Introduction
Benign prostatic hyperplasia (BPH), also known as
benign enlargement of the prostate or adenofibromyomatous
hyperplasia, is a noncancerous enlargement of the prostate gland,
which increases linearly with aging and has become a generally
observed major disease among older males (1,2). An
estimated 50% of males show histological evidence of BPH by the age
of 50 years and 80% by the age of 70 years (2). BPH involves hyperplasia of prostatic
stromal and epithelial cells, resulting in the formation of large,
fairly discrete nodules in the periurethral region of the prostate.
The enlarged prostate gland interferes with the normal flow of
urine. BPH leads to lower urinary tract symptoms (LUTS), including
urinary hesitancy, frequent urination, urgency, thin urine flow and
urinary retention (3,4). These symptoms greatly affect the
physical and mental health of patients, as well as their living
quality. Delayed treatment is likely to cause a number of severe
complications, including bleeding from the prostate, recurrent
infections, renal stones and even kidney failure.
The pathogenic mechanisms underlying BPH development
are complex and are not yet completely understood. However,
accumulating evidence indicates that the development and
progression of BPH relies on angiogenesis to obtain adequate oxygen
and nutrients from the nearby blood vessels by simple passive
diffusion (5–9), which promotes prostatic cell
proliferation and inhibits apoptosis. Angiogenesis is a
physiological process involving the growth of new blood vessels
from endothelial cell precursors or from the pre-existing
vasculature (10,11), and is recognized to play an
important role in various human disease processes, including wound
healing, reproduction, embryonic development and acute and chronic
inflammation (11–13). Increasing evidence has demonstrated
that angiogenesis is a highly sophisticated and coordinated
process. Hypoxia-inducible factor 1 (HIF-1) is hypothesized to be a
key signaling factor required for the activation of the ‘angiogenic
switch’ and the resulting increase in the expression of various
angiogenic growth factors, including vascular endothelial growth
factor (VEGF) and basic fibroblast growth factors (bFGF) (14–17).
HIF-1 is a heterodimeric transcription factor composed of two
polypeptides: HIF-1α and HIF-1β (17). HIF-1α functions as a master
regulator of oxygen homeostasis and accumulates in the cytosol.
Following translocation to the nucleus, it activates
hypoxia-sensitive genes and regulates genes encoding angiogenic
cytokines, including VEGF and bFGF. VEGF or bFGF primarily bind to
the specific receptors located on vascular endothelial cells (ECs),
which induces EC proliferation, migration, survival, sprouting and
eventually extending the primitive vascular network (18–21).
Thus, inhibition of angiogenesis may be a key target for BPH
treatment.
To date, pharmacotherapy remains the modality of
choice for BPH treatment, and can be roughly divided into three
groups: 5α-reductase inhibitors, α-adrenergic blockers and
alternative therapies. The 5α-reductase inhibitors, including
finasteride and dutasteride, inhibit dihydrotestosterone (DHT)
production by suppressing 5α-reductase. The α-adrenergic blockers,
including terazosin, doxazosin and tamsulosin, inhibit α-adrenergic
receptors (22,23), which relaxes smooth muscle in the
prostate and bladder neck, resulting in a decreased blockage of
urine flow. However, these prescription medications can have
troubling side effects, including orthostatic hypotension,
decreased libido and ejaculation or erectile dysfunction (24–27).
Due to these side effects, natural products that appear to have
limited adverse events are becoming increasingly important in the
treatment of BPH, including saw palmetto, pygeum africanum and
hypoxis rooperi (28–30), which have long been used to treat
BPH successfully.
Qianliening capsule (QC) is a traditional Chinese
medicine (TCM) formulation that consists of a combination of
rhubarb, leech, astragalus, achyranthes and dodder. Previous
studies have shown that QC exhibits properties of heat-clearing,
detoxification, promotion of blood circulation and removal of blood
stasis, as well as tonifying the kidney and nourishing vitality
(replenishing the kidney qi in Chinese medicine) (31,32).
QC has been reported to exhibit significant therapeutic effects in
BPH by markedly improving a series of LUTS and the dynamic index of
urine flow in BPH patients (31).
In addition, accumulating evidence has shown that QC markedly
decreases the prostatic volume and weight, inhibits prostatic
hyperplasia, relieves the abnormal ratio of estrogen to androgen,
regulates the expression of the estrogen and androgen receptors,
induces prostatic cell apoptosis and suppresses cell proliferation
by regulating the epidermal growth factor/signal transducer and
activator of transcription 3 signaling pathway and
mitochondrion-dependent apoptosis pathway (32–36).
