Introduction
Prostheses have been used as substitutes for
large-diameter (>6 mm) arteries with satisfactory results since
the 1950s due to the successful development of silk, Dacron and
polytetrafluoroethylene (PTFE) prostheses (1). However, to date, there are no
prostheses that achieve good patency when used to replace
small-diameter (≤6 mm) arteries or veins in vascular surgery.
However, it is possible to achieve improved healing and function of
vascular grafts with a proper seeding technique and an appropriate
cell source (2).
Mature endothelial cells have been shown to
circulate in fresh peripheral blood (3). These cells, detached from the lining
of the cardiovascular tree, may be a source of fallout healing
(4) to endothelialize the flow
surfaces of grafts. Bone marrow contains pluripotent CD34+ cells,
which are known to produce hematopoietic cells. Studies in
vitro have shown that CD34+ cells can differentiate into mature
endothelial cells (5,6). Endothelial cells are known to produce
an antithrombogenic blood-compatible surface; thus, the development
of an endothelial monolayer on the luminal surface of synthetic
vascular grafts is likely to prevent thrombus formation and improve
long-term patency rates.
Prostacyclin (PGI2) exerts its
antithrombotic and vasodilator function and is formed from
arachidonic acid that is present in cellular membranes.
PGI2 is produced by sequential activities of
cyclooxygenases (COX) and PGI2 synthase in healthy
endothelial cells and exerts its function through a paracrine
signaling cascade that involves G-protein-coupled PGI2
receptors on nearby platelets and endothelial cells (7). PGI2 is unstable with a
half-life between 2 and 3 min and is decomposed rapidly to
6-keto-prostaglandin (PG) F1α. Thus, the concentration of
6-keto-PGF1α indirectly reflects the concentration of
PGI2 (8).
In the cardiovascular system, thromboxane (TX)
A2 is predominantly derived from platelet COX-1. The
interaction with the G-protein-coupled TXA2 receptor
elicits not only platelet aggregation and smooth muscle
contraction, but also the expression of adhesion molecules and the
adhesion and infiltration of monocytes/macrophages. TXA2
is in homeostatic balance with PGI2 in the circulatory
system (9). TXA2 is
also unstable with a half-life between 5 and 7 min, and decomposes
rapidly to TXB2. Therefore, the concentration of
TXB2 indirectly reflects the concentration of
TXA2. In this study, we investigatd whether it is
possible to achieve rapid endothelialization in PTFE or Dacron
prostheses by implanting CD34+ cells and the association between
intimal hyperplasia or thrombosis and the concentration of
6-keto-PGF1α and TXB2.
Materials and methods
Animals
A total of 24 healthy young mongrel dogs were
randomly divided into PTFE and Dacron groups. In each group, 8 dogs
were implanted with prostheses that had been seeded with CD34+
cells, while 4 dogs were implanted with prostheses that had been
seeded with autogenous blood only, as a control. The study was
approved by the Animal Care and Use Committee of Shandong
University (Jinan, China).
Bone marrow collection and CD34 cell
isolation
Following intraperitoneal anesthesia with 30 mg/kg
pentobarbital sodium, 15–20 ml bone marrow was aspirated from the
posterior superior iliac spine and mixed with heparin at a dose of
10 IU/ml. The mononuclear cells underwent Ficoll separation
(specific gravity, 1.077) and were then diluted with
phosphate-buffered saline (PBS) to a concentration of
2×107 cells/ml. Cells were incubated with anti-canine
CD34 monoclonal antibodies (BD Biosciences, Franklin Lakes, NJ,
USA) and then CD34+ beads. The cell bead mixture was passed through
the immunomagnetic separation system (immunomagnetic beads;
Miltenyi Biotec, Bergisch Gladbach, Germany) and then washed
several times to remove nonspecifically bound cells. The solution
was released from the magnet and the positively selected CD34+
cells were eluted from the system. The selected cells were assessed
by Trypan blue staining. The CD34 + cells were mixed with 1 ml 1X
phosphate buffer saline (PBS) + 2% horse serum buffer and stained
by CD34-FITC antibody (130-081-001; Miltenyi Biotec) and then Fc
antibody was blocked, finally the CD34+ cells were analyzed by flow
cytometry. Finally, the CD34+ cells were maintained at 4°C
overnight.
Graft preparation
A solution of 0.8 ml plasma, separated from
peripheral venous blood, and 0.1 ml CaCl2 (2.5%) was
injected into the graft lumen to form a layer of fibrin coagulum in
the graft inner wall. The lumen measured 6 mm in diameter and 3 cm
in length. The graft was clipped and rotated for ~5 min to ensure
an adequate spread. The solution was then removed from the lumen
and a mixture of 0.5 ml CD34+ cells, 0.5 ml plasma and 0.1 ml
CaCl2 (2.5%) was injected into the graft lumen with
vascular clamps in place at each end of the graft. The graft was
rotated for ~20 min and maintained in a sterile Petri dish at room
temperature for implantation. Only 1 ml plasma separated from
canine peripheral venous blood was injected into the control grafts
with vascular clamps in place at each end of the graft.
Graft implantation
In total, 24 mongrel dogs underwent surgery with
intraperitoneal anesthesia of 30 mg/kg pentobarbital sodium. A
midline laparotomy incision was performed and the infrarenal
abdominal aorta was isolated by blunt and sharp dissection.
