Introduction
Heart failure (HF) is a complex syndrome,
characterized by symptoms associated with inadequate perfusion of
organs and tissues during exercise, and represents the common
pathway for the majority of primary cardiovascular diseases
(1). Progressive aging of the
population, together with the significant reduction in the rate of
mortalities due to cardiovascular diseases, have been accompanied
by a marked increase in the prevalence of HF, and HF is now
considered a major public health problem (2).
Patients with myocardial infarction (MI) that has
progressed to a late stage develop chronic HF. Chronic HF with
renal insufficiency, which is known as cardiorenal syndrome (CRS),
has gradually become a focus of drug discovery efforts (3). Congestive HF is a progressive
disorder that leads to intense intrarenal vasoconstriction,
elevated renal vascular resistance, reduced renal blood flow
(4), progressive kidney damage and
renal insufficiency in the later stages (5). The incidence of chronic
cardiovascular disease is increasing and, due to the surgical
treatment of numerous patients in the acute phase of the disease,
the number of patients with progression of the disease to end-stage
has also increased markedly; such patients often present with renal
insufficiency and CRS manifestations (6). The pathogenesis of CRS is complex and
studies have identified that excessive activation of the
renin-angiotensin system (RAS) is an important risk factor
(7). The RAS produces angiotensin
II (Ang II) and participates in damaging renal hemodynamic and
non-hemodynamic effects. The non-hemodynamic effects of the RAS,
including the promotion of oxidative stress, are mainly generated
by Ang II local to an organ (8).
Ang II initiates intracellular oxidative stress through the protein
kinase C pathway, stimulates the NADH/NADPH oxidase system, and
produces large amounts of reactive oxygen species resulting in
kidney tissue damage (9).
The mouse model of MI is useful model for studying
the intrarenal abnormalities in congestive HF (10). An in vitro and in
vivo study has shown that olmesartan medoxomil (OLM) blocks Ang
II-induced physiological activity (11). Thus, the present study used a left
anterior descending artery (LAD) ligation MI model and olmesartan
intervention to observe activation of the RAS of the kidney and the
protective effect of olmesartan against renal injury.
Materials and methods
Animal preparation
Ten-week-old male C57/BL/6 mice were used in the
experiments. The mice were housed under climate-controlled
conditions with a 12-h light/dark cycle and were provided with
standard food and water ad libitum. All experimental
procedures were performed under the guidelines for the care and use
of animals as established by the China Medical University
(Shenyang, China). The mice were divided into three groups: The
sham surgery (SHAM) group, MI group and OLM treatment group. The
surgical protocol was performed similar to methods described
previously (12), Briefly, the
mice were anesthetized by 50 mg/kg phenobarbital sodium, then the
mice were orally intubated with polyethylene tubing and connected
to a rodent ventilator (type 845; Harvard Apparatus Ltd., Kent,
UK). Positive-pressure artificial respiration was started
immediately with room air, using a volume of 1.5 ml/100 g
bodyweight at a rate of 90 strokes/min to maintain the normal
PCO2 (40 mmHg), PO2 (100 mmHg) and pH (7.20)
parameters. The chest was opened by a left thoracotomy, followed by
sectioning of the fourth and fifth ribs. The LAD was visualized and
ligated with 7-0 silk suture. The chest wall was closed and the
mice were allowed to recover in a temperature-controlled area. The
mice were included in the MI group if there was a transmural left
ventricular (LV) scar at autopsy obliterating ≥25% LV muscle. The
mice in the OLM treatment group were fed a daily dose of 10 mg/kg
OLM for eight weeks. Sham surgery group mice underwent the same
surgery minus the coronary artery ligation.