However, the precise mechanism underlying the anti-BPH activity
remains largely unclear. To further elucidate the mechanism of QC
activity in BPH, the present study evaluated the effect of QC
extracts on angiogenesis in a rat model of BPH, and investigated
the underlying molecular mechanisms.
Materials and methods
Drugs and reagents
QC (FDA approval no. Z20110009; Fujian, China), as
previously described (35), was
provided by the Academy of Pharmacology of Fujian University of
Traditional Chinese Medicine (Fuzhou, China). The drug powder
inside the QC was dissolved in distilled water and stored at 4°C.
Testosterone propionate injection solution (25 mg/m1) was obtained
from Shanghai GM Pharmaceutical Co., Ltd (Shanghai, China) and
TRIzol reagent was purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). SuperScript II reverse transcriptase was
obtained from Promega Corporation (Madison, WI, USA). Rat VEGF and
bFGF ELISA kits were obtained from Shanghai XiTang Biological
Technology, Co., Ltd. (Shanghai, China). All antibodies were
purchased from Hebei Bohai Biotechnology Development Co., Ltd.
(Shijiazhuang, China) and all other chemicals, unless otherwise
stated, were obtained from Sigma-Adrich (St. Louis, MO, USA).
Animals
In total, 30 specific-pathogen free male
Sprague-Dawley (SD) rats (initial body weight, 200–220 g) were
purchased from Shanghai Si-Lai-Ke Experimental Animal, Co., Ltd.
(Shanghai, China). Rats were housed in clean pathogen-free rooms in
an environment with controlled temperature (22°C), humidity and a
12 h light/dark cycle with free access to water and a standard
laboratory diet. All animal treatments were performed. The study
was conducted in accordance with International Ethical guidelines
and the National Institutes of Health Guide concerning the Care and
Use of Laboratory Animals. Experimental protocols were approved by
the Institutional Animal Care and Use Committee of Fujian
University of Traditional Chinese Medicine.
Grouping and establishing a rat BPH model
and drug administration
A rat model of BPH was generated as previously
described (35). Briefly, the rat
model of BPH was induced by subcutaneous injection of testosterone
propionate following castration. The scrota of 20 rats, out of a
total 30 male SD rats, were castrated. One week after surgery, the
rats were randomly divided into three groups (n=10), termed the
control (10 ml/kg saline), model (10 ml/kg saline) and QC groups,
in which the rats were orally treated with 4.5 g/kg QC. Rats in the
treated groups received the corresponding drug dosage by
gastrogavage, together with subcutaneous injection of testosterone
propionate (5 mg/kg) daily for 28 days.
Sample collection
At the end of the treatments, the animals were
weighed and anesthetized with ketamine-diazepam via intraperitoneal
injection. Blood samples were collected aseptically from the
abdominal aorta and blood-containing tubes were allowed to stand at
room temperature for 2 h. Serum was obtained by centrifugation at
2,000 × g for 20 min at 4°C, and was then stored at −80°C. Intact
prostate tissue was dissociated and removed with caution. The
prostate index (PI) was calculated as follows: Prostate weight
(PW)/body weight (BW) × 100%. One section of prostate tissue was
collected from the same position and fixed with 10% formalin or
stored in liquid nitrogen for later analyses.
Detection of VEGF and bFGF serum levels
by ELISA
ELISA analysis was performed as previously described
(10,35). Serum levels of VEGF and bFGF were
measured using ELISA kits, according to the manufacturer’s
instructions. The concentrations of VEGF and bFGF were determined
by comparison with serial dilutions of VEGF and bFGF purified
standards.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from fresh prostate tissues
using TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
(Promega), according to the manufacturer’s instructions. The
obtained cDNA was used to determine the mRNA expression levels of
VEGF and bFGF by PCR with Taq DNA polymerase (Fermentas;
Thermo Fisher Scientific, Waltham, MA, USA), where β-actin was used
as an internal control. The sequences of the primers used for the
amplification of the VEGF, bFGF and β-actin transcripts were as
follows: VEGF forward, 5′-CAT CCT GGC CTC GCT GTC-3′ and reverse,
5′-CTC GCT CCA ACC GAC TGC-3′ (melting temperature, 61°C; length,
345 bp); bFGF forward, 5′-GCA TGC CCG CAC TGC CGG AGG A-3′ and
reverse, 5′-GCT CAG CTC TTA GCA GAC-3′ (melting temperature, 60°C;
length, 420 bp); β-actin forward, 5′-ACT GGC ATT GTG ATG GAC TC-3′
and reverse, 5′-CAG CAC TGT GTT GGC ATA GA-3′ (melting temperature,
55°C; length, 453 bp). Samples were analyzed by gel electrophoresis
(1.5% agarose) and the DNA bands were examined using a Gel
Documentation System (Model Gel Doc 2000; Bio-Rad, Hercules, CA,
USA).
