Introduction
The traditional Chinese herbal medicine Rhizoma
Pinelliae (RP) is the tuber of Pinellia ternata (Thunb.)
Breit., and is listed in the Dictionary of Traditional Chinese
Medicine (1) as effective in
removing dampness to reduce phlegm, reducing adverse qi for
controlling nausea and vomiting, and relieving distension to
eliminate stagnation, among other effects (2–4). RP
is used alone or in combination with other Chinese medicines to
treat various neoplastic diseases. This plant is also used as folk
medicine for treating cancer in several regions of China (5–8).
There have been a number of studies on the antitumour activity of
Pinellia, such as the inhibitory effects of ethanolic
Pinellia extract on liver cancer cells, of total organic
acids of Pinellia on gastric cancer cells and of
Pinellia protein on ovarian cancer cells (8–12).
However, to the best of our knowledge, the antitumour activity of
low molecular weight components of Pinellia has not been
reported. Trypsin inhibitors (TIs), low molecular weight proteins
that inhibit various serine proteinases, are widely present in
animals, plants and microorganisms. TIs inhibit the catalytic
activity of enzymes or prevent zymogen activation through
combination with the active and allosteric site of the proteinase.
TIs are crucial in regulating physiological and pathological
processes and are an important component of the immune system. TIs
have extensive application prospects in the research and
development of antitumour drugs (13–18).
Studies have demonstrated that TI receptors are present in numerous
types of tumour cell, and TIs exert their antitumour function by
binding with the receptors and regulating the activity of related
proteinases (19–26). In the present study, we isolated a
small water-soluble protein from Pinellia ternata,
investigated its trypsin inhibitory activity and considered the
association of antitrypsin activity with antitumour activity. The
present study aimed to isolate and purify short-chain peptides with
serine proteinase inhibitory activity from the small water-soluble
protein components of raw RP, and to study their physicochemical
properties and their ability to inhibit the proliferation of poorly
differentiated BGC-823 human gastric adenocarcinoma cells in
vivo and in vitro.
Materials and methods
Animals
Specific pathogen-free BALB/c nude mice were
provided by the Experimental Animal Centre at Shandong University
of Traditional Chinese Medicine (Jinan, China). This study was
carried out in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (2010, Eighth edition). The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Shandong University (Jinan, China).
Extraction and separation of RPTI
Fresh Pinellia ternata tuber (100 g; ChengWu
pinellia planting base, Heze, China) was washed, homogenised in
10-fold its volume of buffer solution for extraction (0.05 mol/l,
pH 8.0, Tris HCl) and centrifuged (4,650 × g, 10 min). After
rinsing off the floating solid fatty materials, the supernatant was
collected and then lyophilised using a lyophiliser (FD-1D-50;
Shanghai Bilon Instrument Co., Ltd., Shanghai, China) to obtain the
lyophilised crude RP protein powder. The powder was weighed and its
TI activity was detected.
The lyophilised powder was redissolved in an
appropriate amount of buffer solution for extraction using the
stepwise salting-out method.
(NH4)2SO4 with 40, 60 and 80%
degrees of saturation was gradually added to the solution, which
was left to stand at 4°C for 2 h and then centrifuged (8,000 rpm,
10 min). The precipitate from all steps was collected and dialysed
with distilled water at 4°C for 36 h; during the dialysis, the
dialysate was replaced nine times and the molecular weight of the
proteins retained by the dialysis bag was ≥6,000 Da (Pharmacia
Biotech). After centrifuging (8,000 rpm, 10 min), the supernatant
was lyophilised and weighed. The TI activity was detected and the
dialysed and lyophilised (NH4)2SO4
precipitate with the highest activity was designated as the crude
RPTI product.
Protein content determination
The protein content was determined using the Lowry
protein assay method with bovine serum albumin (Sigma-Aldrich, St.
Louis, MO, USA) as the standard (27).
Preparation of the affinity carrier
CNBr-activated Sepharose CL-4B (Pharmacia Biotech,
Stockholm, Sweden) was coupled with an appropriate amount of
trypsin to prepare the affinity carrier based on the manufacturer’s
instructions (2010).
RPTI purification
The crude RPTI product (20 mg) was dissolved in 200
ml balanced buffer solution (0.05 mol/l, pH 8.0, Tris HCl) and
centrifuged (4°C, 10,464 × g, 10 min). Following maintenance of the
supernatant in the affinity column at 37°C for 1 h, the column was
washed with balanced buffer solution containing 1 mol/l NaCl,
distilled water and hydrochloric acid solution at pH 2.4. The
eluent of the acid solution was collected and was immediately
neutralised dropwise with 2.0 mol/l Tris. Following repeated sample
loading, the eluent was combined and directly filtered with a
Sephadex G-50 chromatographic column (Pharmacia Biotech) and with
0.05 mol/l Tris HCl buffer solution (pH 8.0) as the eluent. The
trypsin inhibition activity peak was determined and the protein
purity of the activity peak in each tube was detected with
SDS-PAGE. The SDS-PAGE index was based on the literature (28). The gel concentration was 12% and
Coomassie Brilliant Blue R-250 was used for staining.
