Introduction
Despite the achievement of an initial complete
remission (CR) in the majority of patients with acute myeloid
leukemia (AML), the therapeutic results at present remain
unsatisfactory. With one or two courses of standard first-line
induction chemotherapy, 60–80% of patients with newly diagnosed AML
achieve CR; however, 50–70% of them eventually relapse. The
disease-free survival (DFS) rate and the overall survival (OS) rate
remain low (1–2). Currently, there are no standard
salvage regimens for relapsed AML; therefore, alternative
treatments that produce improved outcomes for these patients should
be explored.
Cytosine arabinoside (Ara-C) remains one of the most
effective drugs in AML therapy (3). Fludarabine, a purine analog, markedly
increases the intracellular accumulation of Ara-C triphosphate
(Ara-CTP), the active metabolite of Ara-C (4). The combination of a middle dose of
Ara-C along with fludarabine has also achieved a satisfactory CR
rate in refractory or relapsed AML, from 47.5 to 64% (5,6).
Adding another chemotherapeutic agent to the FLAG regimen has
become one of the hot issues of study. A number of studies have
reported on FLAG with mitoxantrone for treating patients with
refractory or relapsed AML. The CR rates were 59 and 67%,
respectively (7,8). However, to date there have been no
reports of this regimen for relapsed AML solely. Since 2003, we
have used fludarabine combined with a middle dose of Ara-C,
mitoxantrone and granulocyte-colony stimulating factor (G-CSF) as a
salvage regimen for patients with relapsed AML in China.
Patients and methods
Patients
Between December 2003 and March 2008, 45 patients
(22 males, 23 females, aged 17–61 years, median 34 years) with
relapsed AML were treated with the Mito-FLAG regimen after
obtaining informed consent. These patients had received standard
first-line induction chemotherapy following initial diagnosis.
Hematologic relapse was defined by recurrence of >5% blasts in
the bone marrow (BM). Diagnosis and classification of AML were
performed according to the French-American-British (FAB)
Corporation criteria (9). The
clinical features of the patients are listed in Table I. The study was approved by the
ethics committee of Wenzhou Medical College (Wenzhou, China).
 | Table I.Clinical features of the patients by
subtype of AML (M1-6). |
Table I.
Clinical features of the patients by
subtype of AML (M1-6).
| Characteristics | All | M1 | M2 | M4 | M5 | M6 |
|---|
| Male/female | 22/23 | 0/1 | 8/11 | 10/7 | 3/4 | 1/0 |
| Age (years) | | | | | | |
| Median | 34 | 48 | 32 | 36 | 28 | 30 |
| Range | 17–61 | - | 18–61 | 22–60 | 17–50 | - |
| Early/late
relapse | 25/20 | 1/0 | 10/9 | 8/9 | 5/2 | 1/0 |
| Duration of prior
remission (months) | | | | | | |
| Median | 10 | 4 | 11 | 10 | 9 | 3 |
| Range | 3–50 | 4 | 4–50 | 5–43 | 6–15 | 3 |
| Karyotype | | | | | | |
| Normal | 29 | 1 | 14 | 11 | 3 | - |
| Abnormala | 16 | - | 5 | 6 | 4 | 1 |
| Number of prior
regimens | | | | | | |
| Median | 3 | 1 | 3 | 3 | 3 | 1 |
| Range | 1–6 | 1 | 1–6 | 2–6 | 2–4 | 1 |
| Number of prior
courses | | | | | | |
| Median | 6 | 3 | 6 | 7 | 6 | 2 |
| Range | 2–17 | 3 | 3–17 | 4–16 | 4–9 | 2 |
| Number of prior
chemotherapeutics | | | | | | |
| Idarubicin | 37 | 1 | 15 | 14 | 6 | 1 |
| Daunomycin | 13 | - | 5 | 6 | 2 | - |
| Mitoxantrone | 17 | - | 7 | 7 | 3 | - |
| Aclacinomycin | 22 | - | 12 | 8 | 2 | - |
|
Homoharringtonine | 14 | - | 6 | 6 | 2 | - |
| Etoposide | 14 | - | 7 | 5 | 2 | - |
| Ara-C
(monotherapy) | 29 | - | 13 | 12 | 4 | - |
Methods
The Mito-FLAG regimen consisted of five days of
treatment with mitoxantrone (7 mg/m2, days 1, 3 and 5),
fludarabine (30 mg/m2, days 1–5), Ara-C (1
g/m2, over 3 h every 12 h, days 1–5) and G-CSF [5
μg/kg/day subcutaneously from day 0 until the white blood
count (WBC) was >20×109/l].
