Introduction
Pressure ulcers, also know as bedsores, occur when
long-term pressure to local tissue causes disruption to blood flow
and tissue nutritional deficiency, which leads to skin ulceration
and necrosis (1). Pressure ulcers
have always been a difficult problem in clinical care and are one
of the most common complications in bedridden patients. Patients
with severe pressure ulcers may develop septicemia and this often
results in mortality. Therefore, it is crucial to discover methods
to prevent and treat pressure ulcers effectively. Interleukin
(IL)-17 is a cytokine which is associated with inflammatory
reactions (2). This study aimed to
investigate the role of IL-17 in pressure ulcers to determine how
they may be related. A mouse model of pressure ulcers was
established and IL-17 expression was observed in an attempt to find
an effective method to prevent and treat pressure ulcers.
Materials and methods
Animals
All methods in this study were approved by the
Ethics Committee of the First Affiliated Hospital of Liaoning
Medical University. Twenty 8-week-old BALB/c mice of either gender
and weighing 25–28 g were purchased from the Experimental Animal
Center, Dalian Medical University [License number: SCXK (Liaoning)
2008–0002].
Apparatus and reagents
A TC-512 gene amplification instrument, a UV
Analyzer, a GeneGenius automated gel imaging system, a BIO-RAD
semi-dry transfer instrument, an IBox 600 in vivo imaging
system, an RNA PCR kit (AMV) Ver 3.0 and Sepharose® were
purchased from Takara Bio, Inc. (Dalian, China). IL-17 and β-actin
primers were synthesized by Shanghai Sangon Biological Engineering
Technology and Service Co., Ltd. (Shanghai, China). IL-17 (H-132)
rabbit polyclonal antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Goat anti-rabbit IgG was
purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.
(Beijing, China). Hematoxylin, 4% formaldehyde and eosin were
purchased from Chemical Reagent Factory (Shanghai, China).
Grouping
Twenty mice were divided into experimental and
control groups (10 mice per group). Ischemia-reperfusion injury was
induced on local tissue in the experimental group.
Preparation of pressure ulcer mouse
model
A mouse model of pressure ulcer was produced in
accordance with previously described methods (3,4).
Mice were fasted for 12 h prior to surgery, anesthetized by
intraperitoneal injection of pentobarbital sodium (0.5 mg/10 g) and
skin preparation was performed. A sterile metal magnetic disk (5×12
mm, 2.4 g, 1000 Gauss) was placed on the skin at the hip joint and
another metal magnetic disk (5×12 mm, 2.4 g, 1000 Gauss) was placed
at the groin to produce a magnetic force of 50 mmHg (1 mmHg, 0.133
kPa, 40.7 g). In the experimental group, 2 h of ischemia and 0.5 h
of reperfusion were employed in a cycle; five cycles were performed
to induce a pressure ulcer. To ensure the balance of water and
electrolytes, 0.5 ml glucose-saline solution was administered via
the caudal vein every 2.5 h (5).
When the experiment had ended, the mice were sacrificed by cervical
dislocation.
Criteria for successful models
The gross appearance of mouse skin was red with
breakages, ulceration and necrosis. Pathological changes, including
muscle fiber atrophy, widened interstitial spaces, inflammatory
cell infiltration and unclear transverse striation, were observed
in mouse pressure ulcers under a light microscope.
Hematoxylin and eosin (H&E) stain for
mouse muscle tissue in pressure ulcer
Muscle tissue in the pressure ulcer was fixed with
4% paraformaldehyde for 12 h, dehydrated with gradient alcohol and
washed twice with xylene for 0.5–1 h. Once it had become
transparent, the muscle tissue was embedded in paraffin and sliced
into sections.
Following deparaffinization with xylene, the
sections were dehydrated with down-gradient alcohol, stained with
hematoxylin for 5–10 min and washed with distilled water for 10
min. Several seconds after the addition of 1% hydrochloric acid
alcohol, the sections were washed with tap water for 30–40 min,
dehydrated with up-gradient alcohol, rendered transparent by
immersion in xylene for 10 min and mounted with neutral gum for
observation under a light microscope.
