Introduction
Budd-Chiari syndrome (BCS) results from venous
obstruction (occlusion or stenosis) of the hepatic veins and/or
retrohepatic inferior vena cava (IVC) and presents clinically as
portal and IVC hypertension (1).
There have been few reports on the subject from the United States
and Europe, but there have been several reports from developing
countries such as China and India. It is common in China’s lower
reaches of the Yellow River such as the Shandong, Jiangsu and Anhui
Provinces (1,2). In the West, most cases result from
thrombosis of the small centrilobular or main hepatic veins
(3,4). In China, however, membranous
obstruction of the IVC or the hepatic vein, or both, is the most
common cause of BCS and accounts for up to 60–70% of the total
patient number (5–7). Thromboses are easily formed in these
patients due to obstruction at the suprahepatic IVC, slow and
reversed blood flow in the IVC, and the hypercoagulable blood
state.
Due to the increase in the understanding and
diagnosis of BCS, a greater number of BCS cases are treated, while
the treatment methods remain varied. Among them, interventional
therapy has been rapidly developed due to being minimally invasive
(8,9). Percutaneous transluminal angioplasty,
including balloon angioplasty and stent placement, has recently
been recommended as a first-line treatment for obstructed IVC at or
above the hepatic level in primary BCS (6,10).
Patients with IVC thrombosis, particularly patients with new
thromboses, remain difficult to treat, however. However, a long
time ago, there was a contraindication of interventional therapy.
There are many therapeutic approaches to treating IVC thrombosis.
These include anticoagulation with warfarin (11), thrombolytic agents (such as the
acylated streptokinase-plasminogen complex, urokinase,
streptokinase and tissue plasminogen activator) administered either
through a peripheral intravenous or catheter-mediated route
(12–14), balloon angioplasty and/or stents
(13,14) or transjugular intrahepatic
portosystemic shunts (15).
Surgical shunts (16) and
orthotopic liver transplantation (17) have also been successfully applied
for the treatment of BCS with thrombosis. They are complicated to
use, however, with a high risk of causing bleeding, and a
significant risk of pulmonary embolism. There has been no guidance