RPTI sequencing
The N-terminal amino acid sequence of RPTI was
performed by Kang Biotechnology Compnay of Shanghai (Shanghai,
China) using the Edman degradation method with an ABI 491A amino
acid sequencer (Applied Biosystems, Foster City, CA, USA) (29).
Determination of TI activity
Based on the literature (30), trypsin activity (Sigma-Aldrich) and
TI activity were detected using BAPNA (Sigma-Aldrich) as the
substrate. The sample to be tested was dissolved in 0.80 ml Tris
HCl buffer solution (0.05 mol/l, pH 8.0), 0.20 ml bovine trypsin
(0.10 mg/ml) was added and the system was maintained at 37°C for 5
min. Subsequently, 2.50 ml BAPNA solution (1 mmol/l) was added, the
system was maintained at 37°C for 5 min and then 0.5 ml 33% acetic
acid was immediately added to terminate the reaction. The
absorbance (A) was detected at 410 nm, with the test sample
without inhibitor as the control sample. One unit of enzyme
activity was defined as the amount of enzyme required to increase
A410 by 0.01, whereas one unit of RPTI-inhibitory activity was
defined as the amount of enzyme required to decrease A410 by
0.01.
Cell culture
Dulbecco’s modified Eagle’s medium (DMEM)
(Gibco-BRL, Carlsbad, CA, USA) was formulated based on the product
description, adjusted to pH 7.2–7.4 with 0.1 mol/l hydrochloric
acid and maintained at 4°C. The foetal bovine serum was deactivated
at 56°C for 30 min and then maintained at −20°C. Trypsin was
diluted to a 2.5 g/l solution with 0.01 mol/l phosphate-buffered
saline (PBS) at pH 7.4 and then maintained at 4°C. The poorly
differentiated BGC-823 human gastric adenocarcinoma cells (Chinese
medicine biotechnology laboratory of Shandong Traditional Chinese
Medicine University) were incubated in DMEM containing 10% refined
calf serum (Gibco-BRL), 100 U/ml penicillin and 100 μg/ml
streptomycin under a 5% CO2 atmosphere at 37°C and were
subcultured following trypsinisation when the BGC-823 cells adhered
to the walls of the flask.
MTT method
Based on the literature (31), 5 g/l MTT (Sigma-Aldrich) solution
was formulated with normal saline, sterilised through filtration
with a 0.22-μm filter (Millipore, Billerica, MA, USA), subpacked
and then maintained at 4°C. The BGC-823 cells in the logarithmic
phase were inoculated into 96-well plates at a density of
1×105 cells/ml and with 100 μl/well and treated
following culture for 24 h. Five RPTI concentrations were
established: 32, 16, 8, 4 and 2 μg/ml and six dual wells were
established for each dose. At 48 and 72 h after treatment, three
dual wells were selected and the culture supernatant was discarded
through aspiration from the wells. Each well was washed with PBS
once and then the supernatant was removed through aspiration.
Subsequently, 100 μl complete DMEM and 10 μl MTT solution were
added into each well and the plates were incubated for 4 h at 37°C
under a saturated 5% CO2 atmosphere. The culture was
then terminated and the cultural supernatant was carefully
discarded by aspiration from the wells. Dimethylsulphoxide (150 μl)
was added into each well and agitated for 10 min for full
dissolution. The absorbance at 490 nm was determined with an
enzyme-labelled instrument (3550 microplate reader; Bio-Rad,
Hercules, CA, USA) to compute the cell growth inhibition rate
according to the following formula: cell growth inhibition rate (%)
= (1 − average A of the treatment group)/average A of the control
group × 100]. The IC50 of RPTI was calculated using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Determination of in vivo antitumour
activity
BGC-823 cells in the logarithmic phase were washed
twice with DMEM and then resuspended in DMEM at a density of
1×106 cells/ml. Subsequently, 0.1 ml of the cell
suspension, i.e., 1xl05 BGC-823 cells, was
subcutaneously (SC) injected into the back of each BALB/C-nu mouse.
Five mice were inoculated, which were regularly observed and fed.