IL-17 mRNA expression in mouse pressure
ulcer tissue determined using real-time (RT)-PCR
Muscle tissue (∼50 mg) was placed in a sterile
Eppendorf (EP) tube and the total RNA was extracted according to
the manufacturer’s instructions. The reverse transcription of cDNA
was performed according to the manufacturer’s instructions for RNA
PCR kit (AMV; Takara, Dalian, China) Ver 3.0. Reverse transcription
was performed in a 10 μl volume containing 1.0 μl 10X RT buffer,
2.0 μl MgCl2, 3.75 μl RNA-free dH2O, 1.0 μl
dNTP mixture, 0.25 μl RNase Inhibitor, 0.5 μl AMV Reverse
Transcriptase, 0.5 μl oligo(dT) adaptor primer and 1 μl total RNA.
The reaction conditions were as follows: 30°C for 10 min, 42°C for
30 min and 99°C for 5 min. Samples were stored at −20°C for future
use. In PCR, the primers used for IL-17 (6) were P1, 5′-AGATCTGGACGCGCAAACATGAG-3′
and P2, 5′-GGGTCGTCGACGGGTCTCTGTTTAG-3′ with an amplified fragment
of 516 bp. The primers used for β-actin (7) were P1, 5′-AGAGGGAAATCGTGCGTGAC-3′ and
P2, 5′-CAATAGTGATGACCTGGCCGT-3′ with an amplified fragment of 138
bp. PCR was performed in a volume of 20 μl containing 2.0 μl 10X
PCR buffer (Mg2+-free), 1.0 μl of 25 mmol/l
MgCl2, 1 μl of 2 mmol/l dNTP, 0.5 μl up- and downstream
IL-17 primer, 0.5 μl up- and downstream β-actin primer, 2 μl cDNA
template, 0.2 μl Ex Taq HS and 11.8 μl ultrapure water.
Reaction conditions were as follows: 94°C for 5 min, 94°C for 30
sec, 51°C for 45 sec and 72°C for 30 sec for 35 cycles. Finally
elongation was carried out at 72°C for 5 min. PCR products
underwent 1.5% agarose gel electrophoresis. Images were obtained
using a UV Analyzer. Grayscale values were obtained and analyzed
with GeneGenius and GeneTool analysis systems.
IL-17 protein expression in mouse
pressure ulcer tissue determined using western blot analysis
Muscle tissue (∼50 mg) was placed in a sterile EP
tube and cell disruption was performed. The protein was extracted
and its concentration was determined using the bicinchoninic acid
(BCA) method. Samples were prepared according to the protein
concentrations and were boiled for 5 min following addition of
reducing sample buffer (RSB) and Tris-buffered saline (TBS). The
samples were stored at −20°C for future use. Each sample (20 μl)
underwent SDS-PAGE and was then transferred to a membrane. The
membrane was sealed with 1% bovine serum albumin (BSA) for 1 h,
followed by the addition of anti-IL-17 polyclonal antibodies for
overnight incubation. The membrane was washed with TBS for 5 min
three times. Horseradish peroxidase-conjugated secondary antibody
was added for a 1-h incubation. The membrane was washed with TBS
for 5 min three times, followed by enhanced chemiluminescence (ECL)
coloration in the dark. Grayscale values were analyzed using an
IBox 600 in vivo imaging system (Tianmei Scientific
Instrument Co., Ltd., Shanghai, China).
Statistical analysis
Statistical analysis was performed using SPSS
software. The data are expressed as mean ± SD. An independent
sample t-test was used for comparisons between the two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Macroscopic observation
In the experimental group, mouse skin integrity was
damaged and exhibited exudation, erosion and necrosis. In the
control group, no marked changes were observed in mouse skin.