for the selection of these methods for use in patients with BCS
combined with IVC thrombosis.
Since 2006, at the vascular surgery centers in Jinan
Central Hospital and Shandong Provincial Hospital, China, a set of
treatment procedures for the interventional therapy of patients
with IVC thrombosis has been implemented. In order to facilitate
clinical standard treatment, the patients with BCS and IVC
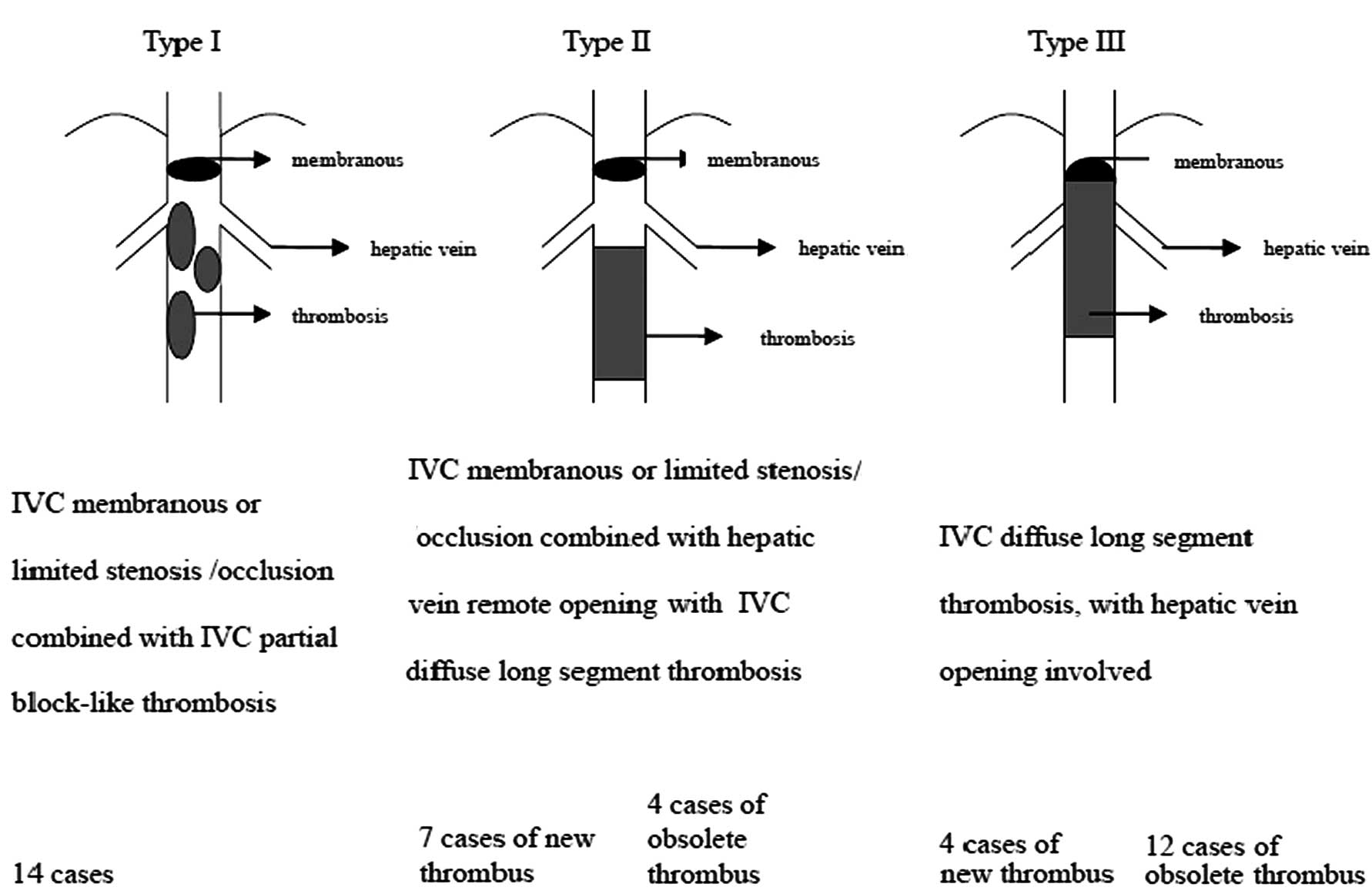
thrombosis were divided into three types (Fig. 1), and different treatments were
employed. The clinical data from 41 patients with BCS and IVC
thrombosis were evaluated. The purpose of this study was to
evaluate the initial results with regard to the clinical safety and
feasibility of the therapeutic approaches, selected according to
our classification of the condition.
Materials and methods
Clinical data
The inclusion criteria were as follows. From
November, 2006 to November, 2010, 41 cases of BCS with IVC
thrombosis underwent vascular surgery at Shandong Provincial
Hospital, Shandong University and Jinan Central Hospital, Shandong
University. All participants were examined initially by color
ultrasonography, enhanced CT, MRI or other imaging methods and
diagnosed to have BCS with IVC thrombosis. The patients had an
average age of 47 years, with clinically refractory ascites,
hepatosplenomegaly, varicose veins of the abdominal wall and the
lower extremities, and leg pigmentation or ulceration. Those
diagnosed as having liver neoplasm or severe heart failure, who had
long segment occlusion of the hepatic vein or whose scope of the
IVC thrombosis involved the iliac and femoral vein were excluded.
The patients were classified based on imaging examination results
(Fig. 1). This study was conducted
in accordance with the Declaration of Helsinki and with approval
from the Ethics Committee of Shandong Provincial Hospital and Jinan
Central Hospital. Written informed consent was obtained from all
participants.
Treatment method
Upon admission, all patients routinely underwent
puncture of the right femoral vein and/or right jugular vein for
orthophoria angiography and lateral radiography. This was to
evaluate the scope of IVC occlusion and show the nature of the
thrombus, the extent of hepatic vein patency, collateral
circulation and other aspects of the disease.