Determination of heart rate (HR),
systolic blood pressure (SBP) and cardiac function
One day prior to sacrifice of the animals, an
intelligent non-invasive blood pressure monitor (Kent Scientific
Corporation, Torrington, CT, USA) was used to measure the SBP and
HR in the awake state. Transthoracic echocardiography was performed
prior to and eight weeks after the surgery using a HP 5500 imaging
system (Hewlett-Packard Co., Palo Alto, CA, USA) equipped with a 15
MHz probe. The M-mode cursor was positioned perpendicular to the
anterior wall in order to measure the interventricular septum, and
the LV end-diastolic and end-systolic diameters (LVDd and LVDs,
respectively) at the level of the papillary muscles below the
mitral valve tip, and the LV ejection fraction (EF) and fractional
shortening (FS) were calculated.
Determination of blood urea nitrogen
(BUN) levels, serum creatinine (SCr) and urinary protein and
albumin concentrations
Following anesthesia, decapitation of the animals
was used, trunk blood was collected in chilled tubes and processed
for measurements of the plasma BUN levels and SCr. The urinary
protein and albumin concentrations were measured using an ELISA kit
(Exocell Inc., Philadelphia, PA, USA) from a one-day timed urine
collection at eight weeks after the surgery.
Determination of plasma and kidney Ang II
levels
Following decapitation, trunk blood was collected
from the mice in chilled tubes and the plasma was separated and
stored. Simultaneously, 50 mg kidney specimens were homogenized in
an ice bath and centrifuged to obtain supernatants. The Ang II
levels in the plasma and renal tissue homogenate were detected with
an radioimmunoassay kit according to the manufacturer’s
instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Determination of the renal pathological
changes by periodic acid-Schiff (PAS) staining
The animals were sacrificed by decapitation,
followed by immediate organ collection for histological analysis.
Fresh kidney sections were fixed in 10% buffered formalin and
embedded in paraffin, and 4-μm sections were stained using the PAS
method. A pathologist blind to the group assignments analyzed the
samples and determined the levels of injury.
Determination of renin, angiotensin II
type 1 receptor (AT1R) and angiotensinogen (AGT) expression levels
in the kidney tissues by quantitative reverse
transcription-polymerase chain reaction (PCR)
The mRNA expression levels of
glyceralde-hyde-3-phosphate dehydrogenase (GAPDH), renin, AT1R and
AGT were analyzed by quantitative PCR using a Light Cycler Fast
Start DNA Master SYBR Green I kit (Applied Biosystems, Foster City,
CA, USA) in ABI PRISM 7900T real-time PCR system (Applied
Biosystems). The primer sequences are shown in Table I. For the PCR, the conditions were
as follows: 30 sec at 94°C; 40 cycles at 94°C for 5 sec, 55°C for 5
sec, and 72°C for 15 sec. All data are expressed as the relative
differences following normalization to the GAPDH expression
levels.
 | Table IOligonucleotide primer sequences. |
Table I
Oligonucleotide primer sequences.
| mRNA | 5′ primer | 3′ primer |
|---|
| Renin |
GAGGCCTTCCTTGACCAATC |
TGTGAATCCCACAAGCAAGG |
| AT1R |
GGAAACAGCTTGGTGGTGATC |
CTGAGACACGTGAGCAGGAAC |
| AGT |
CACCCCTGCTACAGTCCATTG |
GTCTGTACTGACCCCCTCCAG |
| GAPDH | ATCA TCA GCAA
TGCCTCCTG |
CCCTCCGACGCCTGCTT |
Statistical analysis
Data are expressed as mean ± standard error of the
mean of the number of animals. P<0.05 was considered to indicate
a statistically significant difference. Multiple comparisons
between the experimental groups were performed by one-way analysis
of variance with Tukey’s post hoc test.
Results
General state and cardiac function
changes in each group
Eight weeks after the surgery, all animals in the
SHAM group were alive and generally in good condition; in MI group,
three mice had died due to severe HF and the remaining mice
exhibited varying degrees of shortness of breath, wheezing, and
eating and activity reduction; and the mice in the OLM group also
showed slightly reduced eating and activity. The mice in the MI and
OLM groups exhibited anatomical visible LV chamber and liver
enlargement, lung congestion, pleural effusion and ascites. The
transthoracic echocardiography results showed that the cardiac
infarction lead to LV enlargement and systolic dysfunction when
compared with the results of the sham surgery mice (P<0.05).