Following proximal clamping below the renal arteries and distal
clamping above the aorto-iliac bifurcation, a 2-cm segment of the
abdominal aorta was resected. The grafts were then implanted with
5-0 Prolene sutures for end-to-end anastomosis. The same approach
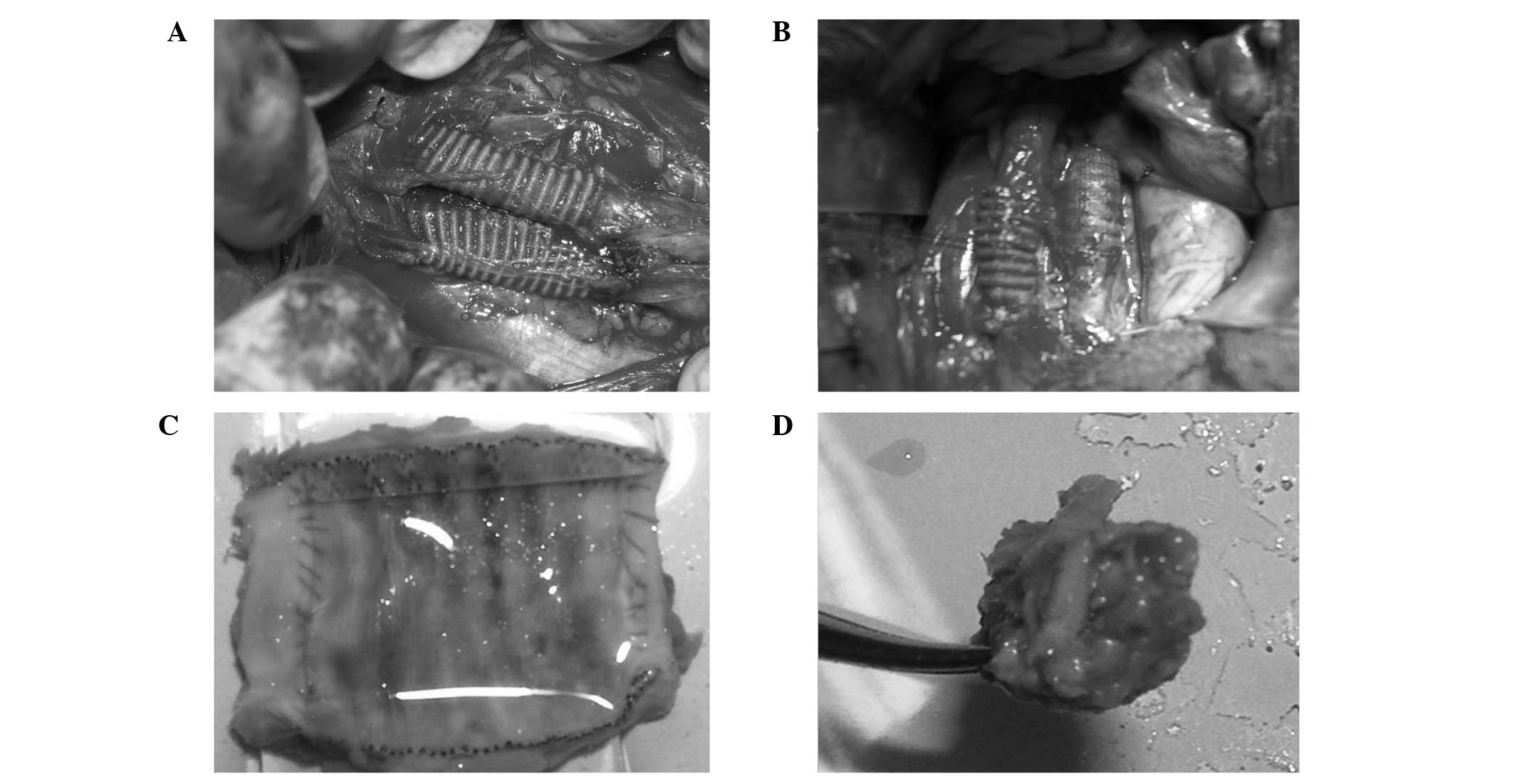
was performed on the inferior vena cava (Fig. 1A). Postoperatively, 100 mg/day
antiplatelet drug (aspirin) was administered for 2 months.
Blood collection and testing
Venous blood samples were collected during surgery
and at days 10, 30 and 60 following surgery. Serum concentrations
of 6-keto-PGF1α and TXB2 were determined by enzyme
immunoassays. Kits for 6-keto-PGF1α (515211.1) and TXB2
(519031.1) were purchased from Cayman Chemical Co. (Ann Arbor, MI,
USA).
Specimen evaluation
At days 10, 30, 60 and 100 following surgery, 2 dogs
from the PTFE experimental group and 2 dogs from the Dacron
experimental group were sacrificed by exsanguination following the
induction of deep anesthesia. At day 60 and 100, 2 dogs from the
PTFE control group and 2 dogs from the Dacron control group were
also sacrificed by exsanguination. The specimens were removed,
opened longitudinally and photographic images were captured. Next,
the tissue samples were evaluated with hematoxylin and eosin
(H&E) staining, immunohistochemical analysis of factor VIII
(FVIII) and CD34 antigen and scanning and transmission electron
microscopy.
H&E staining
The specimens were fixed in 10% formaldehyde
solution and embedded in paraffin. Specimens were stained with
hematoxylin for 5 min and flushed once with PBS. Hydrochloric acid
and ethanol were used for separation and washed once with PBS.
Eosin staining was performed for 3 min followed by PBS flushing.
Finally specimens were dehydrated with alcohol and sealed with
neutral gum seal.