In patients of type I, the direction of membrane
rupturing was selected based on the section shape of the remote and
proximal ends of the IVC obstruction. Membrane rupturing was
successfully coordinated with a guide wire and catheter together (8
cases were from bottom to top and 6 cases were from top to bottom).
A balloon with a diameter of 5–7 mm was inserted through the
blocked section to make a small hole, and a thrombolytic catheter
(5 F) was put into the residual thrombus. Urokinase (100,000 U/q8h)
was injected using a pulse tube, and sodium heparin solution (15
U/kg.h) was continuously injected after urokinase was injected,
during which time coagulation, routine blood tests and liver and
renal function were rechecked. Depending on the results, the dose
was adjusted to limit the activated partial thromboplastin time to
twice the normal value. Every 48 h, the radiography was checked to
observe the thrombolytic effect; the total observation time did not
exceed 10 days. The phlebography was checked to ensure there were
no free thrombi and the IVC thrombolytic catheter was then removed.
If there was residual mural thrombosis on the IVC wall, a stent was
implanted for mechanical compression and changed to a balloon
catheter with a larger caliber (20–30 mm) to fully expand the
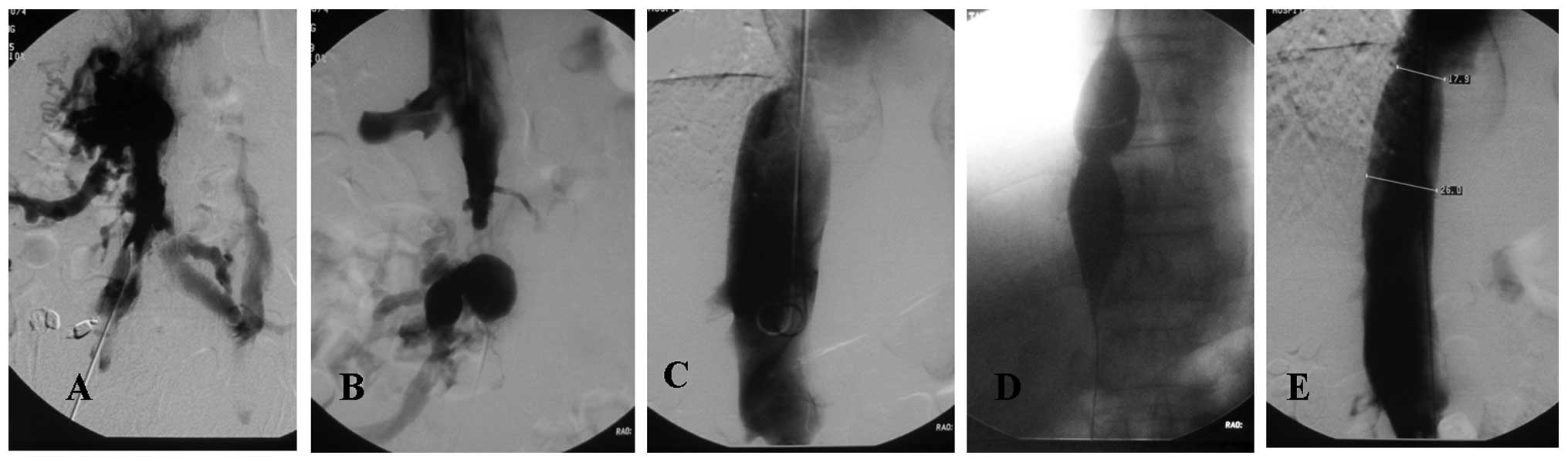
blocked section (Fig. 2). Patients
whose stenosis remained >30% after many expansions, whose IVC
pressure gradient reduced by <40% or who had a floating intimal
flap, were considered for vena cava stent implantation. After
surgery, anticoagulant treatment with oral warfarin was routinely
administered for 3–6 months.