The response criteria were established according to
revised recommendations of the International Working Group for
Diagnosis, Standardization of Response Criteria, Treatment Outcomes
and Reporting Standards for Therapeutic Trials in Acute Myeloid
Leukemia (10). CR was defined as
<5% BM blasts with a neutrophil count of
>1.0×109/l, a platelet count of
>100×109/l and no other diseases. Partial response
(PR) was established as either 5–25% BM blasts, a ≥50% decrease in
BM blasts or <5% BM blasts but with Auer rod presence. No
remission (NR) was established for patients who did not fulfill the
above criteria. BM aspirate was performed following chemotherapy as
soon as the patient achieved peripheral blood morphology values
required for CR; however, this was not performed later than 50 days
from the start of treatment. Early mortality (EM) was defined as
mortality from any cause during the first 30 days from the
beginning of the Mito-FLAG therapy.
Patients in PR received another course of the same
regimen. Patients in CR received allogeneic stem cell
transplantation (allo-SCT) subsequently if aged <50 years with a
suitable donor, whereas those without a donor received maintenance
therapy. Antibiotic treatment and supportive care were administered
according to local guidelines. Patients with treatment failures
received another salvage chemotherapy regimen or palliation therapy
subsequently. Hematological and extra-hematological toxicity were
assessed according to the World Health Organization (WHO) criteria
(11).
DFS was calculated from the first day of remission
until the evidence of progression. OS was calculated from the start
of Mito-FLAG regimen until mortality.
Statistical analysis
Statistical analysis was performed using the
Chi-square test and Kaplan-Meier survival curves were produced. We
considered a two-tailed P-value <0.05 to be an indication of
statistical significance.
Results
Therapeutic effects
Out of the 45 patients, 21 (47%) achieved CR
following the first course of Mito-FLAG and 5 (11%) achieved PR.
All the patients with PR received second Mito-FLAG regimen and 2
patients achieved CR following the second course. Finally, CR and
PR was achieved in 23 (51%) and 3 (7%) patients, respectively, so
the overall response rate was 58%. Fifteen patients (33%) were
refractory and 4 (9%) succumbed early due to cerebral hemorrhage
(n=3) or pulmonary infection (n=1). Patients with abnormal
karyotype (n=16, 36%) had a CR rate of 43.8% and patients with
early relapse (n=25) and late relapse (n=20) had a CR rate of 40.0
and 65.0%, respectively. However, analysis of corresponding
subgroups stratified according to karyotype and early/late relapse
did not show significant differences (Table II).
 | Table II.Outcome of patients treated with
Mito-FLAG. |
Table II.
Outcome of patients treated with
Mito-FLAG.
| CR | % | P-value |
|---|
| Karyotype | | | |
| Normal | 16/29 | 55.2 | 0.463 |
| Abnormal | 7/16 | 43.8 | |
| Relapse | | | |
| Early | 10/25 | 40.0 | 0.095 |
| Late | 13/20 | 65.0 | |
Toxicity associated with Mito-FLAG
regimen
Four patients (9%) succumbed early; three from
cerebral hemorrhage (n=3) and one from pulmonary infection. As
expected, severe myelosuppression was observed in all patients.
Hematological toxicity and infections were the most prominent
toxicities of the Mito-FLAG treatment. The median number of days to
recovery of neutrophil count (>0.5×109/l) and
platelet count (>20×109/l) was 19 days (range, 9–48
days) and 24 days (range, 10–95 days), respectively. Patients
received a median of 5 units (range, 0–12 units) of suspended red
cells and 5 units (range, 3–23 units) of apheresis platelets. The
incidence of serious infections was high. All but 6 patients
experienced fever (temperature >38°C) during the neutropenic
phase. Twenty-one patients (47%) had confirmed grade III–IV
infections (according to the WHO criteria), which included
pneumonia (29%), sepsis (11%), enteritis (7%), perianal abscess
(4%) and stomatitis (2%). The median duration of antibiotic therapy
was 25 days (range, 15–76 days). The other WHO grade III–IV
toxicities included nausea and vomiting (22%), bleeding (18%),
hyperbilirubinemia (4%), renal toxicity (4%) and arrhythmia (2%).