After 20 days, the mice were sacrificed, the tumours were dissected
and their fibrous capsules were removed. The well-grown tumour
tissues were selected, cut into 1-mm3 sections (weighing
~40 mg) and added to 0.2 ml normal saline. One section of the
tumour tissue was transplanted into the left axilla of each nude
mouse. The following day, the inoculated nude mice were randomly
assigned into three groups, with 10 mice in each group: The control
group (SC injection of normal saline at 10 ml/kg once daily); the
cyclophosphamide (CTX) group (SC injection of CTX around the tumour
at 20 mg/kg once every two days); and the RPTI groups (SC injection
of RPTI at 250, 50 and 10 mg/kg once daily). The treatments were
administered for 12 consecutive days. One day after completing the
treatments, the mice were weighed and then sacrificed through
cervical dislocation. The subcutaneous tumours were dissected and
the tumour weights among the groups were compared. The tumour
inhibition rate was then calculated. The spleen was collected under
sterile conditions and weighed to determine the spleen coefficient:
Spleen coefficient = spleen weight (mg)/body weight (g).
Statistical analysis
The different RPTI treatment groups were compared
using SPSS software, a homogeneity test of variance and a t-test.
The data were expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant result.
Results
RPTI extraction and separation
Fresh Pinellia ternata tuber was ground,
homogenised, aqueously extracted and lyophilised to obtain a
water-soluble lyophilised protein powder.
(NH4)2SO4 precipitates of the
total protein of Pinellia ternata at all levels were
obtained by further using the stepwise salting-out method. The
total recovery rate of the precipitate was 96.49%. Analysis
indicated that TI activity was mainly concentrated in the 40%
(NH4)2SO4 precipitate and the
specific trypsin inhibitory activity was 1.83-fold that of the
total protein; the recovery of inhibitory activity was 62.38% and
the total protein content of the precipitate was 123.67 mg,
accounting for 34.13% of the total protein. The majority of the
protein precipitated with 60%
(NH4)2SO4, accounting for 56.62%
of the total protein, and it had partial TI activity; its specific
activity was only 0.50-fold that of the total protein. The 80%
(NH4)2SO4 solution precipitated
the least amount of protein, which exhibited no TI activity
(Table I).
 | Table IIsolation of RPTI crude protein by
salt fractionation with (NH4)2SO4
and determination of its inhibitory activity. |
Table I
Isolation of RPTI crude protein by
salt fractionation with (NH4)2SO4
and determination of its inhibitory activity.
| Component | Total protein
(mg) | Total inhibitory
activity (U) | Inhibition recovery
(%) | Specific inhibitory
activity (U/mg) |
|---|
| Total protein | 362.33 | 34750 | 100.00 | 95.91 |
| 40%
(NH4)2SO4 precipitate | 123.67 | 21677 | 62.38 | 175.81 |
| 60%
(NH4)2SO4 precipitate | 205.14 | 9883 | 28.44 | 48.18 |
| 80%
(NH4)2SO4 precipitate | 20.81 | 0 | 0.00 | 0.00 |
| Total
(NH4)2SO4 precipitate | 349.62 | 31560 | 90.82 | 90.27 |
RPTI purification
The 40% (NH4)2SO4
precipitate was designated as the crude RPTI product and subjected
to trypsin-Sepharose 4B affinity chromatography to form two protein
peaks (A280 nm; Fig.
1A). P1 was a saliferous elution peak that exhibited
no TI activity and P2 was an affinity adsorption
activity peak, which was further separated using Sephadex G-50 to
form four protein peaks (Fig. 1B).
The P1 and P2 formed by Sephadex G-50
separation were TI activity peaks, which were mainly concentrated
in tubes 10–16. The 12% SDS-PAGE purity detection indicated that
only the components in tubes 10–12 (the first half of
P1) exhibited a single protein band and were estimated
to have >90% purity. The eluents of the samples in tubes 10–12
following repeated sample loading were collected and combined to
obtain purified RPTI. Fig. 1C
shows that the crude RPTI protein had numerous bands and a complex
composition. Further affinity chromatography removed the majority
of the hybrid proteins and only left two clear main bands. More
uniform main bands of RPTI were obtained following the gel
filtration, with an apparent relative molecular weight of ~14 kDa.
The activity recovery during each purification step is listed in
Table II. Following the affinity
chromatography, the specific inhibitory activity increased by
3.45-fold. The Sephadex G-50 separation produced a homogenous
component. The activity recovery was 24.40% and 6.02-fold purified
RPTI was obtained.