In the patients of type II who were revealed to have
an obsolete thrombus by radiography, the stenotic section of the
IVC at the end proximal to the hepatic vein was accessed from top
to bottom and a balloon catheter with a large caliber (20–30 mm)
was used to fully expand the stenotic section. The long segment of
IVC thrombosis at the remote end of the hepatic vein opening was
not processed (Fig. 3). If the
condition was combined with a lower extremity refractory ulcer, a
cavoatrial shunt was performed. In the patients who were
demonstrated by radiography to have a new thrombus, a catheter of
large caliber (12–16 F) was inserted into the thrombus via the
right femoral vein. Manual mechanical suction was performed to
remove most of the thrombus. Treatment was administered with a
thrombolytic catheter, in a similar manner to that carried out in
patients of type I before the 10 day cut-off. If there was a
residual mural thrombus on the IVC wall, a stent was implanted for
mechanical compression and changed to a balloon catheter with a
larger caliber (20–30 mm) to fully expand the blocked section.
Anticoagulant treatment with oral warfarin was administered
postoperatively for 6–12 months.
For the patients of type III who were revealed to
have a long segment of obsolete thrombosis by radiography and in
whom the thrombosis was difficult to detect using the guide wire, a
mesocaval shunt or cavoatrial shunt was used instead of
endovascular treatment. The type III patients who were identified
as having a newly developed thrombosis were treated as the patients
of type II. In patients with stenosis or occlusion in the short
segment of the hepatic vein opening, a balloon was applied to
expand the lesion and a stent was implanted. If it was difficult to
enter the hepatic vein from the vena cava, percutaneous hepatic
vein puncture was adopted.
Results
The 14 cases of type I were successfully treated.
One case was implanted with a stent due to a visible floating
intimal flap in the lumen following dilation. Five cases that had
residual mural thrombi in the IVC following thrombolysis were stent
implanted for mechanical compression.
The 7 cases of type II with acute thrombosis of the
IVC were successfully treated following thrombolysis, among which 3
cases had IVC residual mural thrombus, and an inner stent was
pre-implanted for mechanical compression; in the 4 cases of type II
with obsolete thrombosis, the stenotic segment of the IVC at the
end proximal to the hepatic vein was catheterized.
Of the 4 cases of type III with acute thrombosis, 3
cases were successfully treated by thrombolysis (in combination
with percutaneous transhepatic puncture in 2 cases) and one case
was changed to conservative treatment due to the effect of
thrombolysis. In the 12 cases with obsolete thrombosis, 2 cases had
an occluded hepatic vein but unobstructed accessory hepatic vein
and were treated by chamber atrial shunt, while 4 cases with an
occluded hepatic vein and accessory hepatic vein but unobstructed
remote end of the IVC were treated by mesocaval shunt, and 6 cases
were received conservative treatment as a result of poor
health.
No pulmonary embolism, pericardial tamponade or
intra-abdominal bleeding occurred in the successfully treated
patients. All patients of type I and those of types II and III with
acute thrombosis, whose symptoms and signs showed significant
improvement following the successful surgery, demonstrated a
reduction of ascites and the easing of lower limb and abdominal
wall superficial veins. Following treatment of the IVC stenosis at
the end proximal to the hepatic vein, ascites notably subsided in
the type II patients with obsolete thrombosis; one case with
intractable ulcers of both lower extremities was given a cavoatrial
shunt, with the ulcer healing one month after surgery. During the
perioperative period there were no mortalities among those who had
a mesocaval or cavoatrial shunt.
Among the 41 cases studied, 38 cases were followed
up for 1–5 years, with an average follow-up of 2.5 years. Three
cases of type I had IVC restenosis and a secondary balloon
dilation. One case of type II had IVC stent restenosis and was
treated by secondary balloon dilation and one case treated with
intestinal bypass surgery was changed to conservative treatment due
to occlusion of the vascular bypass. In the 7 cases treated in a
conservative manner, 2 cases succumbed to upper gastrointestinal
bleeding and 1 case succumbed to liver and kidney failure.