Immunohistochemical analysis
Tissues were fixed in 10% formaldehyde for 12 h,
paraffin-embedded, sectioned and placed on slides. The slides were
subjected to antigen retrieval and endogenous peroxidase activity
was quenched with hydrogen peroxide. Nonspecific binding was
blocked with normal serum in phosphate-buffered saline (PBS; 0.1%
Tween-20). Polyclonal rabbit anti-rat antibodies against CD31,
HIF-1α, VEGF and bFGF (all at 1:200 dilution) were used to detect
the relevant proteins. Binding of the primary antibody was
demonstrated with a biotinylated secondary horseradish
peroxidase-conjugated streptavidin antibody (Dako UK Ltd,
Cambridge, UK) and diamino-benzidine as the chromogen. The tissues
were counterstained with diluted Harris hematoxylin. Following
staining, five high-power fields (magnification, ×400) were
randomly selected in each slide. The proportion of positive cells
in each field was determined using a true color multifunctional
cell image analysis management system (Image-Pro Plus; Media
Cybernetics, Rockville, MD, USA). To account for nonspecific
staining, PBS was used to replace the primary antibody as a
negative control.
Statistical analysis
Data are presented as the mean ± standard deviation
for the indicated number of independently performed experiments.
Data were analyzed using the SPSS package for Windows (version
17.0; SPSS, Inc., Chicago, IL, USA). Statistical analyses were
conducted with the Student’s t-test and analysis of variance, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of QC on the BW and PI
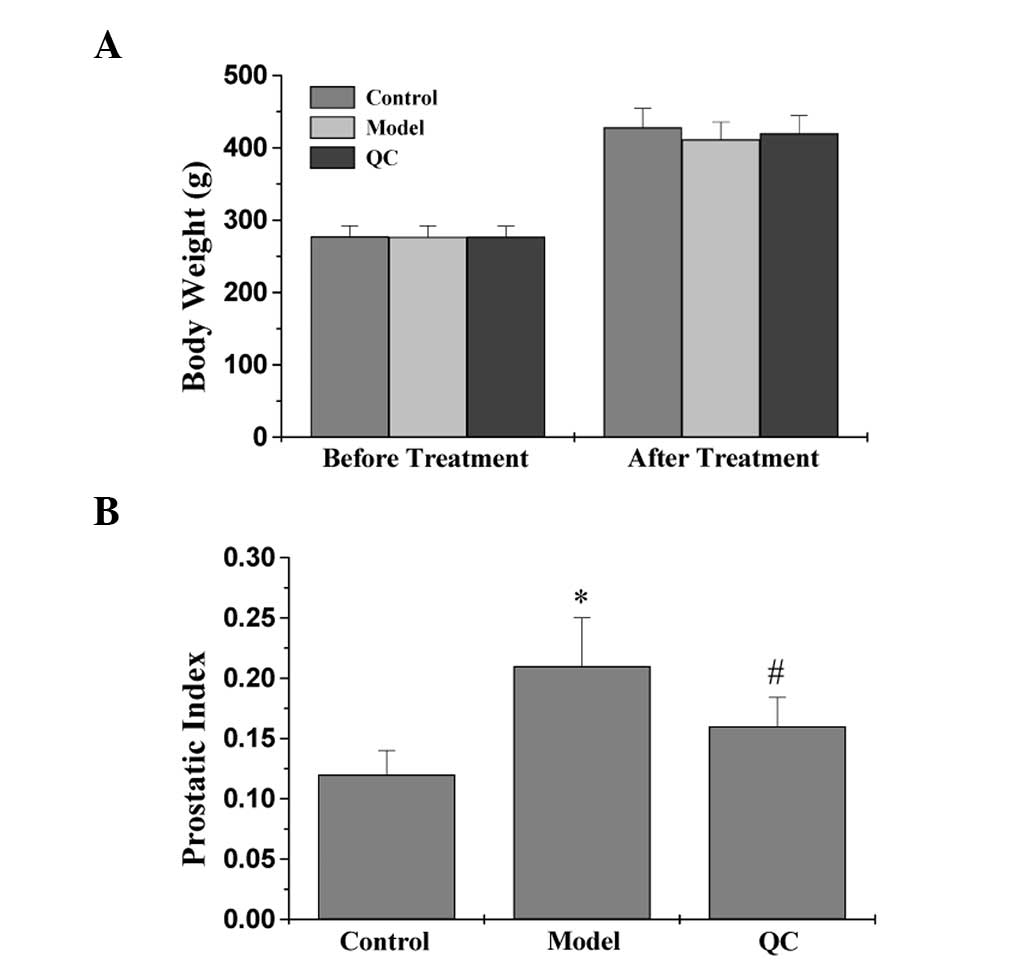
Whether QC treatment caused any adverse health
effects during the study was monitored by measuring BW gain. This
is a relevant and widely used primary indicator to assess the gross
toxicity of testing drugs in intervention studies. As shown in
Fig. 1A, oral administration of QC
did not affect the BW gain and was almost comparable with the
respective control groups (P>0.05), which was consistent with a
previous study of toxicity (37).