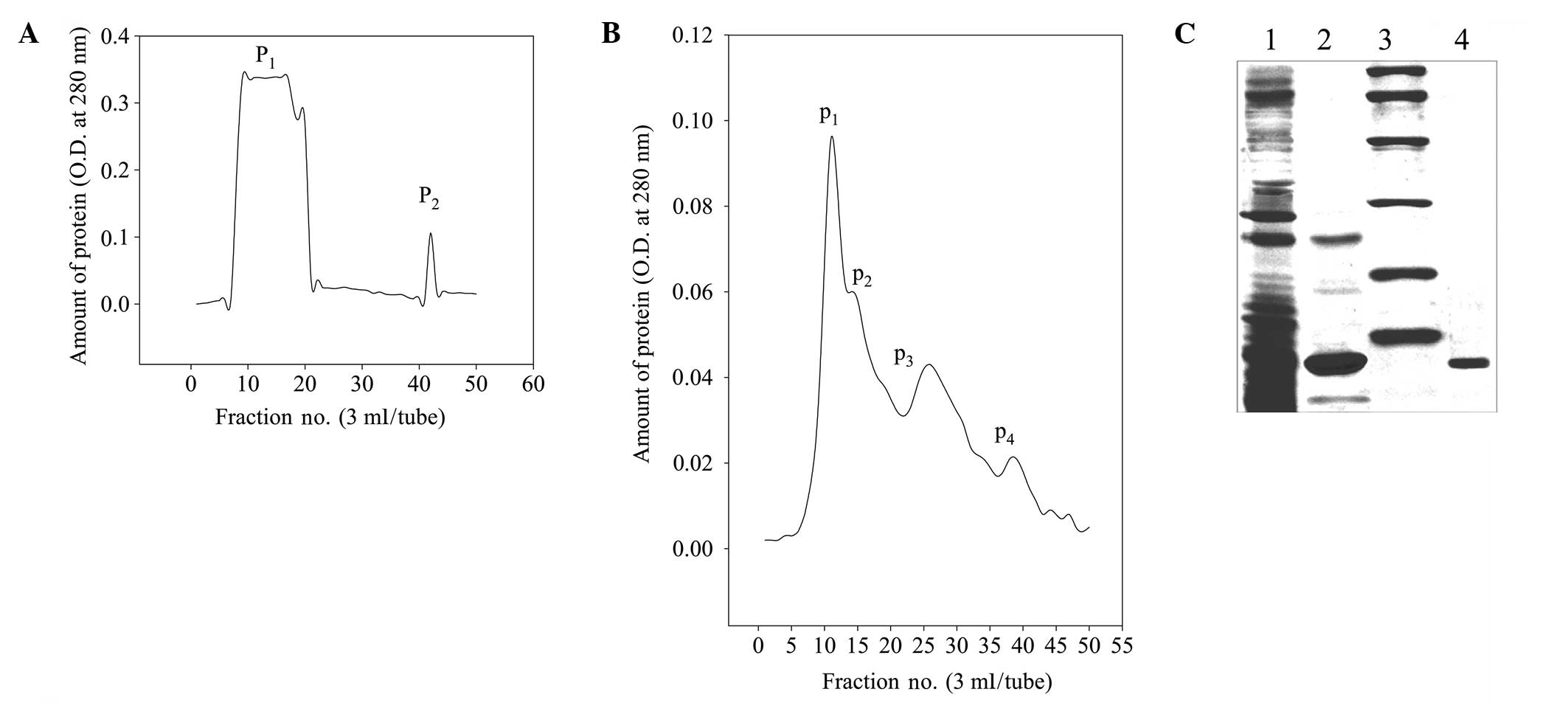 | Figure 1Separation, purification and
electrophoresis of RPTI. (A) Sepharose 4B trypsin-affinity
chromatography. (B) Sephadex G-50 gel filtration chromatography.
(C) SDS-PAGE of RPTI: Lane 1, 40%
(NH4)2SO4 precipitation; 2,
affinity chromatography; 3, molecular weight markers (from top to
bottom: 97.4, 66.2, 43.0, 31.0, 20.1 and 14.4 kDa); and 4, gel
filtration. OD, optical density; RPTI, Rhizoma Pinelliae trypsin
inhibitor. |
 | Table IIPurification steps of RPTI. |
Table II
Purification steps of RPTI.
| Purification
step | Total protein
(mg) | Total inhibition
activity (U) | Specific inhibition
activity (U/mg−1) | Inhibition recovery
(%) |
|---|
| 40%
(NH4)2SO4 precipitate | 20.00 | 3516.20 | 175.81 | 100.00 |
| Affinity
chromatography | 2.19 | 1326.50 | 605.71 | 37.73 |
| Sephadex G-50 gel
filtration | 0.81 | 857.80 | 1059.01 | 24.40 |
N-terminal amino acid sequence and
homology
Following SDS-PAGE and the transmembrane filtration,
the N-terminal amino acid sequence of the pure RPTI was determined.
