Introduction
Systemic lupus erythematosus (SLE) is a prototypic
autoimmune disease that is characterized by humoral autoimmunity
followed by cellular and innate immune responses (1). These responses in turn lead to
inflammation and damage to organs, including the kidney, skin and
joints. Lupus nephritis (LN) affects the majority of patients with
lupus and is associated with poor prognosis in SLE (2). Although the prognosis of patients
with SLE has greatly improved, immune suppression remains evident
and results in higher susceptibility to infectious and malignant
diseases (3). Toxic effects and,
in certain cases, unexpectedly severe complications of the current
therapies are being increasingly reported (3). Thus, more effective and less toxic
therapies for SLE and LN are required.
Nuclear factor (NF)-κB, a key transcription factor
involved in the regulation of immune responses, has been
demonstrated to be a potential candidate for studies concerning the
pathogenesis of autoimmunity (4).
Stimulation of a wide range of receptors, including tumor necrosis
factor (TNF) receptor, interleukin (IL)-1 receptor, Toll-like
receptor, T- and B-cell receptors, B-cell activating factor
receptor, receptor activator of NF-κB and CD40, may lead to the
activation of NF-κB. Thus, NF-κB functions at the crossroads of a
number of signaling pathways (5).
Susceptibility genes involved in the NF-κB signaling pathway may
synergistically contribute to the increased risk of SLE (6). Thus, in the present study, the
potential of a NF-κB inhibitor, dehydroxymethylepoxyquinomicin
(DHMEQ), was investigated as a treatment option for LN and SLE
using a pristane-induced lupus model.
Materials and methods
Animals
Female BALB/c mice (age, 8 weeks; weight, 18±2 g)
were supplied by the Experimental Animal Center of Binzhou Medical
College (Binzhou, China). All experimental protocols reported in
the study were approved by the Ethics Review Committee for Animal
Experimentation of Binzhou Medical College.
Experimental protocols
Female BALB/c mice were randomly divided into
vehicle-treated model and DHMEQ-treated groups (n=12 per group).
The mice were administered an intraperitoneal injection of 0.5 ml
pristane (7). When the mice
reached 16-weeks of age (8 weeks following pristane treatment when
antibody production was significant), intraperitoneal injections of
DHMEQ or vehicle were administered three times a week (8) until the mice were 32 weeks old.
DHMEQ was dissolved in 0.5% carboxymethyl cellulose
(CMC) to a final concentration of 1.2 mg/ml, as described
previously (8). Intraperitoneal
injections of 12 mg/kg DHMEQ or 0.5% CMC (vehicle) were
administered. Serum samples were collected from the tail vein at
weeks 8, 16 and 32 to measure the level of autoantibodies. At week
32 (24 weeks following pristane administration), the mice were
sacrificed under carbon dioxide and nephritic tissues were removed
for immunological detection and examination by light
microscopy.
Determination of autoantibodies
Serum levels of IgG autoantibodies against
nucleosomes, dsDNA and histones were determined by enzyme-linked
immunosorbent assays, as previously described (9,10).
Serum IgG autoantibodies against nucleosomes, dsDNA and histones
were detected at weeks 8, 16 and 32.
Determination of cytokines
Expression levels of mouse IL-1β, 6 and 17 and TNF-α
were measured using a FlowCytomix bead-based assay (Bender
MedSystems, Vienna, Austria), according to the manufacturer’s
instructions. Cytokine concentrations were determined using
FlowCytomixPro 2.2.0 analysis software (Bender MedSystems).
Assessment of kidney disease
To assess urinary protein excretion, mice were
placed in metabolic cages and urine samples were collected over a
24-h period. Protein concentrations were determined using Coomassie
brilliant blue (Pierce Biotechnology, Inc., Rockford, IL, USA),
according to the manufacturer’s instructions. Saline-perfused
kidney sections obtained from the mice and age-matched C57BL/6 mice
were prepared as previously described (11) and analyzed using an Olympus BX60
microscope (Olympus Corporation, Tokyo, Japan). Staining intensity
was quantified using Image J software (National Institutes of
Health, Bethesda, MD, USA).