To evaluate the efficacy of QC in the treatment of BPH, the effect
of QC on the PI was assessed in BPH rats by calculating the ratio
of PW to BW. In the model group, the PI increased significantly
compared with the control group (P<0.01; Fig. 1B), which continued for a period of
28 days, indicating successful model construction. However,
treatment with QC significantly reduced the PI in the BPH rats when
compared with the model group (P<0.01; Fig. 1B). These observations indicated
that QC exhibits efficacy for the treatment of BPH in rats, without
any apparent signs of toxicity.
QC exhibits antiangiogenic activity in
prostatic tissue of BPH rats
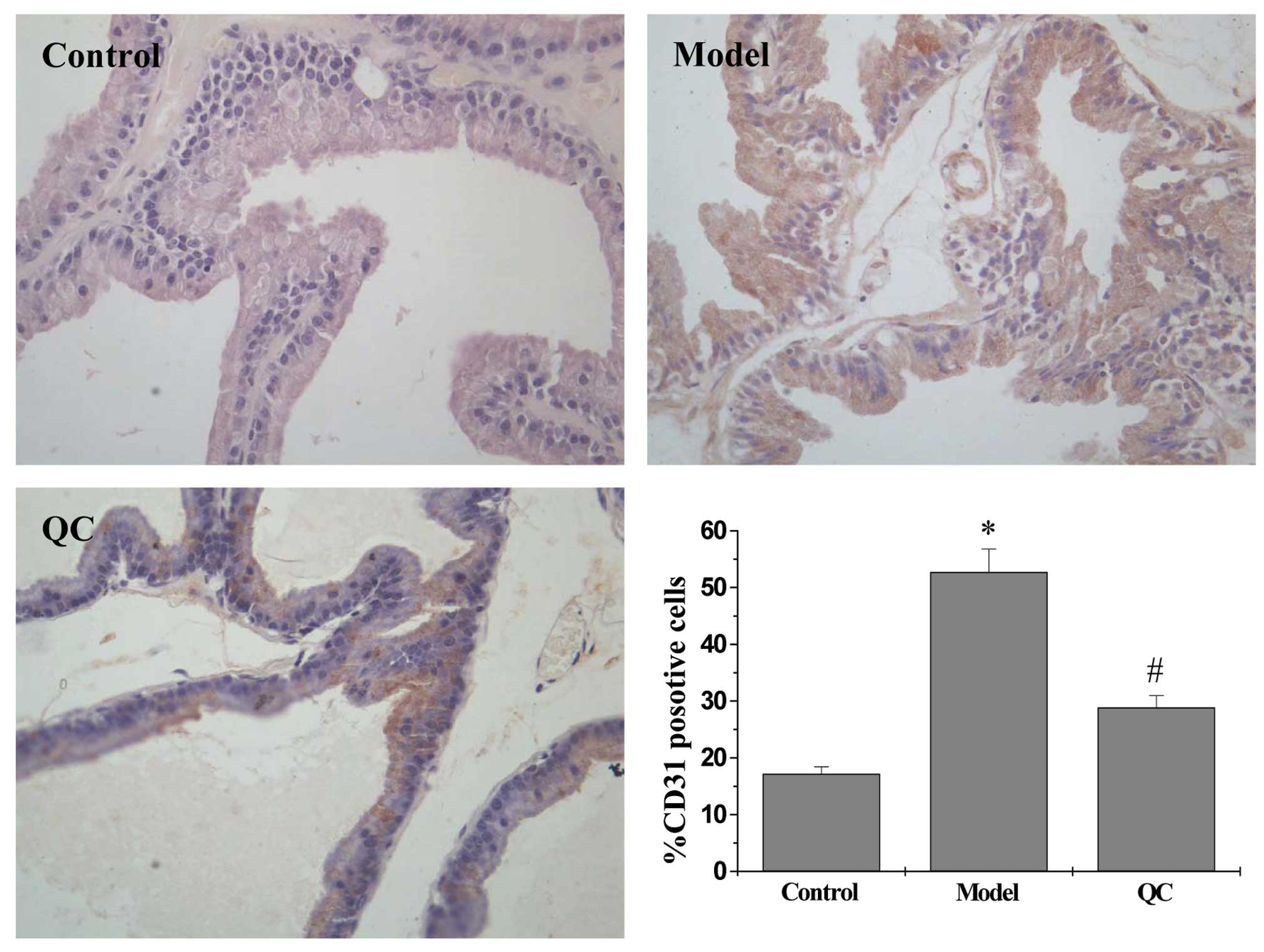
IHC staining for the EC-specific marker, CD31, was
performed to determine the effect of QC on intratumoral microvessel
density (MVD), which is considered to be an indicator of
angiogenesis. As shown in Fig. 2,
the percentage of CD31-positive cells in the model group
significantly increased when compared with the control group
(P<0.01); however, treatment with QC significantly decreased the
number of CD31-positive cells when compared with the model group.