Immunohistochemical detection
Paraffin sections were placed into 3%
H2O2-PBS at room temperature for 15 min, then
washed with PBS three times every 3 min. The microwave antigen
repair was performed as follows: slices were put into the repair of
liquid (citrate buffer) for 10 min at 95°C and 20–30 min at the
room temperature and washed with PBS three times every 3 min, then
blocked by normal sheep serum at wet box of 37°C for 1 h or 4°C
overnight. The sealing fluid was absorbed and primary antibodies
were added at 4°C for the night. PBS wash was performed three times
every 3 min, biotin-labeled secondary antibodies were added and
reacted at 37°C for 30 min to 1 h. PBS wash was performed three
times every 3 min, 35 to 50 μl horseradish peroxidase-labeled
streptavidin was added and reacted at 37°C for 30 min to 1 h. PBS
wash three times every 3 min, DAB was added and color development
was performed without light (PBS 50 ml +20 mg DAB+7 μl 30%
H2O2). Finally, slices were counterstained
with 10% hematoxylin, washed with 1% hydrochloric acid-ethanol,
dehydrated with alcohol and sealed with neutral gum.
Scanning electron microscopy test
Specimens were flushed with heparin saline, fixed by
glutaraldehyde and 1% osmic acid, dehydrated by ethanol, embedded
with epoxy resin, double stained by uranyl acetate-lead citrate and
then photographed with scanning electron microscopy.
Statistical analysis
All experimental parameters were analyzed for
statistical significance using SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). The Student’s t-test or one way analysis
of variance was used to compare the statistical significance of the
differences between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
General observations
The grafts were patent following surgery (Fig. 1A). All the grafts were observed to
adhere tightly to the surrounding tissue. There were no apparent
deformations or signs of infection (Fig. 1B). In the experimental groups, the
longitudinal section at day 10 showed the neointima covering part
of the cavity surface, while at day 30 the neointima completely
covered the graft and there was no thrombosis or stenosis present
(Fig. 1C). Graft stenosis differed
between day 60 and 100 (Table I)
and the stenosis rates from the PTFE and Dacron test groups were
significantly lower compared with those of the respective control
groups at day 60 (P=0.001) and day 100 (P<0.001). All venous
grafts were occlusive (Fig. 1D) in
the control groups at day 100 following surgery. There was no
indication of infarction in the lower limb or other organs in any