The median time of hospitalization was 46 days (range, 18–108
days).
Overall survival and disease-free
survival
Two patients were lost to follow-up shortly after
receiving induction chemotherapy. The median OS for the other 43
patients was 7 months. With a median follow-up of 11 months (95%
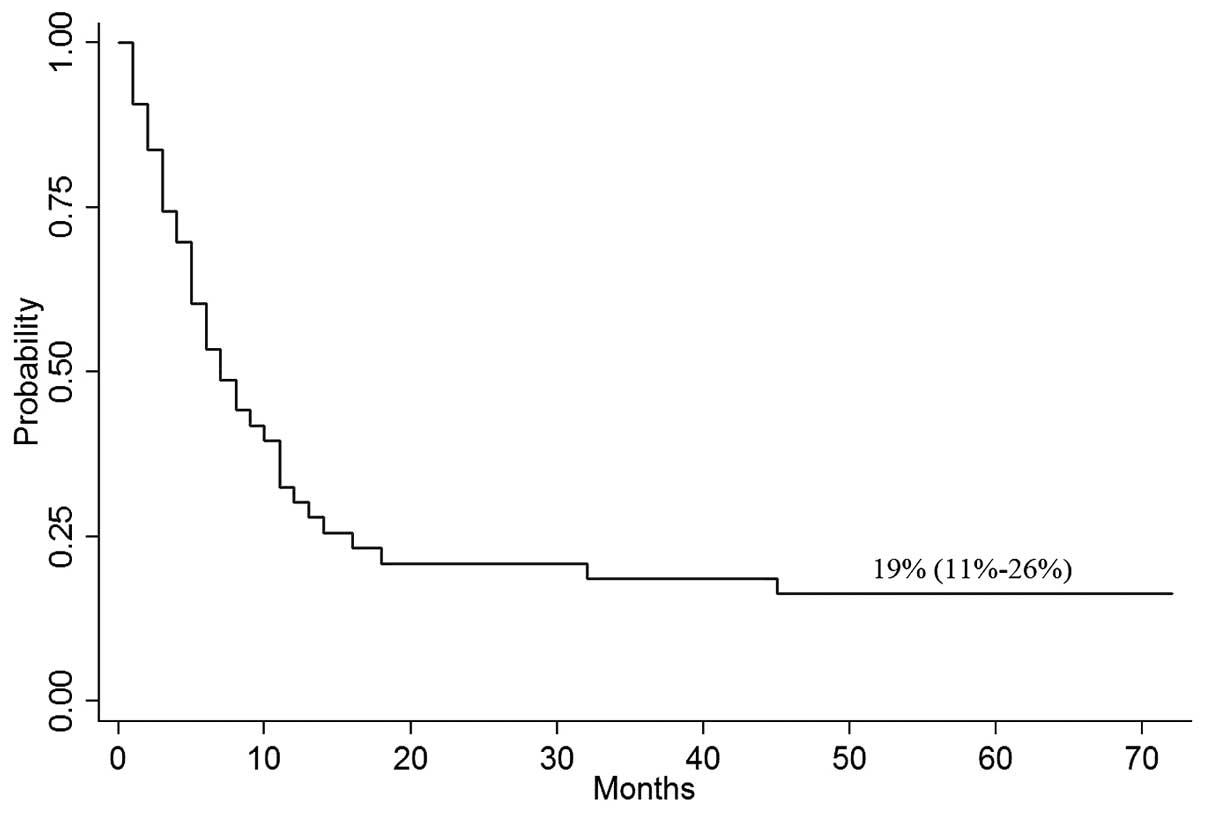
CI, 1–72%), the probability of OS at 4 years was 19% (95% CI,
11–26%; Fig. 1). The median time