These observations indicated that inhibition of BPH tissue
angiogenesis by QC may contribute to the inhibition of prostatic
cell proliferation.
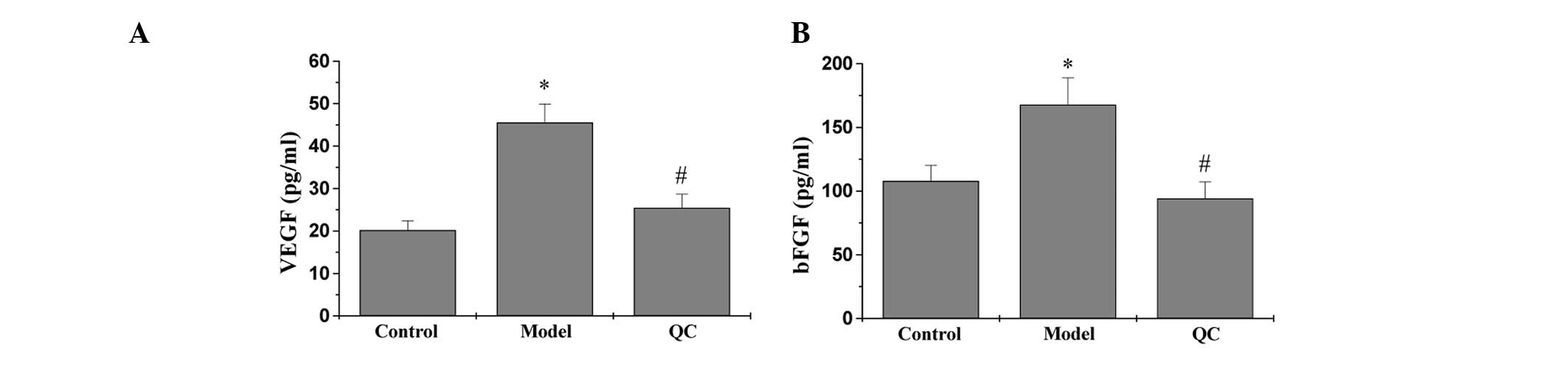
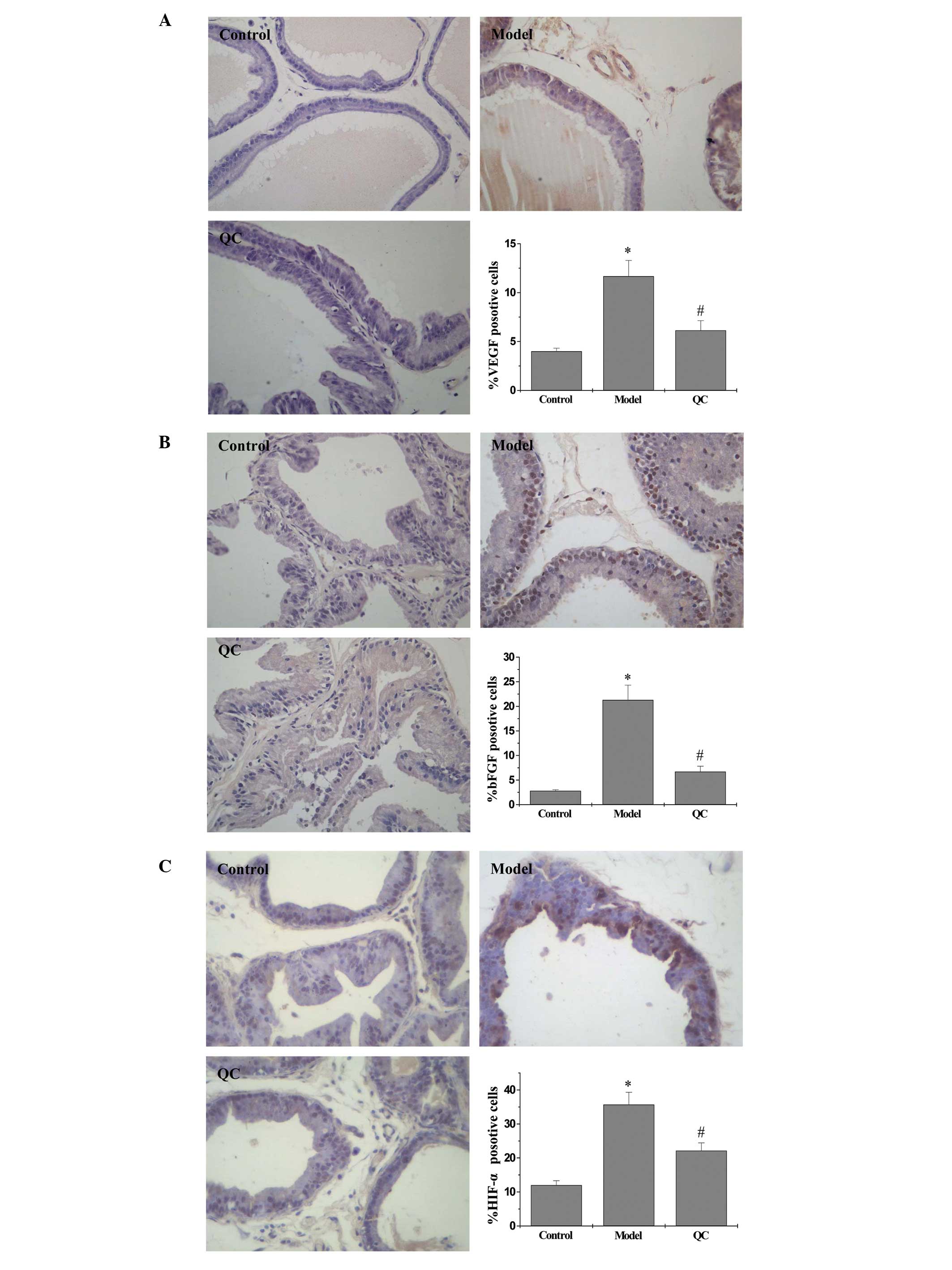
QC downregulates the expression of VEGF
and bFGF in BPH rats
Protein and mRNA expression levels of VEGF and bFGF
in the prostatic tissue of BPH rats were detected using IHC and
qPCR analysis, respectively, while the secretion levels of VEGF and
bFGF in the serum were analyzed by ELISA. The results of the qPCR
assay revealed that the mRNA expression levels of VEGF and bFGF in
the model group were significantly increased when compared with the
control group (P<0.01). However, a marked decrease in expression
was observed following treatment with QC when compared with the
model group (Fig. 3). ELISA and
IHC observations showed that the protein expression patterns of
VEGF and bFGF were similar to the respective mRNA expression levels
(Figs. 4, 5A and B).
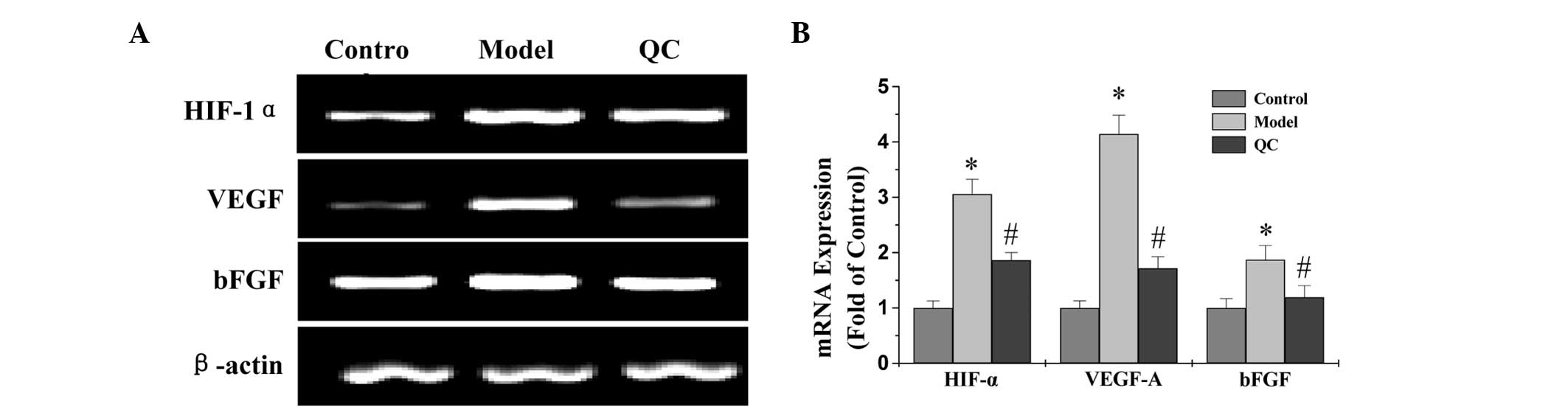 | Figure 3Effect of QC treatment on the mRNA
expression levels of HIF-1α, VEGF and bFGF in prostatic tissue. (A)
mRNA expression levels of HIF-1α, VEGF and bFGF in prostatic tissue
were determined by qPCR and shown by electrophoresis, with β-actin
as an internal control. (B) Densitometric analysis data were
normalized against the mean mRNA expression of the control group
(100%). *P<0.01, vs. control;
#P<0.01, vs. model. QC, qianliening capsule, HIF-1α,
hypoxia-inducible factor-1α; VEGF, vascular endothelial growth
factor; bFGF, basic fibroblast growth factor; qPCR, quantitative
polymerase chain reaction. |
QC suppresses the HIF-1α signaling
pathway in BPH rats
To determine the effect of QC on the HIF-1α
signaling pathway, the mRNA and protein expression levels of HIF-1α
were evaluated in the prostatic tissue of BPH rats using qPCR and
IHC assays. The qPCR results showed that the mRNA expression levels
of HIF-1α in the model group were significantly increased when