Changes in mouse pressure ulcer muscle
tissue viewed under a light microscope
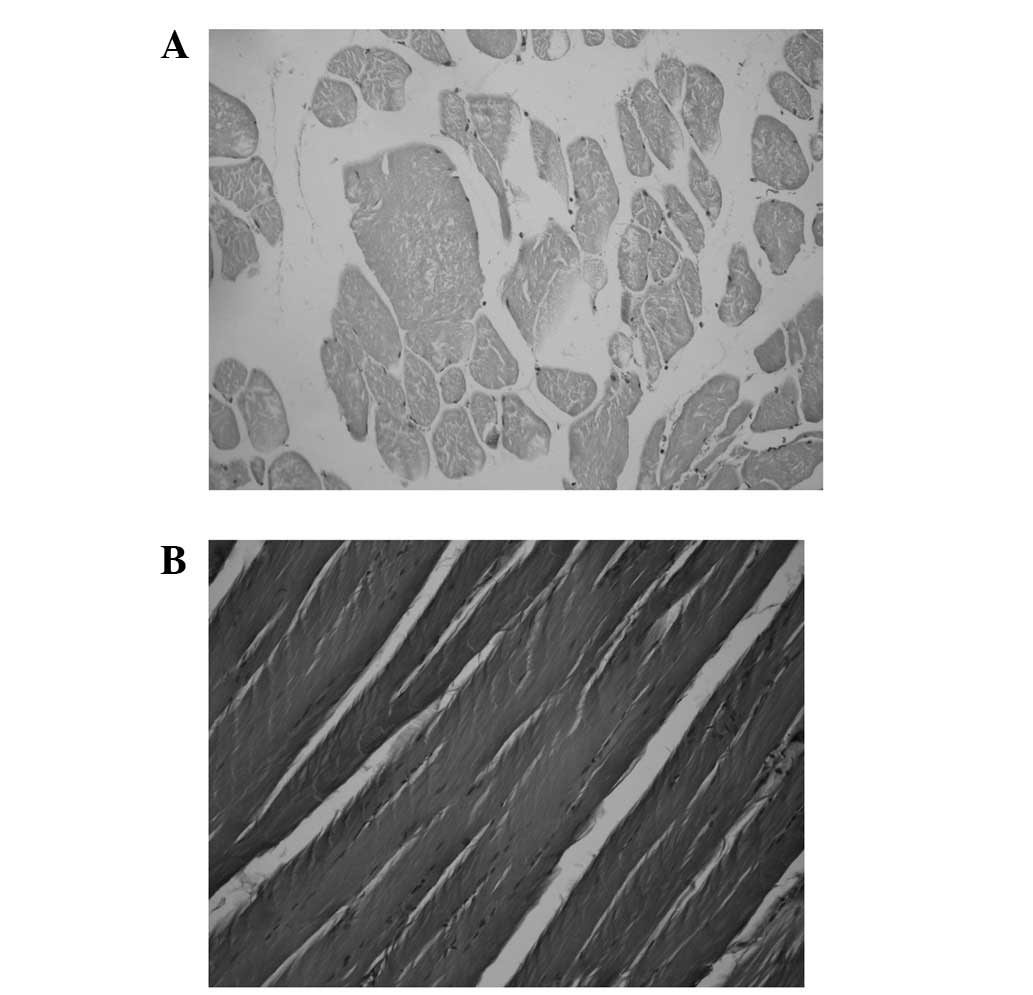
In the experimental group, degenerative tearing,
disappearance of transverse striation, myolysis and inflammatory
cell infiltration were observed in skeletal muscle. Cell
infiltration included neutrophilic granulocytes with pink cytoplasm
and a blue lobulated nucleus and lymphocytes with less cytoplasm
and a large nucleus (Fig. 1A). In
the control group, striated muscle was arranged in order and
cellular structure was intact (Fig.
1B).