of DFS for all 23 patients in CR was 10 months. The probability of
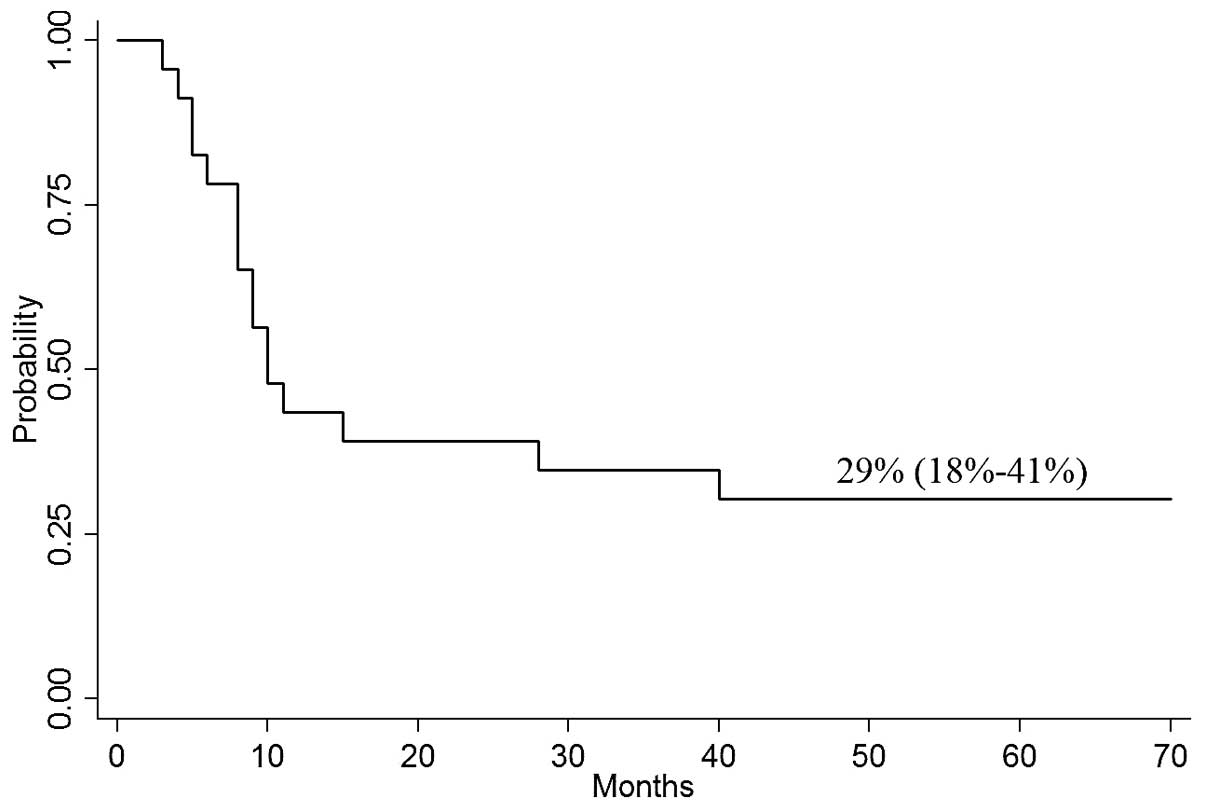
4-year DFS was 29% (95% CI, 18–41%; Fig. 2).
Post-transplant outcome
Out of the 23 patients in CR, 9 (20%) received
allo-SCT. Among them, 2 patients received allo-SCT with a matched
related donor and 7 patients with a matched unrelated donor. Two
patients succumbed due to relapse. Seven patients remained in CR.