The first six amino acid residues at the N-terminal of the RPTI in
the present study were 1-DPVVDG-6. The BLAST database of the NCBI
indicated that the first six amino acids in the N-terminal sequence
of RPTI are the same as those of arrowhead proteinase inhibitors A
and B, with serine replacing glycine at the sixth position being
the only difference. The RPTI sequence was highly homologous to
those of the arrowhead proteinase inhibitors (>80%; Table III).
 | Table IIIN-terminal amino acid sequence of
five protease inhibitors and their homology comparison results. |
Table III
N-terminal amino acid sequence of
five protease inhibitors and their homology comparison results.
| ID | Name | N-terminal amino
acid sequence | Homology (%) |
|---|
| This study | RPTI | 1 DPVVDG 6 | 5/6 (83.33) |
| BAA02972.1 | Precursor of
arrowhead proteinase inhibitor A | 25 DPVVDS 30 | 5/6 (83.33) |
| BAA02973.1 | Precursor of
arrowhead proteinase inhibitor B | 25 DPVVDS 30 | 5/6 (83.33) |
| 1818181A | Arrowhead
proteinase inhibitor A | 1 DPVVDS 6 | 5/6 (83.33) |
| 1208229A | Arrowhead
proteinase inhibitor B | 1 DPVVDS 6 | 5/6 (83.33) |
Inhibitory effect of RPTI on trypsin
activity
A 10-μg/ml RPTI solution was formulated with 0.05
mol/l Tris HCl (pH 8.0) buffer solution and successively diluted to
5.00, 2.50, 1.25 and 0.625 μg/ml. The inhibitory activity of RPTI
on trypsin was determined using the following method: 0.80 ml
diluted sample solution under test is added to 0.20 ml bovine
trypsin and incubated at 37°C for 5 min. Then 2.50 ml BAPNA
solution was added and reacted at 37°C for 5 min, the reaction was
immediately terminated and added to 0.5 ml 33% acetic acid
solution, the absorbance (A) was detected at 410 nm, with the test
sample without inhibitor as the control sample. Table IV shows that at 0–2.5 μg/ml RPTI,
the trypsin inhibition activity had a good linear association with
the concentration of RPTI. The linear regression equation was Y =
−25.709X + 94.862 (R2 = 0.9626). The RPTI concentration
required to completely inhibit 20 μg of trypsin was 3.69 μg/ml and
the inhibition ratio (mass) of RPTI to trypsin was 1:6.78, with a
molar inhibition ratio of 1:1.69.
 | Table IVInhibitory activity of RPTI on
trypsin. |
Table IV
Inhibitory activity of RPTI on
trypsin.
| RPTI concentration
(μg/ml) | A410 nm (mean ± SD,
n=3) | Residual enzyme
activity (%) | Enzyme activity
inhibition rate (%) |
|---|
| 0.000 | 0.218±0.014 | 100.00 | 0.00 |
| 0.625 | 0.166±0.017a | 76.15 | 23.85 |
| 1.250 | 0.123±0.013a | 56.42 | 43.58 |
| 2.500 | 0.075±0.01a | 34.40 | 65.60 |
| 5.000 | 0.047±0.009a | 21.56 | 78.44 |
Inhibitory effect of RPTI on BGC-823 cell
proliferation in vitro
Table V shows that
RPTI significantly inhibited the proliferation of BGC-823 cells and
that the inhibition was concentration-dependent. The
IC50 of RPTI calculated using logistic regression was
16.96 μg/ml within 48 h after treatment and 9.61 μg/ml within 72 h
after treatment.
 | Table VInhibitory effect of RPTI on the
in vitro proliferation of BGC-823 cells. |
Table V
Inhibitory effect of RPTI on the
in vitro proliferation of BGC-823 cells.