When compared with those of the MI mice, the mice in the OLM group
had less LV enlargement (measured as the LVDd and LVDs) and less LV
systolic dysfunction (evaluated using the EF and FS). The MI group
also exhibited increased SBP compared with that of the SHAM group,
while in the OLM group the SBP decreased compared with that in the
MI group. These results are shown in Table II.
 | Table IIHR, SBP and echocardiography
parameters of the mice (mean ± SEM; n=l0 per group). |
Table II
HR, SBP and echocardiography
parameters of the mice (mean ± SEM; n=l0 per group).
| Group | HR (beats/min) | SBP (mmHg) | IVS (mm) | LVDd (mm) | LVDs (mm) | FS (%) | EF (%) |
|---|
| SHAM | 602.5±11.3 | 100.3±1.7 | 0.95±0.02 | 3.29±0.42 | 1.68±0.31 | 42.31±5.09 | 88.91±6.42 |
| MI | 598.7±12.7 | 119.1±2.3a | 0.92±0.01 | 4.23±0.55a | 3.59±0.52a | 19.65±3.42a | 59.16±4.73a |
| OLM | 603.2±13.5 | 101.9±2.2b | 0.91±0.01 | 4.01±0.48a | 2.89±0.58a | 29.69±3.41a | 66.75±5.01a |
Renal injury and renal insufficiency in
each group
In this study, the serum BUN levels, SCr, and
urinary protein and albumin concentrations were examined and the
results are summarized in Table
III. Cardiac infarction lead to slightly increased serum BUN
levels, SCr, and urinary protein and albumin concentrations
compared with those of the SHAM group (P>0.05), and these
increases were slightly attenuated in the OLM group (P>0.05),
but no statistical significance was identified.
 | Table IIISerum BUN levels, SCr, urinary protein
and albumin excretion concentration, and plasma and kidney Ang II
levels (mean ± SEM; n=l0 per group). |
Table III
Serum BUN levels, SCr, urinary protein
and albumin excretion concentration, and plasma and kidney Ang II
levels (mean ± SEM; n=l0 per group).
| Group | BUN (mmol/l) | SCr (μmol/l) | Urinary protein
(mg/24 h) | Urinary albumin
(μg/24 h) | Plasma Ang II
(fmol/ml) | Kidney Ang II
(fmol/g) |
|---|
| SHAM | 18.4±4.9 | 49.6±6.8 | 25.5±0.7 | 17.7±0.9 | 64.3±20.1 | 27.4±11.9 |
| MI | 20.9±4.4 | 54.0±8.7 | 31.4±0.9 | 20.3±5.4 | 98.2±19.2a | 171.3±19.1a |
| OLM | 19.7±8.2 | 50.6±9.3 | 28.3±0.6 | 18.6±3.7 | 218.6±36.6b | 98.7±11.5b |
Plasma and kidney Ang II levels in each
group
MI induced increased Ang II levels in the plasma and
kidney tissues compared with those in the SHAM group (P<0.05).
However, compared with those of the MI group, the renal Ang II
levels were significantly reduced, while the plasma levels were
significantly increased in the OLM group (P<0.05). The results
are shown in Table III.