The median OS time for the patients who underwent allo-SCT was 56
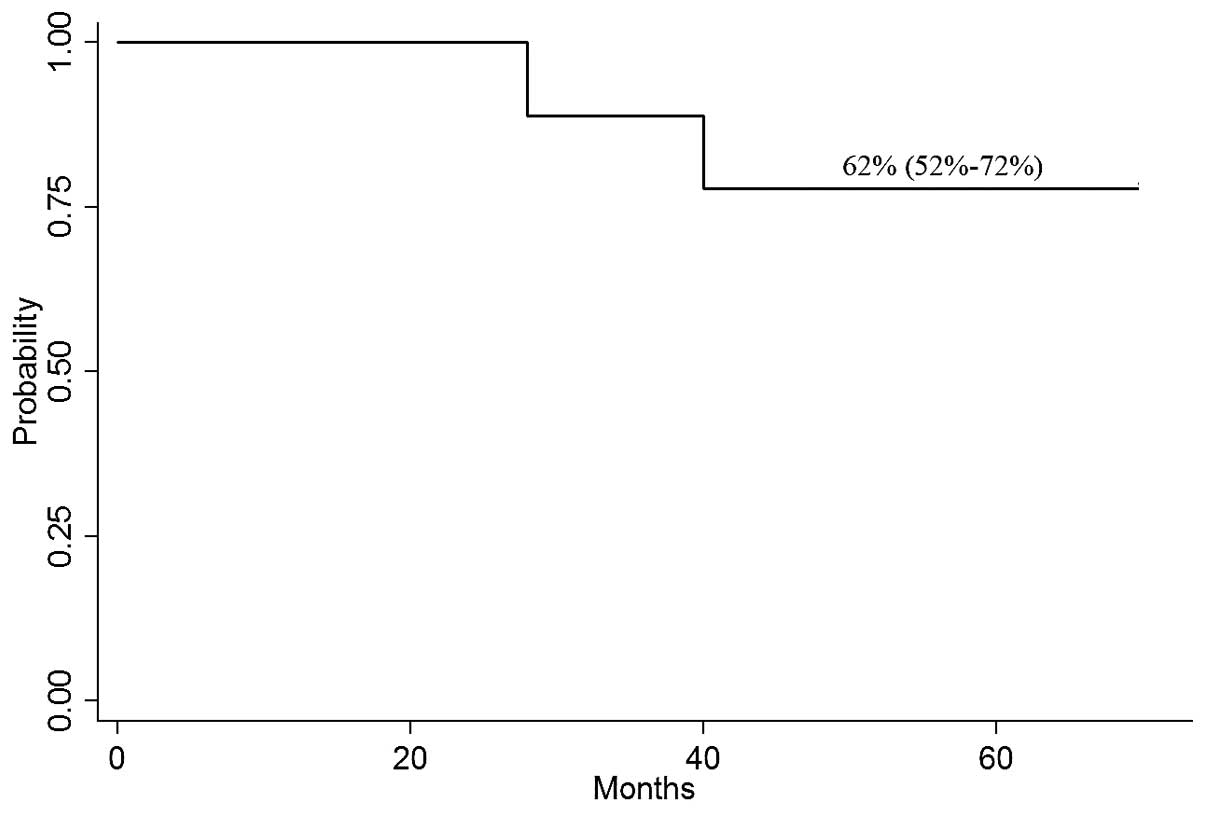
months. The probability of OS at 4 years was 62% (95% CI, 52–72%;
Fig. 3).
Discussion
Ara-C is one of the most effective drugs in AML
therapy (3). It performs antitumor
effects by transforming into 5′-triphosphate Ara-C (Ara-CTP) in
cells. A previous study suggested that patients benefit from
high-dose therapy when an increase in intracellular Ara-CTP occurs
with higher extracellular concentrations of Ara-C (12). Therefore, increased dosages of
Ara-C to produce tumor remission rate improvement are often
utilized doctors in the clinic. However, the dose escalation of
Ara-C did not improve the outcome in practice (13). Thus, the main aim of current
research is to determine how to increase the concentration of
Ara-CTP in leukemic cells to overcome Ara-C resistance.
Fludarabine, a purine analog, which is not susceptible to
degradation by adenosine deaminase, maintains in vivo
activity. By phosphorylation in the plasma, fludarabine transforms
to lipophilic 2-F-Ara-ATP which easily passes through the membrane
of tumor cells. Following phosphorylation by deoxycytidine kinase,
2-F-Ara-ATP turns to activated F-Ara-ATP, which inhibits the
synthesis of DNA, RNA and protein. F-Ara-ATP increases the activity
of deoxycytidine kinase, increasing the rate of Ara-CTP
accumulation by 2–3 fold (14).