of the 24 dogs.
 | Table IGraft stenosis rate following surgery,
%. |
Table I
Graft stenosis rate following surgery,
%.
| Day 60 | Day 100 |
|---|
|
|
|
|---|
| Test | Control | Test | Control |
|---|
|
|
|
|
|
|---|
| Site | PTFE | Dacron | PTFE | Dacron | PTFE | Dacron | PTFE | Dacron |
|---|
| A | 0, 9 | 0, 18 | 36, 48 | 35, 52 | 17, 43 | 12, 64 | 65, 81 | 82, 91 |
| V | 27, 41 | 38, 45 | 67, 83 | 75, 78 | 78, 100 | 87, 100 | 100, 100 | 100, 100 |
Identification of viable CD34+ cells
Cells isolated by the immunomagnetic bead-based
system were identified to be CD34+ cells by flow cytometry
(Fig. 2A). The average number of
viable CD34+ cells isolated in each group was
2.6±0.3×107 cells/ml, as determined by Trypan blue
exclusion prior to seeding.
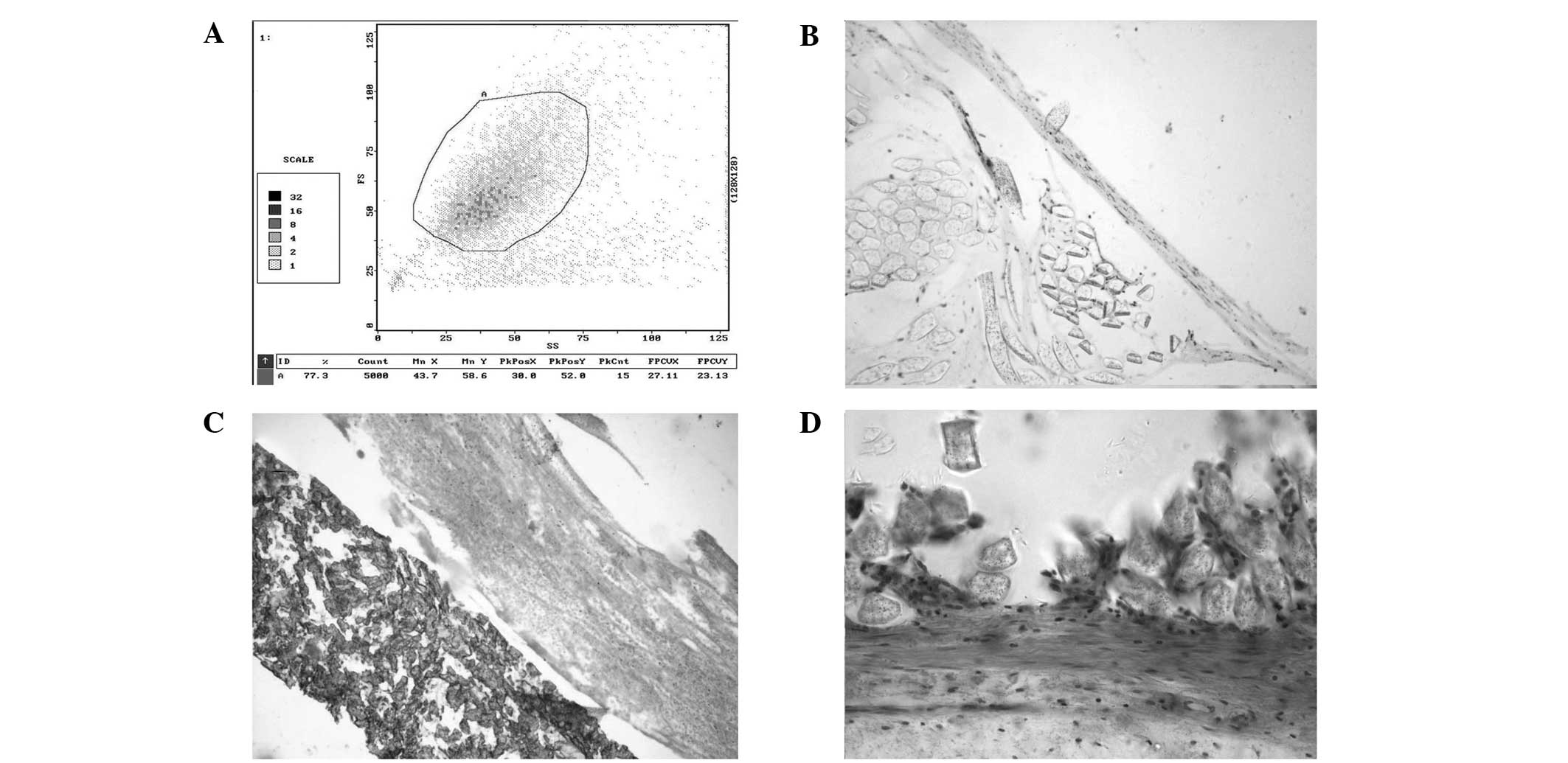 | Figure 2(A) CD34+ cells were identified by
flow cytometry. (B) Immunocytochemical staining with FVIII/vWF and
CD34 antibodies resulted in staining of endothelial cells. (C) With
the PTFE test grafts, there was a layer of neointima consisting of
a single layer of endothelial cells on the surface, shown to be
positive with H&E staining. The subintima was largely composed
of smooth muscle cells, fibroblasts and collagen. (D) With the
Dacron test grafts, the subintima was largely composed of smooth
muscle cells, fibroblasts and collagen. There were no osteocytes,
osteoblasts or microcalcification in the seeded grafts. PTFE,
polytetrafluoroethylene; H&E, hematoxylin and eosin; FVIII,
factor VIII; vWF, von Willebrand factor. |
Identification of endothelial cells
Immunocytochemical staining with FVIII/von
Willebrand factor and CD34 antibodies resulted in staining of
endothelial cells (Fig. 2B). On
the seeded grafts, there was a layer of neointima consisting of a
single layer of endothelial cells on the surface, shown to be
positive with H&E staining. There were varying amounts of
fibrin coagulum with some blood cells (Fig. 2C). The subintima was largely
composed of smooth muscle cells, fibroblasts and collagen. There
were no osteocytes, osteoblasts or microcalcification in the seeded
grafts (Fig. 2D).
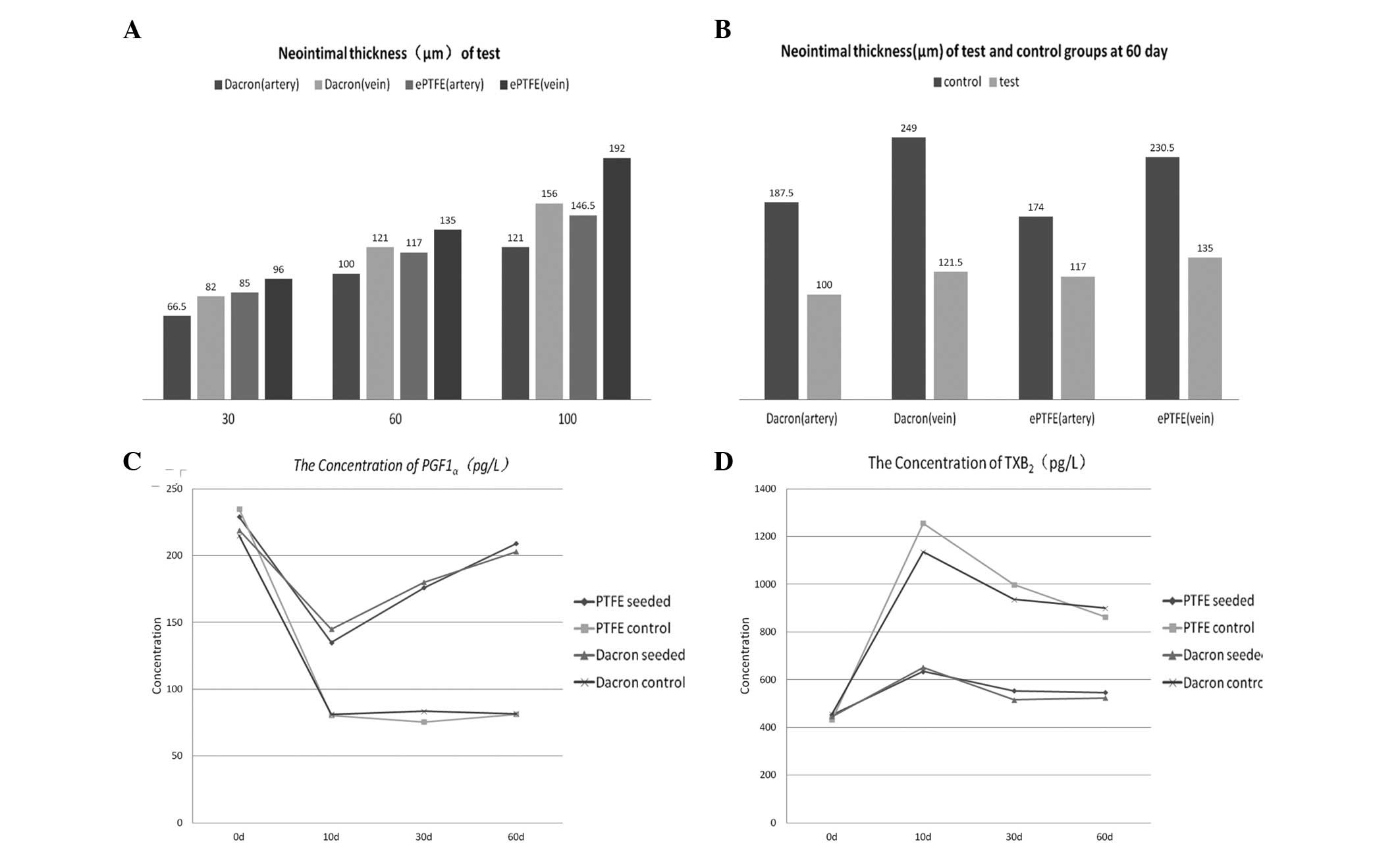
Calculation of neointimal thickness
Endometrial thickness measurements are shown in
Fig. 3A and B. There were
significant differences in neointimal thickness between the
experimental and control groups (PTFE, artery P<0.001 and vein
P<0.001; Dacron, artery P=0.001 and vein P<0.001; Fig. 3B). However, no significant
differences were identified between the two CD34+ cell-seeded
groups (P=0.84; Fig. 3A).