IL-17 mRNA expression in mouse pressure
ulcer tissue determined using RT-PCR
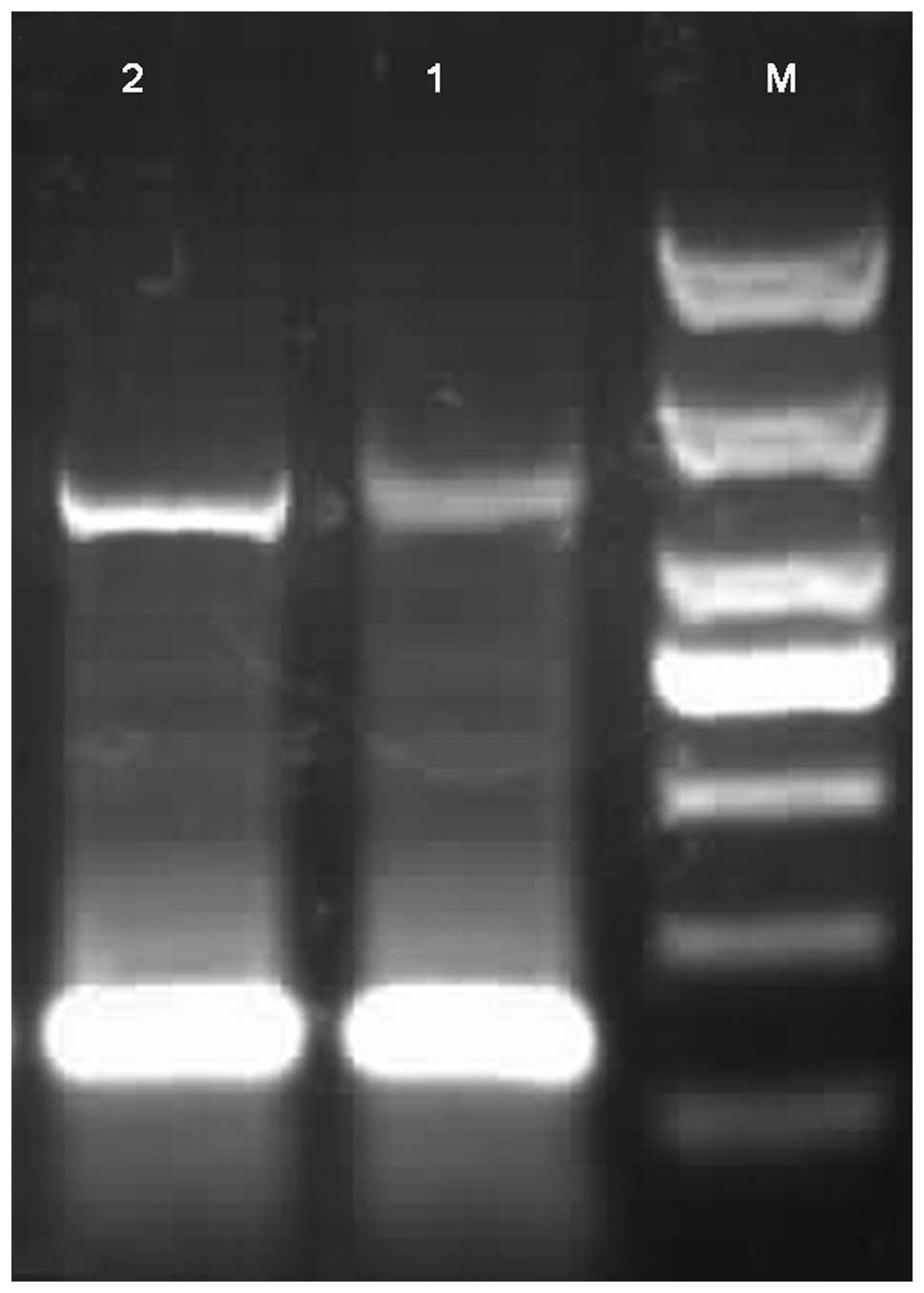
RT-PCR showed the specific band for 516 bp in the
two groups. Compared with the control group, IL-17 mRNA expression
was significantly upregulated in the experimental group (P<0.01;
Table I and Fig. 2).
 | Table I.IL-17 mRNA expression in mouse
pressure ulcer muscle tissue (mean ± SD). |
Table I.
IL-17 mRNA expression in mouse
pressure ulcer muscle tissue (mean ± SD).