Discussion
Obstruction of the hepatic vein by thrombosis in BCS
is prevalent in the United States and Europe. The majority of cases
have clear underlying causes relating to oral contraception,
pregnancy, polycythemia, abnormal bone marrow histiocytosis,
antiphospholipid syndrome, paroxysmal hemoglobinuria and other
blood diseases. In Asia, membranous obstruction of the IVC is
common, but the pathogenesis is unclear (18). Due to the obstruction of the
proximal IVC, blood flow is slow, turbulent or reversed. This leads
to the formation of a thrombus in these patients together with a
hypercoagulable blood state (19).
BCS has been classified in a variety of ways. Wang
et al classified BCS as three distinct types which have been
accepted by the majority of the medical community: type A is
primarily limited to stenosis or obstruction of the membrane, type
B shows diffuse stenosis or obstruction of the IVC and type C shows
hepatic vein occlusion. Interventional techniques have become the
preferred method of treating BCS of type A and partial B and C
types (1,20). There is no explicit classification
to guide the clinical treatment of BCS with IVC thrombosis. The
current classification is a modification of the classification by
Wang et al. This classification is for BCS with IVC
thrombosis.
On the basis of the Wang classification, 41 BCS
patients with IVC thrombosis were divided into the three types
according to the morphology of the thrombus, whether the thrombus
was fresh or obsolete, and the relationship of the thrombus with
the hepatic vein (Fig. 1). These
may all be easily differentiated by color Doppler ultrasound and
digital subtraction angiography of the IVC. On Doppler ultrasound
scans, fresh thrombi are of low-echo density, whereas obsolete
thrombi are of moderate to partially high-echo density (21).
The treatment principle of BCS is firstly the
removal of the hepatic vein obstruction and the reduction of portal
venous pressure, which is the key to preventing further development
of cirrhosis and hepatic dysfunction, reducing or easing esophageal
varices, avoiding rupture and bleeding of esophageal varices, liver
and kidney failure later on and other problems (22). In accordance with this, various
treatment methods were adopted based on the different types of
patient and good treatment effects were achieved in this study.
Twenty-eight patients (68.3%) were treated successfully with
interventional therapy, 7 (17.1%) were given conservative treatment
and 6 (14.6%) were treated with surgical shunts. Interventional
therapy was used in 29 patients, all those of type I, type II and
type III with acute thrombosis, and was successful in 28 (96.6%).
None of these 28 patients had pulmonary embolism, pericardial
tamponade or intra-abdominal bleeding. Four patients (9.8%) had a
second dilation of IVC in the 1–5 year follow-up time.
The following precautions should be considered
during the therapeutic procedure: i) Predilation with a balloon
catheter of small caliber should be performed prior to the
introduction of the thrombolytic catheter. For patients with BCS
combined with IVC thrombosis, one-time angioplasty may cause the
defluvium of large thromboses, leading to fatal pulmonary embolism.
In the present cases, a guide wire and catheter were used to pass
through the IVC occluded section and predilation was conducted with
a balloon catheter of small caliber (5–7 mm diameter). This
prevented pulmonary embolism caused by the defluvium of large
thromboses, and facilitated the functioning of thrombolytic drugs
by facilitating a through blood flow and preparing for the full
dilation. This scheme is especially applicable for patients of type
I. ii) For fresh and diffuse thrombosis, large mechanical catheter
suction should be applied before the thrombolytic catheter is
introduced. In patients of type II and III with IVC and diffuse
thrombosis, mechanical large catheter suction is rapid and
effective, and is able to remove most thrombi. A 12–14 F large
catheter was used for such cases in our group. Direct manual
lymphosuction and repeated operation is capable of removing most of
the soft tissue of a fresh thrombus. The hemorrhage volume caused
by catheter suction of the thrombus was <200 ml. There was no
acute hemorrhage during and after surgery. The thrombus was sucked
out into a thrombolytic catheter. Small doses of thrombolytic drugs
(100,000 units of urokinase diluted in a 50-ml intravenous
injection, 3 times a day) were then given. It is better to use
pulse injection for thrombolytic drugs to increase the contact area
between the drugs and the thrombus. Following thrombus suction and