| 48 h after addition
of RPTI | 72 h after addition
of RPTI |
|---|
|
|
|
|---|
| RPTI (μg/ml) | Absorbance (A) | Inhibition rate
(%) | Absorbance (A) | Inhibition rate
(%) |
|---|
| 0 | 0.163±0.014 | - | 0.178±0.017 | - |
| 2 | 0.147±0.001 | 9.82 | 0.152±0.013a | 14.42 |
| 4 | 0.131±0.007a | 19.63 | 0.132±0.011a | 25.78 |
| 8 | 0.109±0.008a | 33.13 | 0.099±0.009a | 44.26 |
| 16 | 0.082±0.006a | 49.69 | 0.060±0.007a | 66.53 |
| 32 | 0.059±0.005a | 63.80 | 0.037±0.008a | 79.04 |
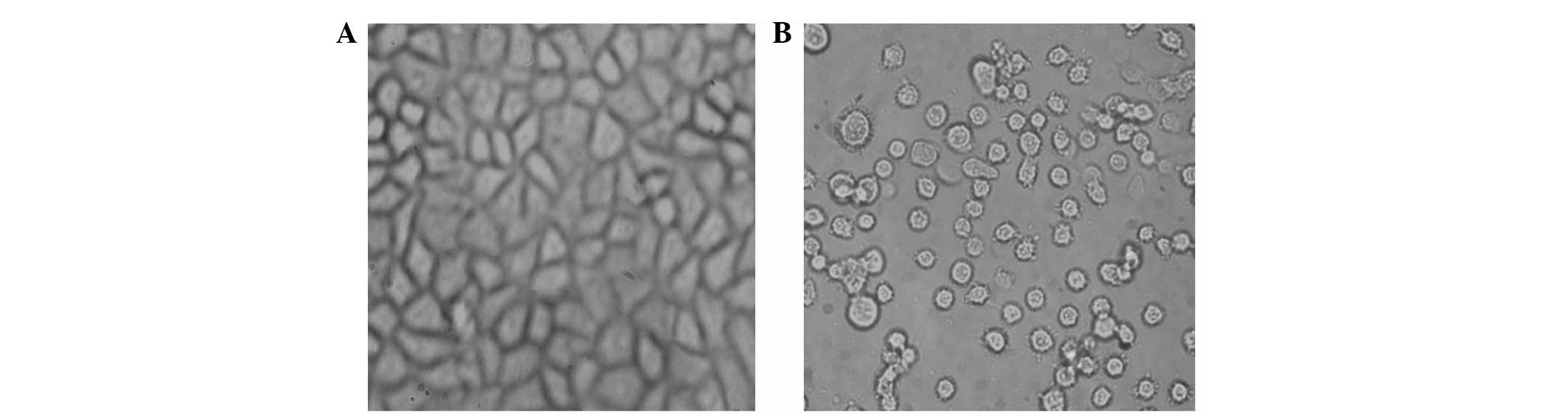
The BGC-823 cells were treated with 10 μg/ml RPTI
(Fig. 2). After 72 h, the changes
in cell shape were observed under an inverted microscope. The
BGC-823 cells in the RPTI group were less shrunken and were
significantly reduced in number compared with those of the control
group, with numerous suspended cells being dead. The cells
exhibited nuclear pyknosis and rough cytoplasms and underwent less
fission. The control cells had regular shapes and good adherent
growth.
Activity of RPTI against BGC-823 cells in
vivo
Table VI shows
that subcutaneous RPTI administration around the tumour
significantly inhibited the growth of the transplanted BGC-823
cells and the inhibition was dose-dependent. The tumour inhibition
rates of the high-dose and medium-dose groups were significantly
higher than that in the control group, but was lower than that of
the CTX positive control group. The tumour inhibition effect in the
low-dose group was not significantly different from that in the
control group. RPTI was not observed to have a significant effect
on the spleen coefficient of the mice.
 | Table VITumour inhibition rate of RPTI on
BGC-823 cells in tumour-bearing mice and the spleen
coefficient. |
Table VI
Tumour inhibition rate of RPTI on
BGC-823 cells in tumour-bearing mice and the spleen
coefficient.
| Group | Dose (mg/kg) | Tumour weight
(g) | Tumour inhibition
rate (%) | Spleen coefficient
(mg/g) |
|---|
| Control | - | 1.44±0.26 | 0.00 | 5.64±0.83 |
| CTX | 20 | 0.28±0.08a | 80.56 | 5.25±0.75 |
| High dose of
RPTI | 250 | 0.41±0.11a | 71.53 | 5.83±1.06 |
| Medium dose of
RPTI | 50 | 0.96±0.19a | 33.33 | 5.78±0.96 |
| Low dose of
RPTI | 10 | 1.18±0.23 | 18.06 | 5.67±0.87 |
Discussion
Pinellia ternata tuber is used for the
clinical treatment of various neoplastic diseases alone or combined
with other Chinese medicines (5–8).
Studies have suggested that the Pinellia protein
significantly inhibits the proliferation of ovarian cancer cells
(11). TIs are widely present in
plants and are widely accepted in the medical field as potential
cancer preventive agents. In the present study, a small protein
from Pinellia ternata tuber was isolated through separation
methods, demonstrated that this component has certain trypsin
inhibitory activity and inferred that RPTI may be a component of
Pinellia ternata that has antitumour activity. To the best
of our knowledge, the present study is the first to isolate and
purify RPTI. The purified RPTI showed a single band under SDS-PAGE,
with a molecular weight of 14 kDa. Its N-terminal amino acid
sequence was DPVVDG, which is highly homologous to that of
arrowhead serine proteinase inhibitors. The inhibition rate (mass)
of RPTI to the trypsin activity was 1:6.78. RPTI markedly inhibited
trypsin activity and its inhibition constant Ki was significantly
lower than the Km of trypsin (data not shown). Therefore, RPTI was
considered as a strong serine proteinase inhibitor.