Concentration of 6-keto-PGF1α and
TXB2
Postoperatively, new endothelial cells in the
experimental groups synthesized significantly higher levels of
PGI2 compared with the levels in the control groups
(PTFE, P=0.001; Dacron, P=0.001; Fig.
3C). In the control groups, vascular damage was not repaired as
quickly, the platelet release reaction was enhanced and TX
synthesis was significantly increased. By contrast, in each of the
experimental groups, due to the protective effect of the
endothelial cells, the TXB2 concentration was
significantly lower compared with that in the respective control
group (PTFE, P<0.001; Dacron, P=0.001; Fig. 3D).
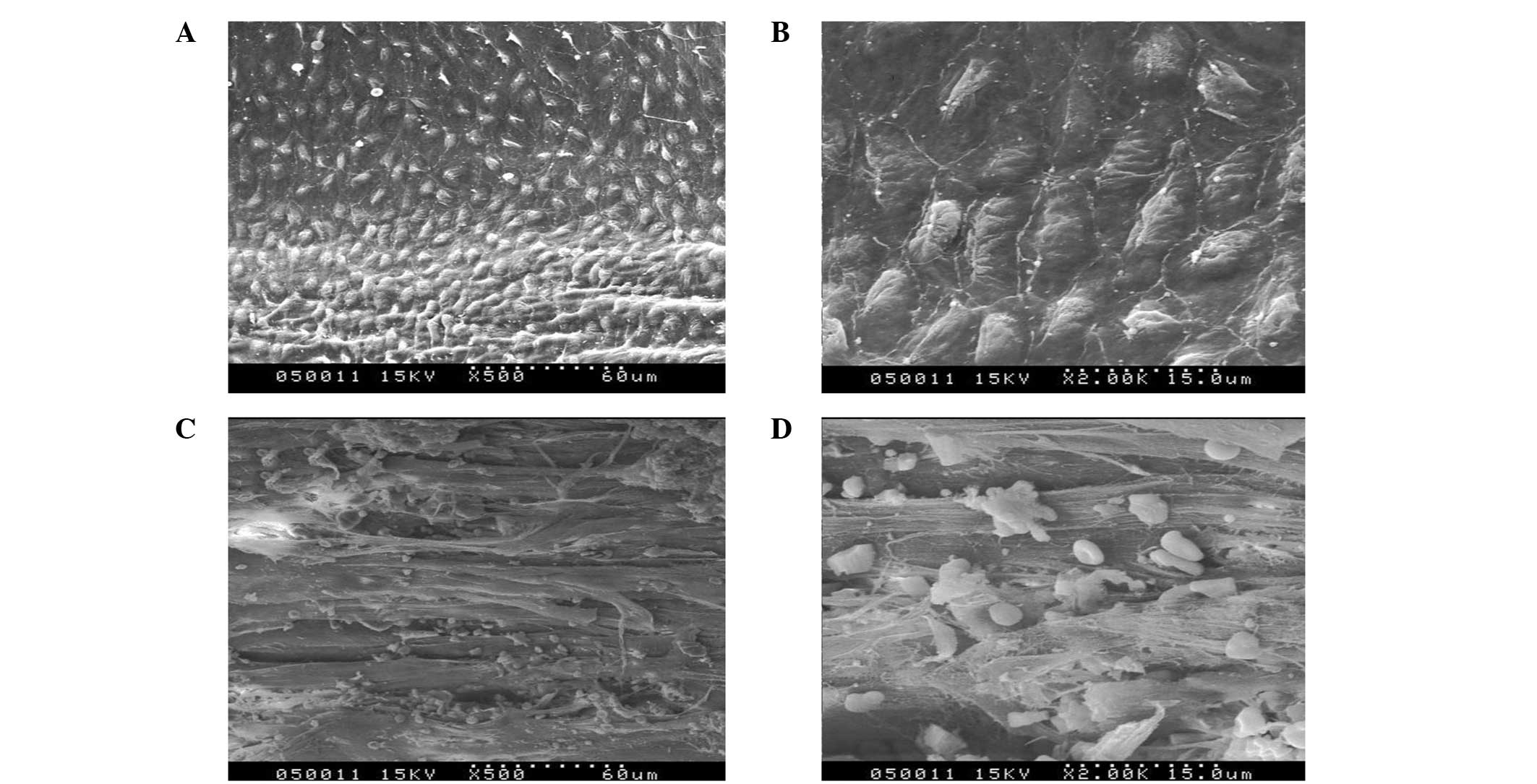
Scanning and transmission electron
microscopy
In the experimental groups at day 10, the density of
the endothelial cells was low and the cells exhibited spindle
morphology. The transformation of specific endothelial cells into
spindles was visible in the images of scanning and transmission
electron microscopy (Fig. 4A and
B). In the experimental groups at day 30, a large number of
endothelial cells appeared at the anastomoses. In addition, the
number of endothelial cells gradually decreased from the ends to
the middle of the graft (Fig. 4C and
D). At day 60, the scanning and transmission electron
microscopy images showed a continuous layer of endothelial cells on
the CD34+ cell-seeded graft surfaces. Additionally, the closer the
anastomoses, the higher the number of endothelial cells (Fig. 5A and B). In the middle of the
grafts, endothelial cells were sparse but almost covered the
surface (Fig. 5C and D). In the
experimental groups at day 100, endothelial cells were arranged
tightly and were more mature (Fig.