thrombolytic therapy, if there is residual mural thrombus, a stent
should be used to apply mechanical compression and avoid its
defluvium. iii) Avoiding the misdiagnosis of type II. For type II
patients with IVC diffuse obsolete thrombosis, it is advisable to
open the IVC at the end proximal to the hepatic vein opening as the
operation is less complex. When performing a color ultrasound
examination of BCS patients with IVC thrombosis, the surgeon and
color ultrasound physicians should pay attention to the IVC near
the opening of the hepatic vein to confirm whether it is type II
and avoid a misdiagnosis of type III. iv) The problems related to
an IVC stent. The clinical significance of maintaining hepatic vein
patency is greater than that of keeping IVC patency. The hepatic
vein occlusion caused by an IVC stent may make future possible
interventions or radical surgery under direct vision more
complicated. The selected stent should, therefore, be as short as
possible for placing mechanical compression on the residual
thrombus. When implanting an internal stent, any affect on the
hepatic vein should be avoided. In patients with an unobstructed
hepatic vein, where IVC occlusion or stenosis remains near the
opening of the hepatic vein or there is residual stenosis even
after balloon dilation, stent implantation should be avoided. If
necessary, dilation may be performed again. v) Color ultrasound
guidance is helpful for passing through the IVC. In cases where
there is difficulty in passing through the IVC occlusion, color
ultrasound guidance may be used together with fluoroscopy. Color
ultrasound is able to show a full view of the pathologic changes
and reveal the thrombi in the IVC and the hepatic vein, making
pathologic changes in the route of the catheter visible. In
addition, the IVC has a certain mobility during aspiration, and the
stent implantation is performed more conveniently and accurately
under the guidance of color ultrasound (23). As a result, in cases where
pathological changes are visible they may be operated on and guided
by color ultrasound. In more complex cases, it is better to combine
the two together; this may increase the success rate and reduce
intra-abdominal hemorrhage, pericardial tamponade and other
complications. In addition, the form of the IVC occluded segment
may also significantly guide the direction of rupture of membranes,
which requires careful observation. The side with conical shape of
the occluded segment is chosen to be punctured. When there are
collateral vessels at the remote end of the occlusion and the
puncture needle mistakenly enters collateral vessels during the
rupture of membranes from bottom to top, the vessel may be ruptured
during the balloon dilation, leading to abdominal hemorrhea.
Consequently, proceeding from top to bottom is a more reliable
method.
There are multiple limitations to this study due to
its retrospective nature, however. The numbers are too small to
draw a conclusion. In future a randomized trial will be performed
in an attempt to verify the classification of the BCS patients with
IVC thrombosis and the therapies indicated according to it.
This study indicates that the classification of BCS
patients with IVC thrombosis is helpful in selecting the best
therapeutic approach. Interventional therapy is the first
therapeutic choice for BCS patients with IVC thrombosis of type I,
type II and type III with acute thrombosis. In patients of type III
with an obsolete thrombus, surgical shunts or conservative
treatment are the main therapeutic methods.
References
|
1
|
Wang ZG, Zhang FJ, Yi MQ and Qiang LX:
Evolution of management for Budd-Chiari syndrome: a team’s view
from 2564 patients. ANZ J Surg. 75:55–63. 2005.
|
|
2
|
Zhang YP, Zu MH and Zhang QQ:
Epidemiological investigation of 1148 patients with Budd-Chiari
syndrome. Chinese Journal of General Surgery. 20:614–617. 2011.
|
|
3
|
Hobbs KE: Budd-Chiari syndrome. Lancet.
339:115–116. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dilawari JB, Bambery P, Chawla Y, et al:
Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with
177 patients and a review of the literature. Medicine (Baltimore).
73:21–36. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu K, Feng B, Zhong H, et al: Clinical
application of interventional techniques in the treatment of
Budd-Chiari syndrome. Chin Med J (Engl). 116:609–615.
2003.PubMed/NCBI
|
|
6
|
Qiao T, Liu CJ, Liu C, Chen K, Zhang XB
and Zu MH: Interventional endovascular treatment for Budd-Chiari
syndrome with long-term follow-up. Swiss Med Wkly. 135:318–326.