Fludarabine, in addition to Ara-C, increases the concentration of
Ara-CTP by almost 5 fold in the regimen of Ara-C and G-CSF
(15). G-CSF mobilizes quiescent
leukemic cells into the cell cycle and increases the sensitivity to
Ara-C, the intake of Ara-C and Ara-C-induced apoptosis. G-CSF also
enhances the activity of topoisomerase II and accelerates
intracellular phosphorylation of fludarabine (16). The FLAG regimen is composed of
fludarabine, Ara-C and G-CSF, which has achieved satisfactory CR
rate in treating refractory or relapsed AML. The FLAG regimen is
repeatedly described as a well-tolerated salvage regimen in AML
with CR rates between 47.5 and 68% (5,18,19).
Adding another chemotherapeutic agent to the FLAG
regimen has become one of the hot issues of study. A number of
studies of FLAG along with idarubicin (IDA-FLAG regimen) for
treating patients of refractory or relapsed AML have been reported.
The CR rates were 42 and 52%, respectively, which were similar to
FLAG (20,21). Mitoxantrone is one of the most
common drugs in AML therapy, particularly in refractory or relapsed
AML. Further studies indicated that doses of mitoxantrone, a
topoisomerase II-directed drug, may be escalated far beyond the
conventional dose schedule, with clinical benefit to patients with
acute leukemia when combined with Ara-C. The effect is satisfactory
and the tolerance is acceptable, even in elderly patients (7,22).
FLAG combined with mitoxantrone strengthens the anti-leukemic
activity. A number of studies of FLAG along with mitoxantrone for
treating patients of refractory or relapsed AML have been reported.
The CR rates were 59 and 67%, respectively (7,8).
In our study, the overall response rate was 58%,
similar to that of IDA-FLAG. However, the price of idarubicin is
more expensive than that of mitoxantrone in China. In addition,
idarubicin has been used in the majority of patients with relapsed
AML previously and reuse of idarubicin may result in serious
cardiac toxicity and other toxicities. Therefore, Mito-FLAG was
selected for relapsed AML in our study. Four patients (9%)
succumbed early. Although hematological toxicity and infections
were the most prominent toxicities of the Mito-FLAG treatment, the
patients recovered quickly. The probability of OS at 4 years was
19% and the probability of 4-year DFS was 29% for all 23 patients
in CR. Nine out of 23 patients in CR received allo-SCT and 7 of
them remained in CR at the end of follow-up. These results indicate
that the Mito-FLAG regimen is a highly effective and well-tolerated
salvage regimen in relapsed AML. The toxicity is acceptable,
enabling the majority of patients to receive further treatment
including allo-SCT.
References
|
1.
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
|
|
2.
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar
|
|
3.
|
Roboz GJ: Novel approaches to the
treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ
Program. 2011:43–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gandhi V, Estey E, Keating MJ and Plunkett
W: Biochemical modulation of arabinosylcytosine for therapy of
leukemias. Leuk Lymphoma. 10:109–114. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee SR, Yang DH, Ahn JS, Kim YK, Lee JJ,
Choi YJ, Shin HJ, Chung JS, Cho YY, Chae YS, Kim JG, Sohn SK and
Kim HJ: The clinical outcome of FLAG chemotherapy without
idarubicin in patients with relapsed or refractory acute myeloid
leukemia. J Korean Med Sci. 24:498–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ferrara F, Palmieri S, Pocali B, Pollio F,
Viola A, Annunziata S, Sebastio L, Schiavone EM, Mele G,
Gianfaldoni G and Leoni F: De novo acute myeloid leukemia with
multilineage dysplasia: treatment results and prognostic evaluation
from a series of 44 patients treated with fludarabine, cytarabine
and G-CSF (FLAG). Eur J Haematol. 68:203–209. 2002. View Article : Google Scholar
|
|
7.
|
Clavio M, Carrara P, Miglino M, Pierri I,
Canepa L, Balleari E, Gatti AM, Cerri R, Celesti L, Vallebella E,
Sessarego M, Patrone F, Ghio R, Damasio E and Gobbi M: High
efficacy of fludarabine-containing therapy (FLAG-FLANG) in poor
risk acute myeloid leukemia. Haematologica. 81:513–520.
1996.PubMed/NCBI
|
|
8.
|
Hänel M, Friedrichsen K, Hänel A, Herbst
R, Morgner A, Neser S, Nicklisch M, Teich M, Ehninger G and Fiedler
F: Mito-flag as salvage therapy for relapsed and refractory acute
myeloid leukemia. Onkologie. 24:356–360. 2001.PubMed/NCBI
|
|
9.