The present study preliminarily demonstrates that
RPTI has a significant inhibitory effect on BGC-823 cell
proliferation in vitro and on the tumour growth of transplanted
BGC-823 cells. The tumour inhibition was concentration- and
dose-dependent. Consequently, RPTI has significant antitumour
activity. A key step in tumour cell growth and infection is
extracellular matrix (ECM) degradation. RPTI likely exerts its
antitumour effects by combining with the serine proteinase or a
specific receptor on the external surface of BGC-823 cell membranes
through different approaches. Thus, the proteinase loses its
ability to hydrolyse the ECM, preventing BGC-823 cell invasion and
tumour growth.
During the separation and purification of RPTI using
affinity chromatography, the samples were maintained at 37°C for 1
h after loading. The column was then washed with a highly
concentrated salt solution (1 mol/l NaCl) to wash off the majority
of nonspecific binding hybrid proteins. The bonding RPTI
ingredients were eluted with aqueous acid so that the purity of the
RPTI, separated through affinity chromatography with trypsin as the
ligand, was significantly improved. The P1 and
P2 activity peaks generated through Sephadex G-50
filtration were not completely separated and the ingredients in
tubes 10–16 were detected to have clear TI activity. Only the first
half of the P1 peak, i.e., the proteins in tubes 10–12,
showed a uniform band in the SDS-PAGE. Following further
electrophoresis and transmembrane separation, the main band of the
purified RPTI ingredient was cut off for sequencing to ensure the
purity of the RPTI.
The serine proteinase inhibitor separated from the
tuber of Pinellia ternata has promising potential for
application in antitumour therapy. The aim of further studies is to
design degenerate primers according to the RPTI N-terminal amino
acid sequence, clone its cDNA sequence, elucidate the complete gene
expression of RPTI and determine its mechanism of action.
References
|
1
|
Zhao GP, Dai S and Chen RS: Dictionary of
traditional Chinese medicine. Shanghai Scientific and Technical
Publishers; Second Edition. pp. 1071–1072. 2005
|
|
2
|
Han MH, Yang XW, Zhong GY and Zhang M:
Bioactive constituents inhibiting TNF-alpha production in fresh
rhizome of Pinellia ternata. Zhongguo Zhong Yao Za Zhi.
32:1755–1759. 2007.(In Chinese).
|
|
3
|
He P, Li S, Wang SJ, Yang YC and Shi JG:
Study on chemical constituents in rhizome of Pinellia
ternata. Zhongguo Zhong Yao Za Zhi. 30:671–674. 2005.(In
Chinese).
|
|
4
|
Lee MY, Shin IS, Jeon WY, Lim HS, Kim JH
and Ha H: Pinellia ternata Breitenbach attenuates
ovalbumin-induced allergic airway inflammation and mucus secretion
in a murine model of asthma. Immunopharmacol Immunotoxicol.
35:410–418. 2013. View Article : Google Scholar
|
|
5
|
Li GL, Gui SQ and Wang L: Effect of
pinellia extract on HeLa cell line in vitro and associated
mechanism. Zhongguo Zhong Xi Yi Jie He Za Zhi. 30:303–307. 2010.(In
Chinese).
|
|
6
|
Lin J, Yao J, Zhou X, Sun X and Tang K:
Expression and purification of a novel mannose-binding lectin from
Pinellia ternata. Mol Biotechnol. 25:215–222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu T, Wang B, Wang L, Zhang Y and Lv Z:
Pinellia ternata agglutinin produced in Bombyx mori
cells exhibits bioactivity. Acta Biochim Pol. 59:231–236. 2012.
|
|
8
|
Lu Q, Li N, Luo J, et al: Pinellia
pedatisecta agglutinin interacts with the methylosome and
induces cancer cell death. Oncogenesis. 1:e292012. View Article : Google Scholar
|
|
9
|
Yoon JS, Seo JC and Han SW: Pinelliae
Rhizoma herbal-acupuncture solution induced apoptosis in human
cervical cancer cells, SNU-17. Am J Chin Med. 34:401–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li GL, Jiang W, Xia Q, et al: HPV E6
down-regulation and apoptosis induction of human cervical cancer
cells by a novel lipid-soluble extract (PE) from Pinellia
pedatisecta Schott in vitro. J Ethnopharmacol. 132:56–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XY, Zhou L and Zheng FY: Proteomica
study of total protein of Pinellia pedactisecta Schott
effect on human ovarian cancer SKOV3 cells. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 31:1651–1656. 2011.(In Chinese).
|
|
12
|
Zhou W, Huang Y, Xu S, et al: Prokaryotic
expression and bioactivity analysis of N-terminus domain of
Pinellia ternata agglutinin using alkaline phosphatase
signal peptide. Protein Expr Purif. 89:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sierko E, Wojtukiewicz MZ, Zimnoch L, et
al: Protein Z/protein Z-dependent protease inhibitor system in
human non-small-cell lung cancer tissue. Thromb Res. 129:e92–e96.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sierko E, Wojtukiewicz MZ, Zimnoch L,
Tokajuk P, Ostrowska-Cichocka K and Kisiel W: Co-localization of
Protein Z, Protein Z-Dependent protease inhibitor and coagulation
factor X in human colon cancer tissue: implications for coagulation
regulation on tumor cells. Thromb Res. 129:e112–e118. 2012.