Western blot analysis of kidney
proteins
For western blot analysis, the kidneys were removed
and snap-frozen in liquid nitrogen for protein extraction using
RIPA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Next,
20 μg crude protein was subjected to 12.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Following
electrophoresis, the proteins were transferred onto a
nitrocellulose membrane (GE-Healthcare, Little Chalfont, UK). The
blots were blocked with 2% bovine serum albumin in
phosphate-buffered saline (PBS), which was followed by 1 h
incubation with 1:2,000 diluted primary antibodies against
phosphorylated-p38 mitogen-activated protein kinase (p-p38 MAPK),
phosphorylated-c-Jun N-terminal kinase (p-JNK) or NF-κB p65 (Santa
Cruz Biotechnology, Inc.). Following washing with PBS, the reacted
blots were incubated with peroxidase-conjugated secondary
antibodies (BioSource International, Inc., Camarillo, CA, USA).
Antigen-antibody complexes were identified by enhanced
chemiluminescence (Pierce Biotechnology, Inc.) and the photographic
density was quantified using an image analysis system (Fujifilm,
Tokyo, Japan). GAPDH and lamin B1 were used as internal
controls.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as mean ± SEM. Data were compared by one-way analysis of
variance and P<0.01 was considered to indicate a statistically
significant difference.
Results
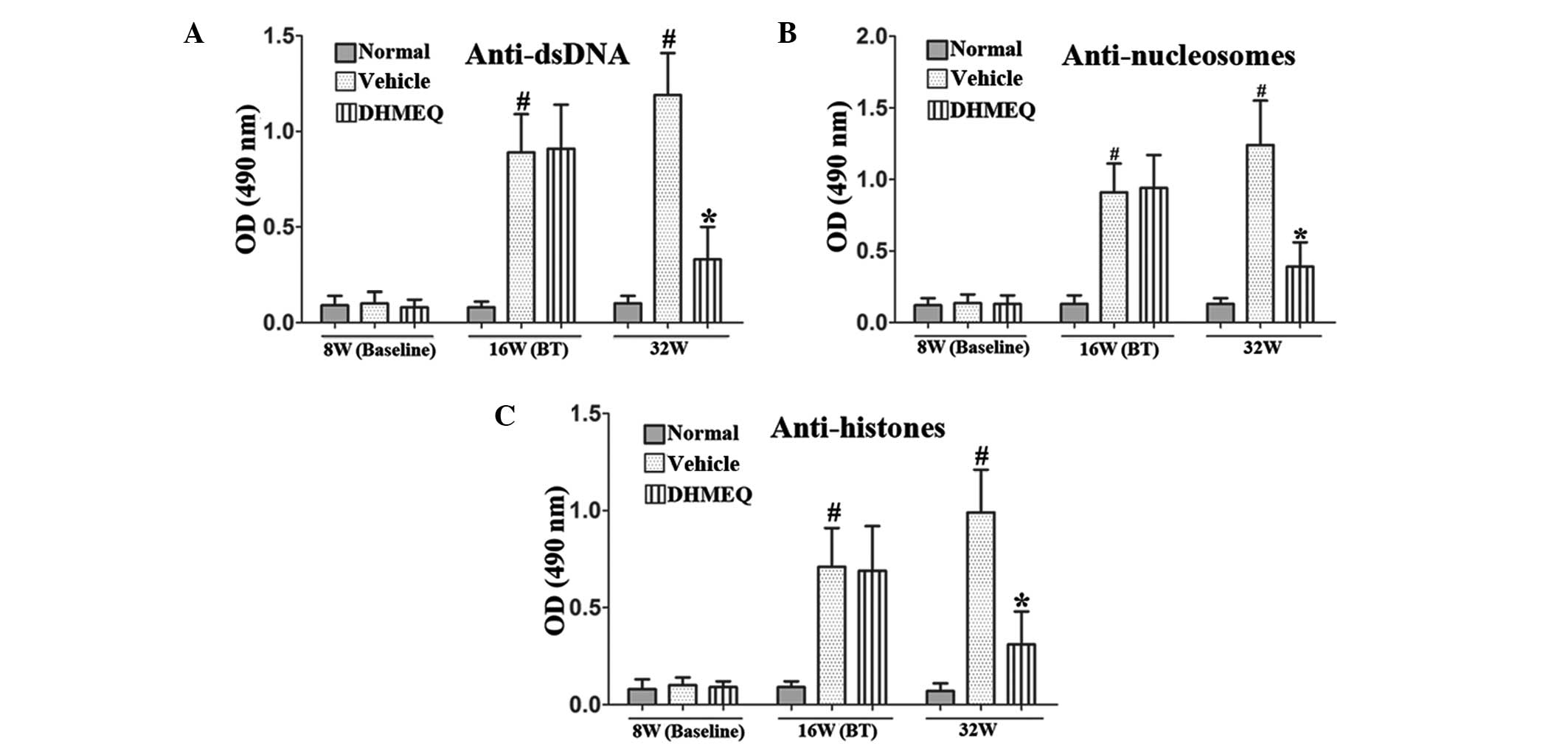
DHMEQ reduces the serum levels of
anti-dsDNA, anti-nucleosome and anti-histone antibodies in mice
with pristane-induced lupus
The production of numerous autoantibodies is a key
feature of SLE and is associated with renal damage. Serum IgG
autoantibodies against nucleosomes, dsDNA and histones were
detected at weeks 8, 16 and 32. There were marked increases in the
serum anti-dsDNA antibody levels of the vehicle-treated model group
at weeks 16 and 32, whereas the DHMEQ-treated group had markedly
reduced anti-dsDNA antibody levels compared with those in the
vehicle-treated model group at week 32 (Fig. 1A). Similarly, serum anti-nucleosome
and anti-histone antibody levels were also significantly reduced by
DHMEQ treatment when compared with those in the vehicle-treated
control group at week 32 (Fig. 1B and
C).