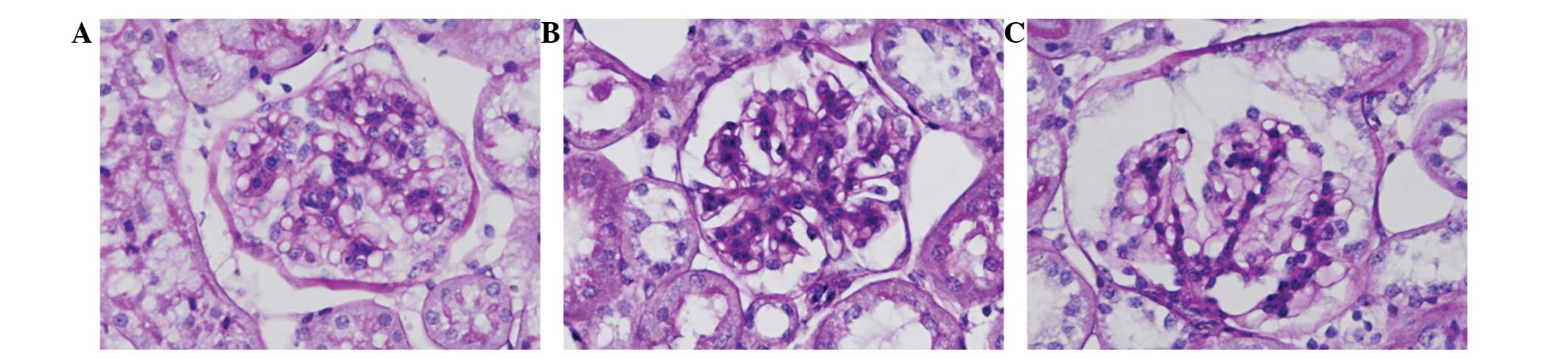
Renal pathological changes shown by PAS
staining
In the SHAM group, the PAS staining indicated normal
glomerular and tubular structures, renal tubular epithelial cells
arranged in neat rows and no inflammatory cell infiltration in the
stroma. By contrast, in the MI group, glomerular mesangial and
tubular lumen expansion and irregular thickening of the basement
membrane were observed, and the renal interstitial PAS positive
staining was markedly increased compared with that in the SHAM
group. These changes were clearly reduced in the OLM group. The
results are shown in Fig. 1.
Intrarenal RAS activation during MI
The quantitative PCR results showed that the
expression levels of renin, AT1R and AGT were increased in the MI
group compared with those in the SHAM group (P<0.05). However,
compared with those of the MI group, the levels were significantly
reduced in the OLM group (P<0.05). The results are shown in
Table IV.
 | Table IVExpression levels of renin, AT1R and
AGT in the kidney tissue, detected by quantitative PCR (mean ± SEM;
n=l0 per group). |
Table IV
Expression levels of renin, AT1R and
AGT in the kidney tissue, detected by quantitative PCR (mean ± SEM;
n=l0 per group).
| Group | Renin | AT1R | AGT |
|---|
| SHAM | 0.20±0.02 | 1.55±0.07 | 1.00±0.10 |
| MI | 1.31±0.07a | 2.21±0.10a | 1.74±0.07a |
| OLM | 0.56±0.07b | 1.84±0.07b | 1.36±0.09b |
Discussion
Reduced cardiac mass following MI commonly leads to
congestive HF in humans and experimental animal models (12). In patients with congestive HF,
renal perfusion and function are often compromised, which is known
as CRS (13,14). Cardiac and renal dysfunctions often
occur simultaneously as they share causes and pathogenetic
mechanisms. There is a close association between renal and cardiac
function in acute and chronic diseases. Cardiovascular disease
causes >50% of the mortalities of patients with renal failure,
while poor renal function increases mortality in patients with HF
(15). Traditionally, renal
impairment has been attributed to renal hypoperfusion due to
reduced cardiac output and systemic pressure. The hypovolemia leads
to sympathetic activity, increased renin-angiotensin-aldosterone
pathway activity and arginine-vasopressin release. In addition, as
well as ischemia, inflammation and oxidative stress are considered
the main determinants of cardiac and renal dysfunction (16).
During HF, renal perfusion reduction and RAS
activation lead to progressive decline of renal function (17). An increasing number of studies have
demonstrated that local RAS activation and the production of Ang II
mediate numerous important roles, including cell growth, the
long-term effects of cardiac and vascular hypertrophy, ventricular
and vascular remodeling and even renal fibrosis (18,19).
Existing studies have verified that the effects of Ang II
associated with excessive activation of the kidney RAS include the
generation of oxygen free radicals, the activation of NF-κB in
fibroblast proliferation, inflammation and exaggerated
extracellular matrix deposition (20,21,22).