6A). The endothelial cells were arranged more closely at the
anastomoses than elsewhere. At high magnification, it was evident
that the cells were spread well with rich pseudopodia (Fig. 6B).
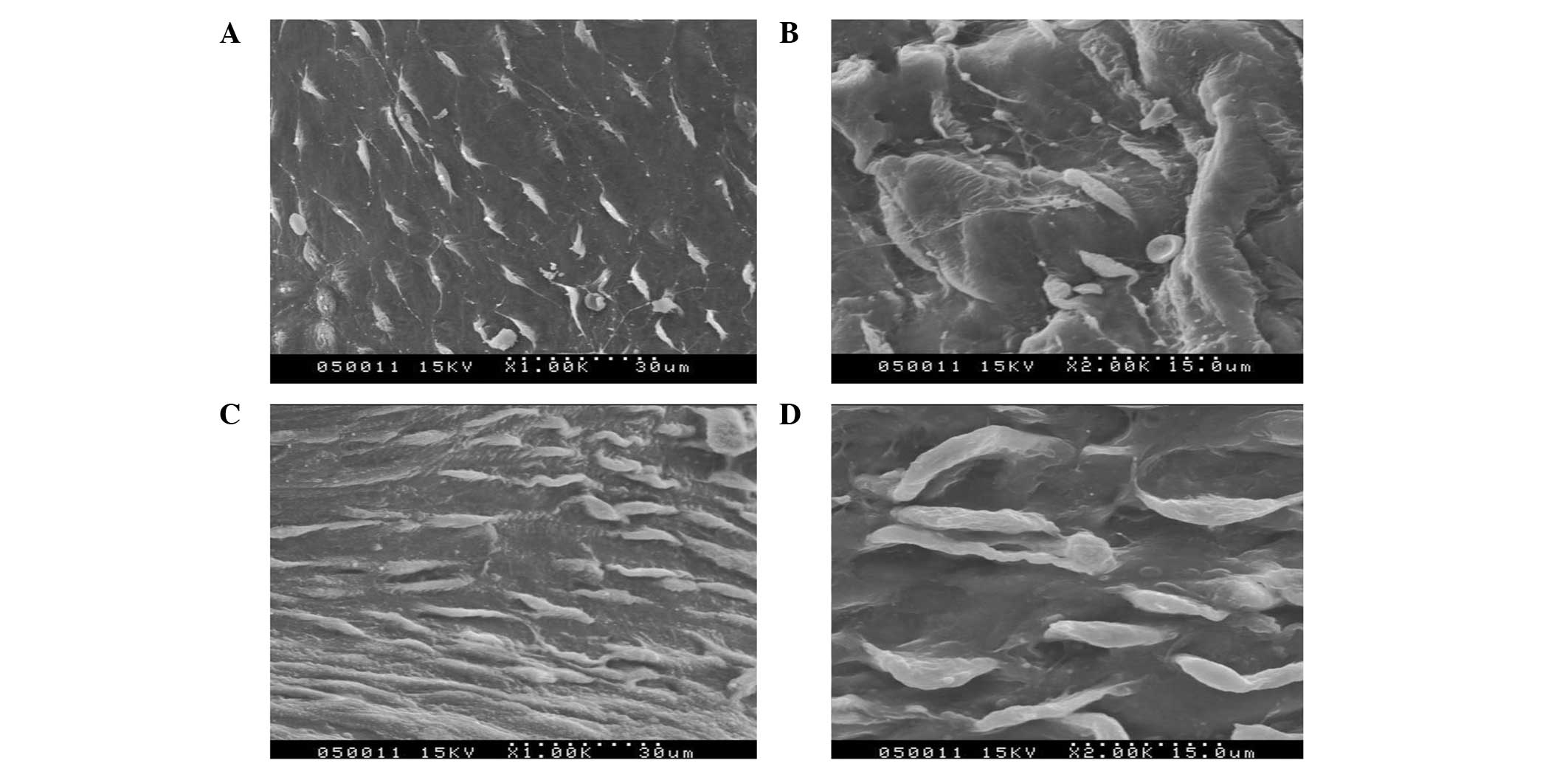 | Figure 4Scanning and transmission electron
microscopy images of the (A) Dacron test group at day 10
(magnification, ×1,000), (B) PTFE test group at day 10
(magnification, ×2,000), (C) Dacron test group at day 30
(magnification, ×1,000) and (D) PTFE test group at day 30
(magnification, ×2,000). PTFE, polytetrafluoroethylene. |
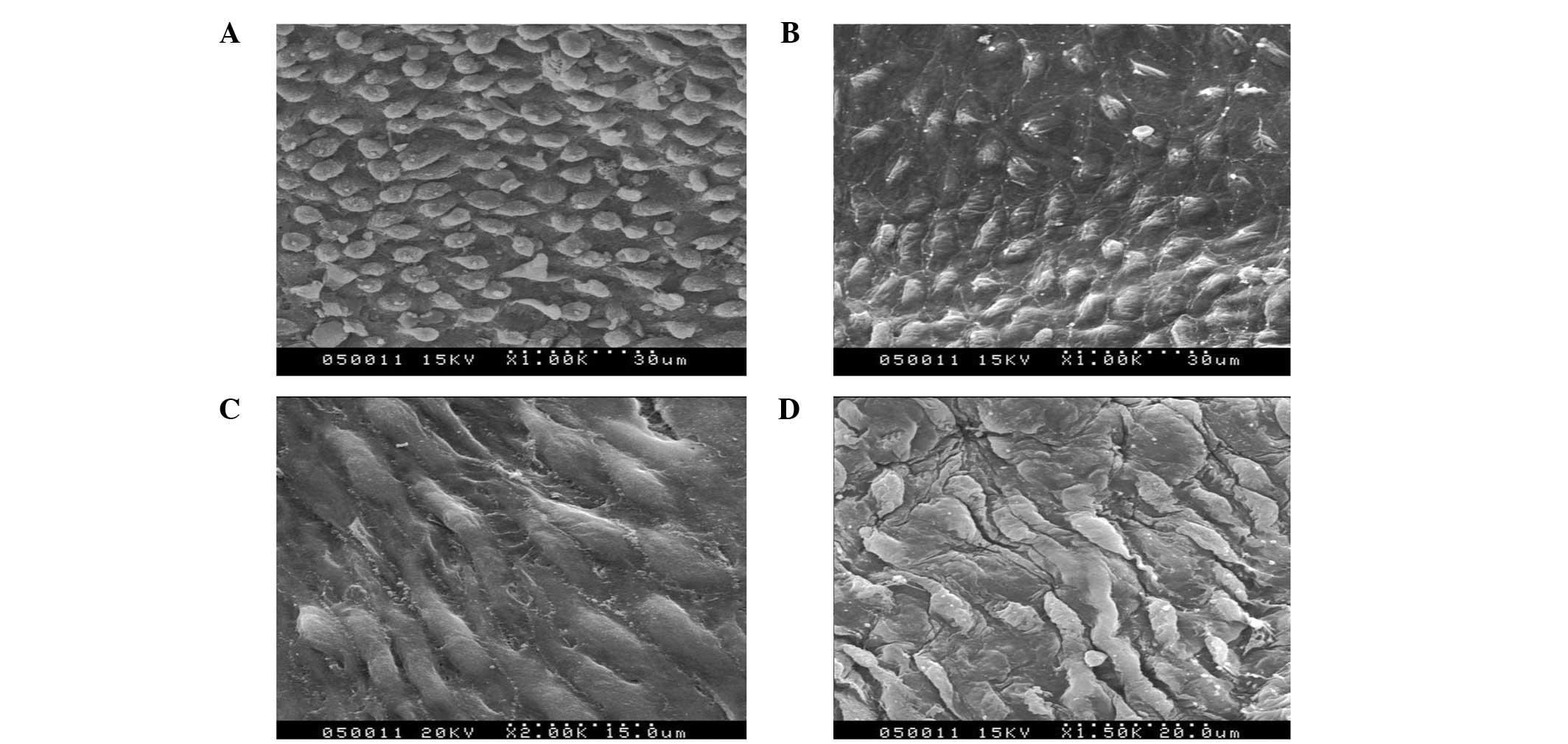 | Figure 5Scanning and transmission electron
microscopy images of the (A) Dacron test group at day 60
(magnification, ×1,000), (B) PTFE test group at day 60
(magnification, ×1,000), (C) Dacron test group at day 60
(magnification, ×2,000) and (D) PTFE test group at day 60
(magnification, ×1,500). PTFE, polytetrafluoroethylene. |
On the control grafts, the majority of the surface
was covered with a film of fibrin material mixed with blood cells.
In addition, a small amount of necrosis and senescence was present
in the endothelial cells at day 60 (Fig. 6C and D).
Discussion
Vessel prostheses have been widely used in vascular
surgery; however, small-caliber artificial blood vessels have not
produced satisfactory results. Bypass graft failure, as a result of
acute thrombosis and intimal hyperplasia, has been the major
challenge for surgical procedures involving small-diameter vascular
prostheses (10). A lack of
endothelial cells in the inner surface of the prostheses is the
main reason for the lower patency rates (11).
In 1984, Civin et al (12) identified CD34+ cells for the first
time. CD34+ cells include pluripotent hematopoietic progenitor
cells and are defined by the expression of their surface antigen,
which is a mucin-like cell surface glycoprotein (13). Previous studies (14,15)
with human peripheral blood and umbilical cord blood support the
derivation of circulating outgrowth endothelial cells from a small
subset of CD34+ cells.
In previous years, endothelialization with CD34+
cells seeded in artificial vasculature has shown enormous
potential. However, the experiments used short experimental times
and in vitro cultures, which may be affected by a number of
factors; thus, the procedure is yet to be applied in clinical
practice (16). Based on these
circumstances, the present study investigated the medium-term
results of CD34+ cell seeding in small-diameter artificial
vessels.