compared with the normal group (P<0.01); however, expression was
downregulated following treatment with QC (Fig. 3). Protein expression levels of
HIF-1α in the prostatic tissue of BPH rats were determined using
IHC. As shown in Fig. 5C, the
positive expression levels of HIF-1α in the model group were
markedly increased compared with the normal group (P<0.01);
however, treatment with QC significantly inhibited the effect that
the model construction had on HIF-1α expression. Therefore, these
observations indicated that QC inhibits HIF-1α signaling in BPH
rats.
Discussion
BPH is the most common proliferative disease of the
prostate in males, greater than 90% of men are affected by the age
of 80. The pathogenesis of BPH is complex and not yet elucidated.
Major theories of BPH pathogenesis include DHT,
interstitium-epithelial cell interaction, androgen-estrogen
coordination, embryo rearousal, cell proliferation and apoptosis
disorder (38,39). Recently, angiogenesis has become an
attractive target for BPH treatment due to its essential role in
the progression and development of BPH. Angiogenesis is a complex
process and regulated by a variety of molecules. Therefore, BPH
treatment reagents that affect a single target may be insufficient
and long-term administration may generate side effects. These
problems highlight the urgency for the development of multi-target
agents with minimal side effects and toxicity. Natural products,
including TCMs, have been used clinically to treat various types of
diseases, including BPH (28,29,40).
QC, a TCM formulation, is a complex combination of numerous natural
products, each of which contains a number of chemical compounds.
Therefore, QC is considered to be a multi-component and
multi-target agent that exerts a therapeutic function in a more
holistic manner; and is a promising approach for BPH treatment.
However, the mechanism underlying the anti-BPH activity remains
largely unknown.
In the present study, QC was confirmed to
significantly reduce the PI in BPH rats without affecting the BW,