Excessive production of Ang II also affects glomerular mesangial
cells and podocytes, leading to glomerulosclerosis and renal
dysfunction (23).
The model of coronary ligation in mice was selected
as a previous study has documented the typical hemodynamic changes
of MI in this model (24). In the
present study, the LAD of mice was occluded for eight weeks, and
the mice exhibited significant LV enlargement and systolic
dysfunction of the heart, accompanied by renal dysfunction which
presented as slightly increased plasma BUN levels, SCr, and urinary
protein and albumin concentrations with no statistical
significance. The PAS staining results identified
glomerulosclerosis accompanied by intrarenal RAS activation, which
manifested as increased levels of Ang II in the plasma and kidney,
and increased levels of renal renin, AT1R and AGT expression, which
was consistent with the results of previous study (25).
There are four subtypes of Ang II receptor, but the
physiological effect is usually mediated by the AT1R. Studies have
shown that the AT1R is widely distributed in various cells of
kidney tissue. The AT1R is usually combined with Ang II and results
in renal vasoconstriction, reduced renal blood flow and increased
glomerular pressure and mesangial extracellular matrix production,
and simultaneously stimulates the production of certain growth
factors (26). Angiotensin II type
2 receptors (AT2Rs) are mainly distributed in the glomerular
afferent arterioles and mesangial cells, which usually dilate
afferent arterioles, and inhibit the growth of mesangial cells and
stimulate apoptosis. AT2Rs in the kidney stimulate the generation
of NO, so these two types of receptor have opposite physiological
roles (27). OLM is a prodrug,
which is absorbed via the gastrointestinal tract and hydrolyzed to
form olmesartan. Olmesartan is a selective AT1R antagonist, which
antagonizes the effects of Ang II by selectively blocking the
binding site at which Ang II and AT1R combine. The results of the
present study showed that OLM combined with AT1R substantially
suppressed excessive activation of intrarenal RAS, thereby blocking
Ang II damage to the kidneys. The study also demonstrated that OLM
significantly increased the plasma Ang II levels, which may be
combined with AT2R in the circulation and play a protective
role.
In conclusion, MI activates the intrarenal RAS and
leads to glomerulosclerosis, and OLM protects the kidneys by
inhibiting the effects of Ang II.
Acknowledgements
This study was supported in part by the grants from
the National Natural Science Foundation of China (no.
81100109).
References
|
1
|
Wang L, Shi J and Zhang Y: Influences of
simvastatin on vascular endothelial function of patients with
coronary heart disease complicated with congestive heart failure.
Eur Rev Med Pharmacol Sci. 17:1590–1593. 2013.PubMed/NCBI
|
|
2
|
Hayashi H, Fukuma N, Kato K, Kato Y,
Takahashi H and Mizuno K: Clinical backgrounds and the time course
of sleep-disordered breathing in patients after myocardial
infarction. J Nippon Med Sch. 80:192–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhini C, Molino C and Fabbian F:
Cardiorenal syndrome: still not a defined entity. Clin Exp Nephrol.
14:12–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ronco C, Kaushik M, Valle R, Aspromonte N
and Peacock WF 4th: Diagnosis and management of fluid overload in
heart failure and cardio-renal syndrome: the ‘5B’ approach. Semin
Nephrol. 32:129–141. 2012.
|
|
5
|
Ronco C: Cardiorenal syndromes: definition
and classification. Contrib Nephrol. 164:33–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shchekochikhin D, Schrier RW and
Lindenfeld J: Cardiorenal syndrome: pathophysiology and treatment.
Curr Cardiol Rep. 15:3802013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weir MR: Effects of renin-angiotensin
system inhibition on end-organ protection: can we do better? Clin
Ther. 29:1803–1824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bader M and Ganten D: Update on tissue
renin-angiotensin systems. J Mol Med (Berl). 86:615–621. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plumb RD, El-Sherbeeny NA, Dixon LJ, et
al: NAD(P)H-dependent superoxide production in platelets: the role
of angiotensin II and protein kinase C. Clin Biochem. 38:607–613.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parlakpinar H, Ozer MK, Cicek E, Cigremis
Y, Vardi N and Acet A: Renal damage in rats induced by myocardial
ischemia/reperfusion: Role of nitric oxide. Int J Urol.