In the present study, endothelialization and
stenosis were investigated in prostheses seeded with CD34+ cells.
Confluent endothelial cells appeared on the neointima of prostheses
seeded with CD34+ cells, as shown by light and electron microscopy.
In addition, there were fewer endothelial cells and a film of
fibrin material mixed with blood cells in the control group.
Therefore, the results indicate that pre-seeding with CD34+ cells
in vascular prostheses may result in rapid endothelialization and
prevent platelet aggregation and thrombus formation
effectively.
Scanning electron microscopy observations revealed
that the PTFE grafts had a less complete neointima compared with
that on the Dacron grafts, as cellulose and blood cells were
attached to the surface. In addition, following H&E staining,
the PTFE neointima was shown to separate partly from the vascular
surface and the cell density was lower compared with that in the
Dacron experimental group. This may be associated with the
structure of the Dacron artificial blood vessels, as the inner
surface is relatively rough compared with that of the PTFE
grafts.
Serum concentrations of 6-keto-PGF1α and
TXB2 following surgery were determined. In the control
groups, the 6-keto-PGF1α concentrations decreased significantly
(P=0.01) and were then maintained at a lower level. By contrast,
the 6-keto-PGF1α concentrations in the experimental groups were
significantly higher compared with those in the control groups.
This may be due to the neovascular endothelial cells synthesizing
more PGI2, which is unstable and decomposes rapidly to
6-keto-PGF1α.
The TXB2 concentration exhibited a marked
increase (P=0.01) that remained at a high level, which indicated
that platelets were activated and synthesizing greater quantities
of TXA2. Higher TXB2 levels were observed in
the control groups than in the experimental groups. This may be due
to: i) the missing intima induced activated platelets to produce
more TXA2. ii) the protective effect of the neointima
inhibited platelet adhesion and activation; and/or iii) high
PGI2 levels may have antagonized the production of
TXB2 in the experimental group.
In the present study, artificial vessel
endothelialization was shown to be viable with CD34+ cell seeding.
In addition, the procedure inhibited excessive reductions in the
concentration of 6-keto-PGF1α and increases in the concentrations
of TXB2. Excessive intimal hyperplasia and thrombosis
were also inhibited. Improved stenosis rates were obtained in the
medium-term, which provides a theoretical basis for the clinical
application of artificial vessel endothelialization.
References
|
1
|
Kidd K, Patula VB and Williams SK:
Accelerated endothelialization of interpositional 1-mm vascular
grafts. J Surg Res. 113:234–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhattacharya V, McSweeney PA, Shi Q, et
al: Enhanced endothelialization and microvessel formation in
polyester grafts seeded with CD34(+) bone marrow cells. Blood.
95:581–585. 2000.PubMed/NCBI
|
|
3
|
Solovey A, Lin Y, Browne P, Choong S,
Wayner E and Hebbel RP: Circulating activated endothelial cells in
sickle cell anemia. N Engl J Med. 337:1584–1590. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Q, Wu MH, Hayashida N, Wechezak AR,
Clowes AW and Sauvage LR: Proof of fallout endothelialization of
impervious Dacron grafts in the aorta and inferior vena cava of the
dog. J Vasc Surg. 20:546–557. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi Q, Rafii S, Wu MH, et al: Evidence for
circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
6
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moncada S, Gryglewski R, Bunting S and
Vane JR: An enzyme isolated from arteries transforms prostaglandin
endoperoxides to an unstable substance that inhibits platelet
aggregation. Nature. 263:663–665. 1976. View Article : Google Scholar
|
|
8
|
Wen SJ, Zhao LM, Wang SG, et al: Human
vascular smooth muscle cells and endothelial cells cocultured on
polyglycolic acid (70/30) scaffold in tissue engineered vascular
graft. Chin Med J (Engl). 120:1331–1335. 2007.PubMed/NCBI
|
|
9
|
Nakahata N: Thromboxane A2:
physiology/pathophysiology, cellular signal transduction and
pharmacology. Pharmacol Ther. 118:18–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ranjan AK, Kumar U and Hardikar AA, Poddar
P, Nair PD and Hardikar AA: Human blood vessel-derived endothelial
progenitors for endothelialization of small diameter vascular
prosthesis. PLoS One. 4:e77182009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CM, Wang ZG, Gu YQ, et al: Preliminary
investigation of seeding mesenchymal stem cells on biodegradable
scaffolds for vascular tissue engineering in vitro. ASAIO J.
55:614–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Civin CI, Strauss LC, Brovall C, et al:
Antigenic analysis of hematopoiesis. III A hematopoietic progenitor
cell surface antigen defined by a monoclonal antibody raised
against KG-la cells. J Immunol. 133:157–165. 1984.
|
|
13
|
Yoder MC: Is endothelium the origin of
endothelial progenitor cells? Arterioscler Thromb Vasc Biol.
30:1094–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Case J, Mead LE, Bessler WK, Prater D,
White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS and
Ingram DA: Human CD34+AC133+VEGFR-2+ cells are not endothelial
progenitor cells but distinct, primitive hematopoietic progenitors.
Exp Hematol. 35:1109–1118. 2007.
|
|
15
|
Timmermans F, Van Hauwermeiren F, De Smedt
M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J and
Vandekerckhove B: Endothelial outgrowth cells are not derived from
CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb
Vasc Biol. 27:1572–1579. 2007.
|
|
16
|
van der Zijpp YJ, Poot AA and Feijen J:
Endothelialization of small-diameter vascular prostheses. Arch
Physiol Biochem. 111:415–427. 2003.PubMed/NCBI
|