consistent with the observations of a previous study (35). Notably, through IHC staining for
the endothelial cell-specific marker, CD31, QC was found to
significantly reduce the intraprostatic MVD, demonstrating an in
vivo antiangiogenic activity. Angiogenesis is tightly regulated
by the HIF-1α signaling pathway, since activation of HIF-1α
signaling upregulates the expression of VEGF and bFGF, which are
strong angiogenesis stimulators. VEGF and bFGF exert a
proangiogenic function via binding to specific receptors, leading
to a series of angiogenic processes (18,41).
In the present study, QC treatment was shown to inhibit the
activation of the HIF-1α pathway in prostatic hyperplasia tissues,
with QC significantly suppressing the mRNA and protein expression
of HIF-1α. Consistently, administration of QC significantly
decreased the serum levels of VEGF and bFGF in BPH rats, as well as
downregulated the mRNA and protein expression levels of VEGF and
bFGF in prostatic tissue.
Angiogenesis is regulated by multiple pathways,
including the Ras/extracellular signal-regulated kinase,
phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin,
Hedgehog and Wnt signaling pathways. In addition, QC is composed of
rhubarb, leech, astragalus, achyranthes and dodder, which contain a
number of chemical compounds, including emodin, calycosin, hirudin,
D-β-asparagine, astragalan, rhein, chrysophanol, oleanolic acid,
ursolic acid, ecdysterone, isocyasterone, quercetin, coumarin and
stigmasterol. It is unknown which of these compounds contribute to
the anti-BPH activity, or whether the compounds of QC target
various sites individually or function on a single site additively
or synergistically. It also remains unclear which signaling
pathways these compounds are involved in to exert their
bioactivity. Future study should focus on addressing these
questions in order to completely elucidate the molecular mechanism
by which QC treats BPH, with the aim of developing improved
multi-target drugs for BPH therapy.