13:1327–1332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koike H, Sada T and Mizuno M: In vitro and
in vivo pharmacology of olmesartan medoxomil, an angiotensin II
type AT1 receptor antagonist. J Hypertens Suppl. 19:S3–S14. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venugopal J, Rajeswari R, Shayanti M,
Sridhar R, Sundarrajan S, Balamurugan R and Ramakrishna S: Xylan
polysaccharides fabricated into nanofibrous substrate for
myocardial infarction. Mater Sci Eng C Mater Biol Appl.
33:1325–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ronco C, McCullough P, Anker SD, et al;
Acute Dialysis Quality Initiative (ADQI) consensus group.
Cardio-renal syndromes: report from the consensus conference of the
acute dialysis quality initiative. Eur Heart J. 31:703–711. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shah BN and Greaves K: The cardiorenal
syndrome: a review. Int J Nephrol. 2011:9201952010.PubMed/NCBI
|
|
15
|
Coresh J, Astor BC, Greene T, Eknoyan G
and Levey AS: Prevalence of chronic kidney disease and decreased
kidney function in the adult US population: Third National Health
and Nutrition Examination Survey. Am J Kidney Dis. 41:1–12. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ritz E: Heart and kidney: fatal twins? Am
J Med. 119(5 Suppl 1): S31–S39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raizada V, Skipper B, Luo W and Griffith
J: Intracardiac and intrarenal renin-angiotensin systems:
mechanisms of cardiovascular and renal effects. J Investig Med.
55:341–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Souza ÁP, Sobrinho DB, Almeida JF, et al:
Angiotensin II type 1 receptor blockade restores
angiotensin-(1–7)-induced coronary vasodilation in hypertrophic rat
hearts. Clin Sci (Lond). 125:449–459. 2013.
|
|
19
|
Shao W, Seth DM, Prieto MC, Kobori H and
Navar LG: Activation of the renin-angiotensin system by a low-salt
diet does not augment intratubular angiotensinogen and angiotensin
II in rats. Am J Physiol Renal Physiol. 304:F505–F514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishiyama A: Mechanisms responsible for
the renoprotective effects of renin-angiotensin inhibitors.
Yakugaku Zasshi. 132:455–459. 2012.(In Japanese).
|
|
21
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panda SS1, Bajpai M, Sinha A, Mallick S
and Sharma MC: Effect of ipsilateral ureteric obstruction on
contralateral kidney and role of renin angiotensin system blockade
on renal recovery in experimentally induced unilateral ureteric
obstruction. J Indian Assoc Pediatr Surg. 18:58–61. 2013.
View Article : Google Scholar
|
|
23
|
Wolf G: Renal injury due to
renin-angiotensin-aldosterone system activation of the transforming
growth factor-beta pathway. Kidney Int. 70:1914–1919.
2006.PubMed/NCBI
|
|
24
|
Maki T, Nasa Y, Tanonaka K, Takahashi M
and Takeo S: Beneficial effects of sampatrilat, a novel
vasopeptidase inhibitor, on cardiac remodeling and function of rats
with chronic heart failure following left coronary artery ligation.
J Pharmacol Exp Ther. 305:97–105. 2003. View Article : Google Scholar
|
|
25
|
Wen ZZ, Cai MY, Mai Z, et al: Angiotensin
II receptor blocker attenuates intrarenal renin-angiotensin-system
and podocyte injury in rats with myocardial infarction. PLoS One.
8:e672422013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Volpe M, Savoia C, De Paolis P, et al: The
renin-angiotensin system as a risk factor and therapeutic target
for cardiovascular and renal disease. J Am Soc Nephrol. 13(Suppl
3): S173–S178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berk BC: Angiotensin type 2 receptor
(AT2R): a challenging twin. Sci STKE. 181:PE162003.PubMed/NCBI
|