DHMEQ reduces the expression of
proinflammatory cytokines in mice with pristane-induced lupus
To investigate the effects of DHMEQ on cytokines in
SLE, the expression levels of IL-1β, 6 and 17 and TNF-α were
analyzed. The results revealed that the expression levels of TNF-α,
IL-1β, 6 and 17 were significantly increased in the vehicle-treated
SLE model mice (P<0.01; Fig.
2). By contrast, in the DHMEQ treatment group, the cytokine
levels were almost normal and markedly lower compared with those in
the vehicle-treated model mice (P<0.01; Fig. 2).
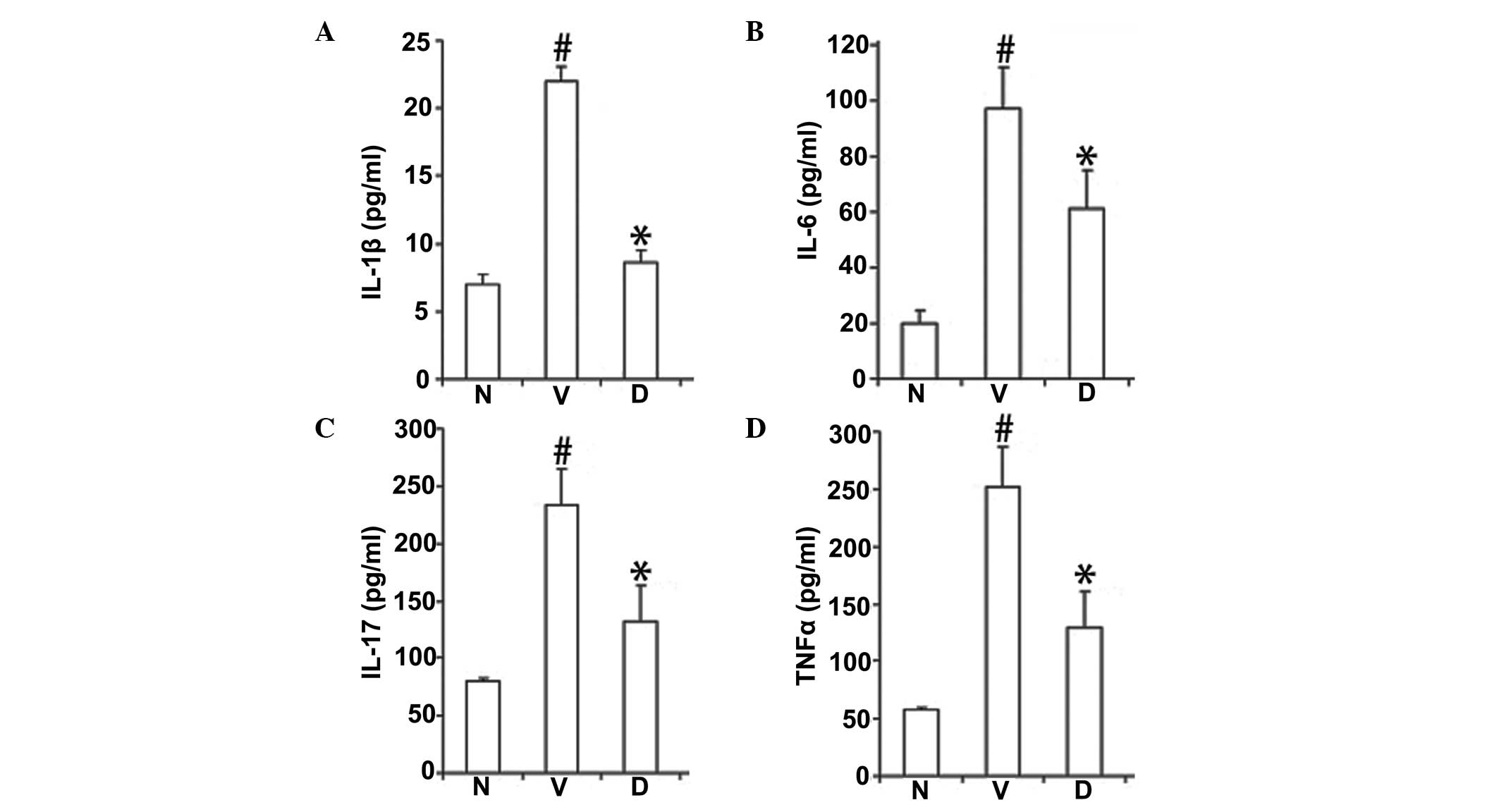 | Figure 2DHMEQ reduced the expression levels of