2005.PubMed/NCBI
|
|
7
|
Zhang CQ, Fu LN, Xu L, et al: Long-term
effect of stent placement in 115 patients with Budd-Chiari
syndrome. World J Gastroenterol. 9:2587–2591. 2003.PubMed/NCBI
|
|
8
|
Horton JD, San Miguel FL, Membreno F, et
al: Budd-Chiari syndrome: illustrated review of current management.
Liver Int. 28:455–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beckett D and Olliff S: Interventional
radiology in the management of Budd-Chiari syndrome. Cardiovasc
Intervent Radiol. 31:839–847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee BB, Villavicencio L, Kim YW, et al:
Primary Budd-Chiari syndrome: outcome of endovascular management
for suprahepatic venous obstruction. J Vasc Surg. 43:101–108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao T, Liu CJ, Liu C, et al:
Interventional endovascular treatment for Budd-Chiari syndrome with
long-term follow-up. Swiss Med Wkly. 135:318–326. 2005.PubMed/NCBI
|
|
12
|
He XH, Li WT, Peng WJ, Li YD and Tan HQ:
Anticoagulation with warfarin for Budd-Chiari syndrome with chronic
inferior vena cava thrombosis: an initial clinical experience. Ann
Vasc Surg. 25:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo GP, Brodsky RA and Kim HS:
Catheter-directed thrombolysis and thrombectomy for the Budd-Chiari
syndrome in paroxysmal nocturnal hemoglobinuria in three patients.
J Vasc Interv Radiol. 17:383–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishiguchi T, Fukatso H, Itoh S, Shimamoto
K and Sakuma S: Budd-Chiari syndrome with long segmental inferior
vena cava obstruction: treatment with thrombolysis, angioplasty and
intravascular stents. J Vasc Interven Radiol. 3:421–425. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han XW, Ding PX, Li YD, Wu G and Li MH:
Retrieval stent filter: treatment of Budd-Chiari syndrome
complicated with inferior vena cava thrombosis-initial clinical
experience. Ann Thorac Surg. 83:655–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garcia-Pagán JC, Heydtmann M, Raffa S, et
al: TIPS for Budd-Chiari syndrome: long-term results and
prognostics factors in 124 patients. Gastroenterology. 135:808–815.
2008.PubMed/NCBI
|
|
17
|
Ilkgul O, Kilic M, Içöz G, et al:
Experience with mesocaval shunt with autologous jugular vein
interposition in patients with Budd-Chiari syndrome.
Hepatogastroenterology. 52:662–665. 2005.PubMed/NCBI
|
|
18
|
Orloff MJ, Daily PO, Orloff SL, Girard B
and Orloff MS: A 27-year experience with surgical treatment of
Budd-Chiari syndrome. Ann Surg. 232:340–352. 2000.PubMed/NCBI
|
|
19
|
Darwish Murad S, Plessier A,
Hernandez-Guerra M, et al: Etiology, management, and outcome of the
Budd-Chiari syndrome. Ann Intern Med. 151:167–175. 2009.PubMed/NCBI
|
|
20
|
Ding PX, Li YD, Han XW, Wu G, Shui SF and
Wang YL: Budd-Chiari syndrome with fresh inferior vena cava
thrombosis: agitation thrombolysis and balloon dilation. Vasa.
40:57–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZG, Zhang FJ, Li XQ and Meng QY:
Management of Budd-Chiari syndrome: what is the best approach. J
Gastroenterol Hepato. 119:212–218. 2004. View Article : Google Scholar
|
|
22
|
Chaubal N, Dighe M, Hanchate V, Thakkar H,
Deshmukh H and Rathod K: Sonography in Budd-Chiari syndrome. J
Ultrasound Med. 25:373–379. 2006.PubMed/NCBI
|
|
23
|
Meng QY, Sun NF, Wang JX, Wang RH and Liu
ZX: Endovascular treatment of Budd-Chiari syndrome. Chin Med J
(Engl). 124:3289–3292. 2011.PubMed/NCBI
|