| Group | Mice (n) | IL-17 mRNA | t | P-value |
|---|
| Experimental | 10 | 0.307±0.058 | 8.595 | 0.000 |
| Control | 10 | 0.112±0.042 | | |
IL-17 protein expression in mouse
pressure ulcer tissue determined using western blot analysis
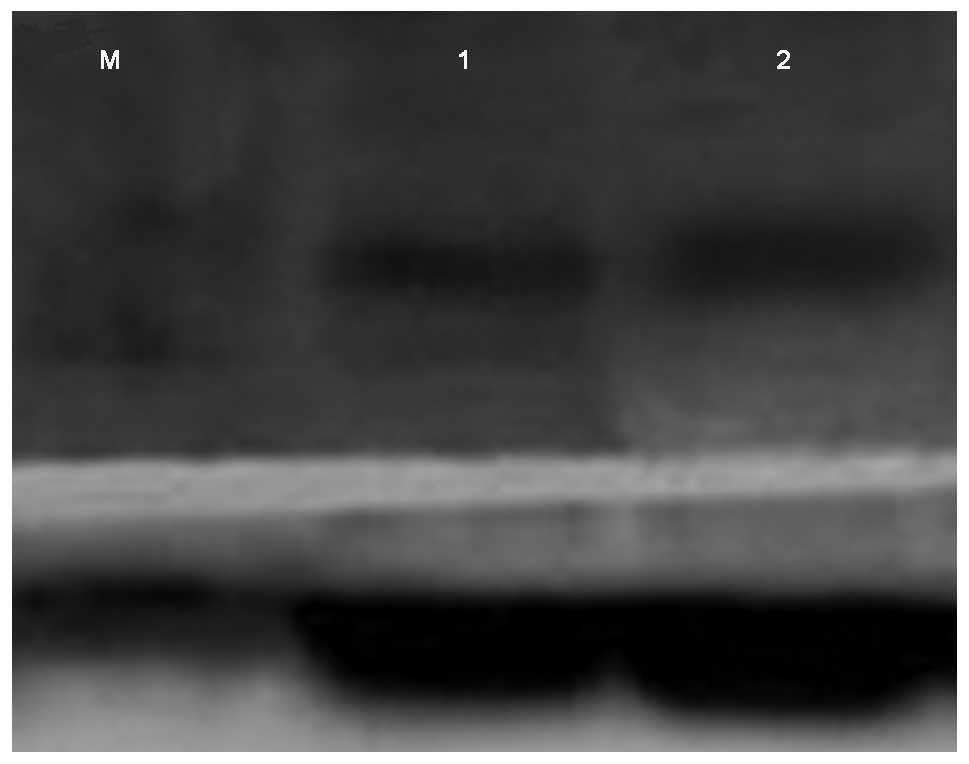
Western blot analysis showed the specific band for
15 kDa in the two groups. Compared with the control group, IL-17
protein expression was significantly upregulated in the
experimental group (P<0.01; Table
II and Fig. 3).
 | Table II.IL-17 protein expression in mouse
pressure ulcer muscle tissue (mean ± SD). |
Table II.
IL-17 protein expression in mouse
pressure ulcer muscle tissue (mean ± SD).
| Group | Mice (n) | IL-17 | t | P-value |
|---|
| Experimental | 10 | 0.434±0.097 | 7.608 | 0.000 |
| Control | 10 | 0.181±0.040 | | |
Discussion
Long-term pressure on soft tissue causes disruption
to blood circulation and vascular endothelial cell injury.
Continuous platelet agglomeration leads to the occurrence of
microcirculation thrombosis, which aggravates local tissue ischemia
(8). Long-term tissue ischemia and
hypoxia may induce metabolic disorders in tissue and cells and
changes in plasma colloid osmotic pressure, which leads to cellular
edema and perhaps even cell rupture (9). Damage to the tissue produces
self-defensive and protective reactions to recover normal function.
However, metabolic compensation is not able to maintain normal
function for long, due to long-term demands on the local tissue and
when metabolic disruption results in the production of oxygen free
radicals and their derivatives, tissue damage is aggravated
(10). This results in severe
tissue damage which causes infection and inflammation to occur
repeatedly. In this study, pressure ulcer mouse models were
established and IL-17 expression was observed in an attempt to find
an effective method to prevent and treat pressure ulcers.
H&E staining results revealed neutrophil and
lymphocyte infiltration of mouse muscle tissue in the experimental
group and well-arranged striated muscle and intact cells in the
control group. IL-17 (IL-17A) is mainly secreted by Th17 cells of
the CD4+ T lymphocyte subset. Th17 cells exert their
biological effects via the secretion of IL-17A, IL-17F, IL-6, IL-22
and TNF-α, which signals neutrophils to move towards the site of
inflammation and play an infection-fighting role in the early stage
of the immune response (11–13).