serum (A) IL-1β, (B) IL-6, (C) IL-17 and (D) TNF-α proinflammatory
cytokines. Data are expressed as mean ± SEM (n=12 per group).
#P<0.01, vs. baseline at week 8;
*P<0.01, vs. vehicle-treated model control mice. N,
normal; V, vehicle-treated model control; D, DHMEQ-treated model
mice; DHMEQ, dehydroxymethylepoxyquinomicin; IL, interleukin; TNF,
tumor necrosis factor. |
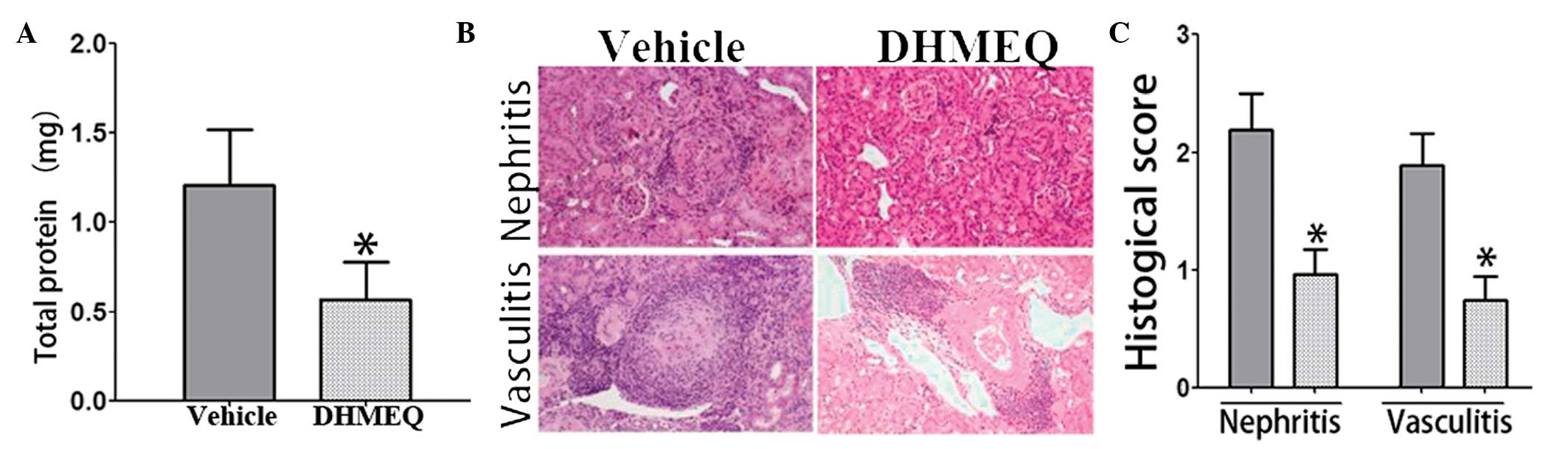
DHMEQ reduces LN in mice with
pristane-induced lupus
To quantify urinary protein excretion, urine samples
from individual mice in metabolic cages were collected over 24 h.
