peroxisomal proliferator-activated
receptor gamma (PPARgamma) expression and reduction of MMP-9
activity through PPARgamma activation in mononuclear phagocytes in
vitro. Am J Pathol. 153:17–23. 1998.
|
|
10.
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Delerive P, De Bosscher K, Besnard S, et
al: Peroxisome proliferator-activated receptor alpha negatively
regulates the vascular inflammatory gene response by negative
cross-talk with transcription factors NF-kappaB and AP-1. J Biol
Chem. 274:32048–32054. 1999. View Article : Google Scholar
|
|
12.
|
Chakrabarti R, Vikramadithyan RK, Misra P,
et al: Ragaglitazar: a novel PPAR alpha PPAR gamma agonist with
potent lipid-lowering and insulin-sensitizing efficacy in animal
models. Br J Pharmacol. 140:527–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mittra S, Sangle G, Tandon R, et al:
Increase in weight induced by muraglitazar, a dual PPARalpha/gamma
agonist, in db/db mice: adipogenesis/or oedema? Br J Pharmacol.
150:480–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Calkin AC, Allen TJ, Lassila M, Tikellis
C, Jandeleit-Dahm KA and Thomas MC: Increased atherosclerosis
following treatment with a dual PPAR agonist in the ApoE knockout
mouse. Atherosclerosis. 195:17–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Calkin AC, Cooper ME, Jandeleit-Dahm KA
and Allen TJ: Gemfibrozil decreases atherosclerosis in experimental
diabetes in association with a reduction in oxidative stress and
inflammation. Diabetologia. 49:766–774. 2006. View Article : Google Scholar
|
|
16.
|
Calkin AC, Forbes JM, Smith CM, et al:
Rosiglitazone attenuates atherosclerosis in a model of insulin
insufficiency independent of its metabolic effects. Arterioscler
Thromb Vasc Biol. 25:1903–1909. 2005. View Article : Google Scholar
|
|
17.
|
Nissen SE, Wolski K and Topol EJ: Effect
of muraglitazar on death and major adverse cardiovascular events in
patients with type 2 diabetes mellitus. JAMA. 294:2581–2586. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tordjman K, Bernal-Mizrachi C, Zemany L,
et al: PPARalpha deficiency reduces insulin resistance and
atherosclerosis in apoE-null mice. J Clin Invest. 107:1025–1034.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mardones P, Pilon A, Bouly M, et al:
Fibrates down-regulate hepatic scavenger receptor class B type I
protein expression in mice. J Biol Chem. 278:7884–7890. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Saha SA, Kizhakepunnur LG, Bahekar A and
Arora RR: The role of fibrates in the prevention of cardiovascular
disease - a pooled meta-analysis of long-term randomized
placebo-controlled clinical trials. Am Heart J. 154:943–953. 2007.
View Article : Google Scholar
|
|
22.
|
Zeng X, Dai J, Remick DG and Wang X:
Homocysteine mediated expression and secretion of monocyte
chemoattractant protein-1 and interleukin-8 in human monocytes.
Circ Res. 93:311–320. 2003. View Article : Google Scholar
|
|
23.
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Laakso M: Hyperglycaemia and
cardiovascular disease in type 2 diabetes. Diabetes. 48:937–942.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tenenbaum A, Motro M, Fisman EZ, et al:
Peroxisome proliferator-activated receptor ligand bezafibrate for
prevention of type 2 diabetes mellitus in patients with coronary
artery disease. Circulation. 109:2197–2202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Jones IR, Swai A, Taylor R, Miller M,
Laker MF and Alberti KG: Lowering of plasma glucose concentrations
with bezafibrate in patients with moderately controlled NIDDM.
Diabetes Care. 13:855–863. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sobel BE: Optimizing cardiovascular
outcomes in diabetes mellitus. Am J Med. 120(9 Suppl 2): S3–S11.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Liuzzo G, Goronzy JJ, Yang H, et al:
Monoclonal T-cell proliferation and plaque instability in acute
coronary syndromes. Circulation. 101:2883–2888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors (PPARs): nuclear
receptors with functions in the vascular wall. Z Kardiol. 90(Suppl
3): 125–132. 2001.PubMed/NCBI
|
|
30.
|
Wang G, Wei J, Guan Y, Jin N, Mao J and
Wang X: Peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone reduces clinical inflammatory responses in type 2
diabetes with coronary artery disease after coronary angioplasty.
Metabolism. 54:590–597. 2005. View Article : Google Scholar
|
|
31.
|
Wang TD, Chen WJ, Lin JW, Cheng CC, Chen
MF and Lee YT: Efficacy of fenofibrate and simvastatin on
endothelial function and inflammatory markers in patients with
combined hyperlipidemia: relations with baseline lipid profiles.
Atherosclerosis. 170:315–323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Han SH, Quon MJ and Koh KK: Beneficial
vascular and metabolic effects of peroxisome proliferator-activated
receptor-alpha activators. Hypertension. 46:1086–1092. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mohanty P, Aljada A, Ghanim H, et al:
Evidence for a potent antiinflammatory effect of rosiglitazone. J
Clin Endocrinol Metab. 89:2728–2735. 2004. View Article : Google Scholar : PubMed/NCBI
|