The results of this study indicated that the expression of IL-17
mRNA and protein occurred in the two groups, however these
expression levels were significantly increased in the experimental
group compared with the control group (P<0.01). This is
suggestive of overexpression of IL-17 in mouse pressure ulcer
muscle tissue, which is associated with the occurrence and
development of inflammatory lesions. IL-17 stimulates the secretion
of IL-6, TNF-α, granulocyte macrophage colony-stimulating factor
(GM-CSF), IL-8, IL-6, IL-1β and G-CSF, which allows neutrophils,
polymorphonuclear cells, T cells and macrophages to move to the
inflammation site and carry out an immune function. In this study,
H&E staining results indicated that neutrophil and lymphocyte
infiltration occurs in mouse pressure ulcer muscle tissue,
suggesting that IL-17 may contribute to an immune response in the
development of pressure ulcers. Differentiation occurs earlier in
Th17 cells than in Th1 and Th2 cells, there are fewer Th17 cells
than Th1 and Th2 cells and the effective response time is shorter
in Th17 cells than in Th1 and Th2 cells, therefore it is crucial to
extend the effective response time and to increase the number of
Th17 cells to improve the immune effects of IL-17. When the number
of Th17 cells reaches a certain threshold, Th1, Th2 and Treg cells
inhibit Th17 cell differentiation. Therefore, in the persistent
infection of pressure ulcers, a replacement for the role of IL-17
secreted by Th17 cells is required. Studies have shown that IFN-γ
is increased in the late phase of inflammation (14), however, whether IFN-5 plays a major
role in severe pressure ulcers remains to be confirmed by future
studies.
Pressure ulcer is a complex problem in clinical
care. Further studies are required to discover better methods to
prevent and treat pressure ulcer.
References
|
1.
|
No authors listed:. Pressure ulcer
prevalence, cost and risk assessment: consensus development
conference statement - The National Pressure Ulcer Advisory Panel.
Decubitus. 2:24–28. 1989.
|
|
2.
|
Kramer JM and Gaffen SL: Interleukin-17: a
new paradigm in inflammation, autoimmunity, and therapy. J
Periodontol. 78:1083–1093. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Stadler I, Zhang RY, Oskoui P, et al:
Develoment of a simple, noninvasive, clinically relevant model of
pressure ulcers in the mouse. J Invest Surg. 17:221–227. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Peirce SM, Skalak TC and Rodeheaver GT:
Ischemia-reper-fusion injury in chronic pressure ulcer formation: a
skin model in the rat. Wound Repair Regen. 8:68–76. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Salcido R, Fisher SB, Donofrio JC, et al:
An animal model and computer controlled surface pressure delivery
system for the production of pressure ulcers. J Rehabil Res Dev.
32:149–161. 1995.
|
|
6.
|
Wang Dong-hai: IL-17 regulates T
cell-mediated type I diabetes in NOD mice; long-term alcohol
consumption reduces the expression of PBR and StAR in rat leydig
cells. Shandong: Internal Medicine of Shandong University; 2008,
(In Chinese).
|
|
7.
|
Liu Shuang: Inhibitory effects of IL-23
gene on breast cancer in mice and its mechanism. Hebei: Hebei
Medical University. Immunology,. 2008, (In Chinese).
|
|
8.
|
Witzigmann H, Ludwig S, Armann B, et al:
Endothelin(A) receptor blockade reduces ischemia/reperfusion injury
in pig pancreas tansplantation. Ann Surg. 238:264–274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jiang LP, Tu Q, Wang Y and Zhang E:
Icshemia-reperfusion injury-induced histological changes affecting
early stage pressure ulcer development in a rat model. Ostomy Wound
Manage. 57:55–56. 2011.
|
|
10.
|
Saito Y, Hasegawa M, Fujimoto M, et al:
The Loss of MCP-1 attenuates cutaneous ischemia-reperfusion injury
in a mouse model of pressure ulcer. J Invest Surg. 128:1838–1851.
2008.PubMed/NCBI
|
|
11.
|
Ouyang W, Kolls JK and Zheng Y: The
biological funcations of Thelper 17 cell effector cytokines in
inflammation. Immunity. 28:454–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fontao L, Bremblilla NC, Masouyé I, et al:
Interleukin-17 expression in neutrophils and Th17 cells in
cutaneous T-cell lymphoma associated with neutrophilic infiltrate
of the skin. Br J Dermatol. 166:687–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Korn T, Oukka M, Kuchroo V, et al: Th17
cell: effector T cell with inflammatory properties. Semin Immunol.
19:362–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sheng Xu: High expression of
interleukin-17 secreted by CD4<+> memory T cells and its
mechanism. Shanghai: Immunology.Second Military Medical Univesity;
2008, (In Chinese).
|