View Article : Google Scholar
|
|
15
|
Sun L, Niu L, Zhu X, Hao J, Wang P and
Wang H: Antitumour effects of a protease inhibitor, nelfinavir, in
hepatocellular carcinoma cancer cells. J Chemother. 24:161–166.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tamburino R, Pizzo E, Sarcinelli C, et al:
Enhanced cytotoxic activity of a bifunctional chimeric protein
containing a type 1 ribosome-inactivating protein and a serine
protease inhibitor. Biochimie. 94:1990–1996. 2012. View Article : Google Scholar
|
|
17
|
Gocho T, Uwagawa T, Furukawa K, et al:
Combination chemotherapy of serine protease inhibitor nafamostat
mesilate with oxaliplatin targeting NF-κB activation for pancreatic
cancer. Cancer Lett. 333:89–95. 2013.PubMed/NCBI
|
|
18
|
Morjen M, Kallech-Ziri O, Bazaa A, et al:
PIVL, a new serine protease inhibitor from Macrovipera lebetina
transmediterranea venom, impairs motility of human glioblastoma
cells. Matrix Biol. 32:52–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi H, Gotoh J, Hirashima Y and
Terao T: Inter-alpha-trypsin inhibitor bound to tumor cells is
cleaved into the heavy chains and the light chain on the cell
surface. J Biol Chem. 271:11362–11367. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baba T, Kawaguchi M, Fukushima T, et al:
Loss of membrane-bound serine protease inhibitor HAI-1 induces oral
squamous cell carcinoma cells’ invasiveness. J Pathol. 228:181–192.
2012.PubMed/NCBI
|
|
21
|
Brandi G, Tavolari S, De Rosa F, et al:
Antitumoral efficacy of the protease inhibitor gabexate mesilate in
colon cancer cells harbouring KRAS, BRAF and PIK3CA mutations. PLoS
One. 7:e413472012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen DY, Lee Y, Van Tine BA, et al: A
pharmacologic inhibitor of the protease Taspase1 effectively
inhibits breast and brain tumor growth. Cancer Res. 72:736–746.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magee PJ, Owusu-Apenten R, McCann MJ, Gill
CI and Rowland IR: Chickpea (Cicer arietinum) and other
plant-derived protease inhibitor concentrates inhibit breast and
prostate cancer cell proliferation in vitro. Nutr Cancer.
64:741–748. 2012.
|
|
24
|
Palavalli MH, Natarajan SS, Wang TT and
Krishnan HB: Imbibition of soybean seeds in warm water results in
the release of copious amounts of Bowman-Birk protease inhibitor, a
putative anticarcinogenic agent. J Agric Food Chem. 60:3135–3143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rengan R, Mick R, Pryma D, et al: A phase
I trial of the HIV protease inhibitor nelfinavir with concurrent
chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell
lung cancer: a report of toxicities and clinical response. J Thorac
Oncol. 7:709–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shim JS, Rao R, Beebe K, et al: Selective
inhibition of HER2-positive breast cancer cells by the HIV protease
inhibitor nelfinavir. J Natl Cancer Inst. 104:1576–1590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loewenberg JR: Cyanide and the
determination of protein with the Folin phenol reagent. Anal
Biochem. 19:95–97. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Tubuly AA: SDS-PAGE and western
blotting. Methods Mol Med. 40:391–405. 2000.
|
|
29
|
Edman P, Högfeldt E, Sillén LR and Kinell
P-O: Method for determination of the amino acid sequence in
peptides. Acta Chemica Scandinavica. 4:283–293. 1950. View Article : Google Scholar
|
|
30
|
Dudai M, Mayer M and Kidron M: Protease
inhibitor activity in rat skeletal muscle. Hoppe Seylers Z Physiol
Chem. 363:651–654. 1982.PubMed/NCBI
|
|
31
|
Berg K, Hansen MB and Nielsen SE: A new
sensitive bioassay for precise quantification of interferon
activity as measured via the mitochondrial dehydrogenase function
in cells (MTT-method). APMIS. 98:156–162. 1990. View Article : Google Scholar
|