At week 32 (24 weeks following pristane injection), urinary protein
excretion in the DHMEQ-treated mice was significantly lower
compared with that in the vehicle-treated model mice (Fig. 3A).
In addition, the renal histology of the mice with
pristane-induced lupus was investigated. Consistent with the
aforementioned observations, hematoxylin and eosin-stained sections
of the kidneys from mice in the DHMEQ-treated group exhibited
reduced glomerular damage compared with that observed in the
vehicle-treated lupus model mice (Fig.
3B and C).
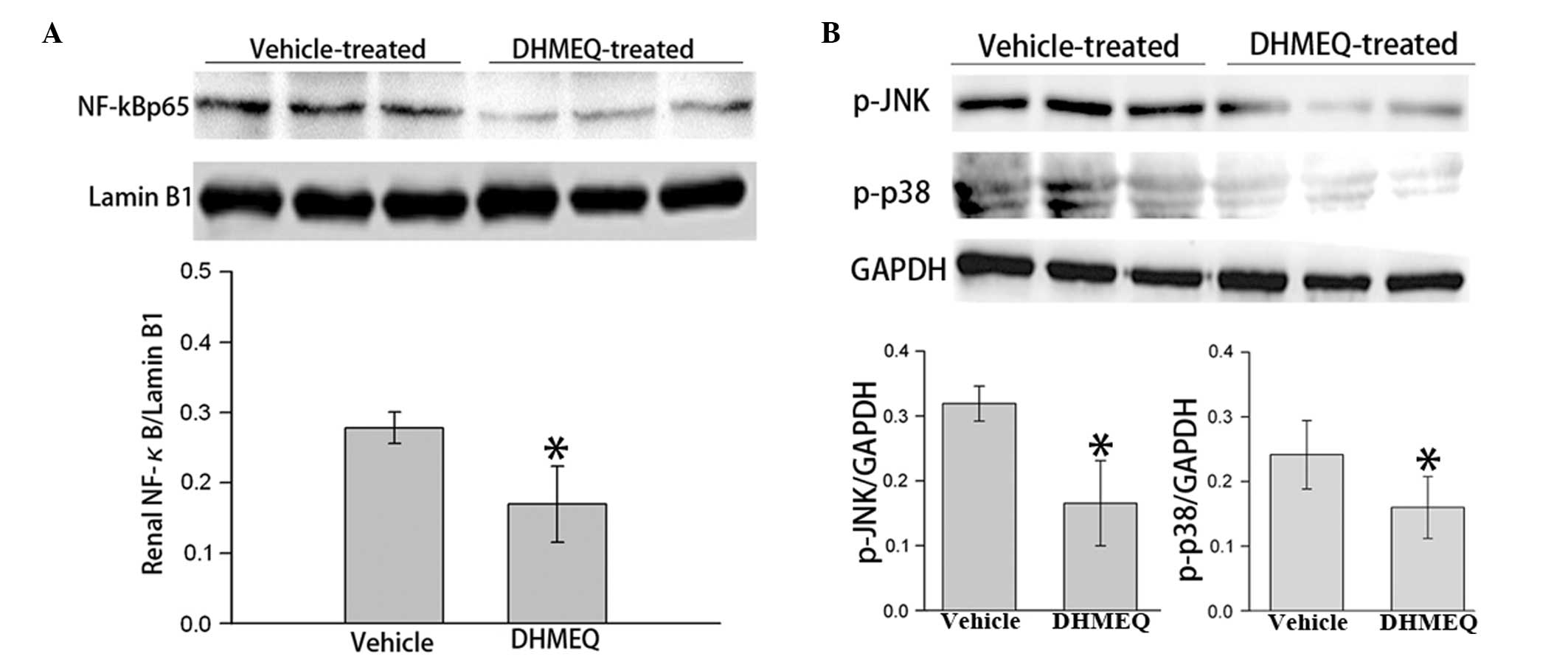
DHMEQ inhibits renal NF-κB and JNK/p38
MAPK pathways in mice with pristane-induced lupus
To further explore the underlying mechanism, the
expression levels of associated signaling molecules in the renal
tissue were analyzed. A previous study indicated that the
inflammation-mediated activation of the JNK and p38 MAPK signaling
pathways may be the underlying intracellular mechanism causing
lymphocyte hyperactivity in SLE (12). NF-κB p65, an indicator of NF-κB
signaling activation, was found to be downregulated by DHMEQ
treatment (Fig. 4A). In addition,
the phosphorylation levels of JNK and p38 MAPK in the renal tissues
of the mice with pristane-induced lupus were significantly
decreased following DHMEQ treatment (Fig. 4B). These results indicate that the
treatment effects of DHMEQ are associated with JNK/p38 MAPK
signaling in mouse SLE.