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposed revised
criteria for the classification of acute myeloid leukemia. A report
of the French-American-British Cooperative Group. Ann Intern Med.
103:620–625. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA,
Löwenberg B, Sanz MA, Head DR, Ohno R and Bloomfield CD: Revised
recommendations of the International Working Group for Diagnosis,
Standardization of Response Criteria, Treatment Outcomes and
Reporting Standards for Therapeutic Trials in Acute Myeloid
Leukemia. J Clin Oncol. 21:4642–4649. 2003. View Article : Google Scholar
|
|
11.
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rustum YM, Slocum HK, Wang G, Bakshi D,
Kelly E, Buscaglia D, Wrzosek C, Early AP and Preisler H:
Relationship between plasma Ara-C and intracellular Ara-CTP pools
under conditions of continuous infusion and high-dose Ara-C
treatment. Med Pediatr Oncol. 10(Suppl 1): 33–43. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kern W and Estey EH: High-dose cytosine
arabinoside in the treatment of acute myeloid leukemia: Review of
three randomized trials. Cancer. 107:116–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gandhi V, Estey E, Keating MJ and Plunkett
W: Fludarabine potentiates metabolism of cytarabine in patients
with acute myelogenous leukemia during therapy. J Clin Oncol.
11:116–124. 1993.PubMed/NCBI
|
|
15.
|
Ossenkoppele GJ, Graveland WJ, Sonneveld
P, Daenen SM, Biesma DH, Verdonck LF, Schaafsma MR, Westveer PH,
Peters GJ, Noordhuis P, Muus P, Selleslag D, van der Holt B,
Delforge M, Löwenberg B and Verhoef GE: The value of fludarabine in
addition to ARA-C and G-CSF in the treatment of patients with
high-risk myelodysplastic syndromes and AML in elderly patients.
Blood. 103:2908–2913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hubeek I, Litvinova E, Peters GJ,
Broekhuizen R, Haarman EG, Huismans DR, Cloos J, Zwaan CM,
Fleischhack G, Creutzig U and Kaspers GJ: The effect of G-CSF on
the in vitro cytotoxicity of cytarabine and fludarabine in the FLAG
combination in pediatric acute myeloid leukemia. Int J Oncol.
25:1823–1829. 2004.PubMed/NCBI
|
|
17.
|
Carella AM, Cascavilla N, Greco MM,
Melillo L, Sajeva MR, Ladogana S, D’Arena G, Perla G and Carotenuto
M: Treatment of “poor risk” acute myeloid leukemia with
fludarabine, cytarabine and G-CSF (flag regimen): a single center
study. Leuk Lymphoma. 40:295–303. 2001.
|
|
18.
|
Wang SH, Yu L, Wang QS, Li HH, Zhao Y and
Li F: Effects of FLAG protocol in treatment of the first time
induced non-remission acute myeloid leukemia. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 15:1297–1299. 2007.(In Chinese).
|
|
19.
|
Yavuz S, Paydas S, Disel U and Sahin B:
IDA-FLAG regimen for the therapy of primary refractory and relapse
acute leukemia: a single-center experience. Am J Ther. 13:389–393.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Pastore D, Specchia G, Carluccio P, Liso
A, Mestice A, Rizzi R, Greco G, Buquicchio C and Liso V: FLAG-IDA
in the treatment of refractory/relapsed acute myeloid leukemia:
single-center experience. Ann Hematol. 82:231–235. 2003.PubMed/NCBI
|
|
21.
|
Feldman EJ, Seiter K, Damon L, Linker C,
Rugo H, Ries C, Case DC Jr, Beer M and Ahmed T: A randomized trial
of high- vs standard-dose mitoxantrone with cytarabine in elderly
patients with acute myeloid leukemia. Leukemia. 11:485–489. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Feldman EJ, Alberts DS, Arlin Z, Ahmed T,
Mittelman A, Baskind P, Peng YM, Baier M and Plezia P: Phase I
clinical and pharmacokinetic evaluation of high-dose mitoxantrone
in combination with cytarabine in patients with acute leukemia. J
Clin Oncol. 11:2002–2009. 1993.PubMed/NCBI
|