Discussion
DHMEQ, a 5-dehydroxymethyl derivative of
epoxyquinomicin C (13), is an
antibiotic originally isolated from Amycolatopsis sp.
(14). The majority of NF-κB
inhibitors target IκBα phosphorylation, whereas DHMEQ inhibits the
nuclear translocation of p65, a component of NF-κB (15). DHMEQ has not exhibited evident
toxicity in animals (13,15), indicating the tolerance of this
compound for NF-κB. In the present study, the anti-lupus property
of DHMEQ was investigated in a pristane-induced lupus mouse model.
DHMEQ was shown to antagonize the increasing levels of
anti-nucleosome, anti-dsDNA and anti-histone autoantibodies, as
well as the levels of TNF-α, IL-1β, 6 and 17. In addition, DHMEQ
reduced the number of renal lesions caused by pristane, as
reflected by milder proteinuria and reduced glomerular pathology.
Renal expression levels of p-p38MAPK, p-JNK and NF-κB p65 were
significantly downregulated. These results indicate that DHMEQ has
a beneficial effect on pristane-induced lupus through regulating
the levels of cytokines and the MAPK/JNK/NF-κB signaling
pathway.
Elevated constitutive levels of active NF-κB are
associated with chronic inflammatory diseases (16). The NF-κB family of transcription
factors is regulated by inhibitors, including IκBα. Lower mRNA
expression levels of IκBα have been observed in spleens and
dendritic cells (DCs) derived from lupus-prone mice, as compared
with wild-type mice (6),
indicating an abnormal activation of NF-κB in lupus mice. NF-κB can
affect the function of DCs and their capacity to regulate adaptive
immunity (6). Previous studies
have shown that an NF-κB blockade interferes with unwanted T-cell
responses, as observed in experimental autoimmune encephalomyelitis
(17). In spontaneous lupus model
MRL/lpr mice, inhibiting the NF-κB-mediated inflammatory response
was shown to be effective for LN (18). The present study has provided new
evidence indicating that the pharmacological inhibition of NF-κB in
mice with pristane-induced lupus may significantly reduce the
effects of lupus disease.
Accumulating evidence has demonstrated the
involvement of NF-κB in self-reactive T- and B-lymphocyte
development, survival and proliferation, as well as the maintenance
of chronic inflammation due to cytokines, including TNF-α, IL-1, 6
and 17 (19). Thus, an
NF-κB-mediated inflammatory response may contribute to organ damage
in SLE (18,19). In the present study, DHMEQ was
found to antagonize the increasing levels of IL-1β, 6 and 17 and
TNF-α. In addition, a number of studies indicate that the p38
MAPK/JNK signaling pathway plays an important role in the
regulation of cellular and humoral autoimmune responses (20,21).
DHMEQ, a specific inhibitor of p38 MAPK, has shown to be effective
in an MRL/lpr mouse model of SLE, as demonstrated by improved renal
function and the attenuation of histological damage (21). The results of the present study
demonstrate that the MAPK/JNK signaling pathway is inhibited by
DHMEQ treatment. These results indicate that DHMEQ plays a
therapeutic role in SLE by blocking the NF-κB/MAPK/JNK-mediated
inflammatory response.
In conclusion, the results of the present study
demonstrate that DHMEQ has a beneficial effect on pristane-induced
lupus through regulating cytokine levels and the MAPK/JNK/NF-κB
signaling pathway. The results support the hypothesis that NF-κB
blockade may be an important pharmacological approach for the
downmodulation of detrimental autoimmune responses.
References
|
1
|
Perl A, Fernandez DR, Telarico T, Doherty
E, Francis L and Phillips PE: T-cell and B-cell signaling
biomarkers and treatment targets in lupus. Curr Opin Rheumatol.
21:454–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dooley MA, Hogan S, Jennette C and Falk R;
Glomerular Disease Collaborative Network. Cyclophosphamide therapy
for lupus nephritis: poor renal survival in black Americans. Kidney
Int. 51:1188–1195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monneaux F and Muller S: Molecular
therapies for systemic lupus erythematosus: clinical trials and
future prospects. Arthritis Res Ther. 11:2342009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuryłowicz A and Nauman J: The role of
nuclear factor-kappaB in the development of autoimmune diseases: a
link between genes and environment. Acta Biochim Pol. 55:629–647.
2008.PubMed/NCBI
|
|
5
|
Moynagh PN: The NF-kappaB pathway. J Cell
Sci. 118:4589–4592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalergis AM, Iruretagoyena MI, Barrientos
MJ, et al: Modulation of nuclear factor-kappaB activity can
influence the susceptibility to systemic lupus erythematosus.
Immunology. 128(1 Suppl): e306–e314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng D, Yang L, Bi X, Stone RC, Patel P
and Barnes BJ: Irf5-deficient mice are protected from
pristane-induced lupus via increased Th2 cytokines and altered IgG
class switching. Eur J Immunol. 42:1477–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohsugi T, Horie R, Kumasaka T, et al: In
vivo antitumor activity of the NF-kappaB inhibitor
dehydroxymethylepoxyquinomicin in a mouse model of adult T-cell
leukemia. Carcinogenesis. 26:1382–1388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wellmann U, Letz M, Schneider A, Amann K
and Winkler TH: An Ig mu-heavy chain transgene inhibits systemic
lupus erythematosus immunopathology in autoimmune (NZB × NZW)F1
mice. Int Immunol. 13:1461–1469. 2001.PubMed/NCBI
|
|
10
|
Comby E, Tanaff P, Mariotte D,
Costentin-Pignol V, Marcelli C and Ballet JJ: Evolution of
antinuclear antibodies and clinical patterns in patients with
active rheumatoid arthritis with longterm infliximab therapy. J
Rheumatol. 33:24–30. 2006.PubMed/NCBI
|
|
11
|
Hoffmann A, Kerr S, Jellusova J, et al:
Siglec-G is a B1 cell-inhibitory receptor that controls expansion
and calcium signaling of the B1 cell population. Nat Immunol.
8:695–704. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niculescu F, Nguyen P, Niculescu T, Rus H,
Rus V and Via CS: Pathogenic T cells in murine lupus exhibit
spontaneous signaling activity through phosphatidylinositol
3-kinase and mitogen-activated protein kinase pathways. Arthritis
Rheum. 48:1071–1079. 2003. View Article : Google Scholar
|
|
13
|
Matsumoto N, Ariga A, To-e S, et al:
Synthesis of NF-kappaB activation inhibitors derived from
epoxyquinomicin C. Bioorg Med Chem Lett. 10:865–869. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumoto N, Iinuma H, Sawa T, et al:
Epoxyquinomicins A, B, C and D, new antibiotics from
Amycolatopsis. II Effect on type II collagen-induced
arthritis in mice. J Antibiot (Tokyo). 50:906–911. 1997.PubMed/NCBI
|
|
15
|
Ariga A, Namekawa J, Matsumoto N, Inoue J
and Umezawa K: Inhibition of tumor necrosis factor-alpha-induced
nuclear translocation and activation of NF-kappa B by
dehydroxymethylepoxyquinomicin. J Biol Chem. 277:24625–24630. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iruretagoyena MI, Tobar JA, González PA,
et al: Andrographolide interferes with T cell activation and
reduces experimental autoimmune encephalomyelitis in the mouse. J
Pharmacol Exp Ther. 312:366–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang T, Tian F, Zheng H, et al: Nrf2
suppresses lupus nephritis through inhibition of oxidative injury
and the NF-κB-mediated inflammatory response. Kidney Int.
85:333–343. 2014.PubMed/NCBI
|
|
19
|
Brown KD, Claudio E and Siebenlist U: The
roles of the classical and alternative nuclear factor-kappaB
pathways: potential implications for autoimmunity and rheumatoid
arthritis. Arthritis Res Ther. 10:2122008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mavropoulos A, Rigopoulou EI, Liaskos C,
Bogdanos DP and Sakkas LI: The role of p38 MAPK in the
aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev
Immunol. 2013:5697512013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin N, Wang Q, Zhang X, Jiang D, Cheng H
and Zhu K: The selective p38 mitogen-activated protein kinase
inhibitor, SB203580, improves renal disease in MRL/lpr mouse model
of systemic lupus. Int Immunopharmacol. 11:1319–1326. 2011.
View Article : Google Scholar : PubMed/NCBI
|