In conclusion, for the first time, the present study
has demonstrated that QC inhibits angiogenesis in prostatic tissue
of BPH rats via the inhibition of the HIF-1α signaling pathway,
which may in part explain the mechanism underlying the activity of
QC in BPH treatment.
Acknowledgements
The study was supported by grants from the Nature
Science Foundation of China (nos. 81173433 and 81373817), the
Natural Science Foundation of Fujian Province of China (no.
2010J01199) and the Research Foundation of Education Bureau of
Fujian Province of China (no. JA09128).
Abbreviations:
|
QC
|
qianliening capsule
|
|
BPH
|
benign prostatic hyperplasia
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
VEGF
|
vascular endothelial growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
BW
|
body weight
|
|
PI
|
prostatic index
|
|
LUTS
|
lower urinary tract symptoms
|
|
EC
|
endothelial cell
|
|
DHT
|
dihydrotestosterone
|
|
SD
|
Sprague-Dawley
|
|
PW
|
prostate weight
|
|
RT-PCR
|
reverse transcription- polymerase
chain reaction
|
|
PBS
|
phosphate-buffered saline
|
|
MVD
|
microvessel density
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
O’Malley KJ, Dhir R, Nelson JB, Bost J,
Lin Y and Wang Z: The expression of androgen-responsive genes is
up-regulated in the epithelia of benign prostatic hyperplasia.
Prostate. 69:1716–1723. 2009.PubMed/NCBI
|
|
2
|
Paolone DR: Benign prostatic hyperplasia.
Clin Geriatr Med. 26:223–239. 2010. View Article : Google Scholar
|
|
3
|
Roehrborn CG: Male lower urinary tract
symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin
North Am. 95:87–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh SJ: Unsolved issues in managing benign
prostatic hyperplasia. Korean J Urol. 54:349–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Is tissue mass regulated by
vascular endothelial cells? Prostate as the first evidence.
Endocrinology. 139:441–442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soulitzis N, Karyotis I, Delakas D and
Spandidos DA: Expression analysis of peptide growth factors VEGF,
FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic
hyperplasia. Int J Oncol. 29:305–314. 2006.PubMed/NCBI
|
|
7
|
Ren J, Huan Y, Wang H, et al: Dynamic
contrast-enhanced MRI of benign prostatic hyperplasia and prostatic
carcinoma: correlation with angiogenesis. Clin Radiol. 63:153–159.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucia MS and Lambert JR: Growth factors in
benign prostatic hyperplasia: basic science implications. Curr Urol
Rep. 9:272–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bostanci Y, Kazzazi A, Momtahen S, Laze J
and Djavan B: Correlation between benign prostatic hyperplasia and
inflammation. Curr Opin Urol. 23:5–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JM, Zhao JY, Zhuang QC, Hong ZF and
Peng J: Xiongshao capsule promotes angiogenesis of HUVEC via
enhancing cell proliferation and up-regulating the expression of
bFGF and VEGF. Chin J Integr Med. 17:840–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar
|
|
12
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CM, Chiu JH, Wu IH, Wang BW, Pan CM
and Chen YH: Ferulic acid augments angiogenesis via VEGF, PDGF and
HIF-1 alpha. J Nutr Biochem. 21:627–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi YH, Bingle L, Gong LH, Wang YX, Corke
KP and Fang WG: Basic FGF augments hypoxia induced HIF-1-alpha
expression and VEGF release in T47D breast cancer cells. Pathology.
39:396–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andrikopoulou E, Zhang X, Sebastian R, et
al: Current insights into the role of HIF-1 in cutaneous wound
healing. Curr Mol Med. 11:218–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong XY, Wang J and Li Z: AGR2 expression
is regulated by HIF-1 and contributes to growth and angiogenesis of
glioblastoma. Cell Biochem Biophys. 67:1487–1495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin J, Wei L, Xu W, Hong Z, Liu XX and
Peng J: Effect of Hedyotis Diffusa Willd extract on tumor
angiogenesis. Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
20
|
Goto F, Goto K, Weindel K and Folkman J:
Synergistic effects of vascular endothelial growth factor and basic
fibroblast growth factor on the proliferation and cord formation of
bovine capillary endothelial cells within collagen gels. Lab
Invest. 69:508–517. 1993.
|
|
21
|
Prior BM, Yang HT and Terjung RL: What
makes vessels grow with exercise training? J Appl Physiol (1985).
97:1119–1128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roehrborn CG, Nuckolls JG, Wei JT and
Steers W; BPH Registry and Patient Survey Steering Committee. The
benign prostatic hyperplasia registry and patient survey: study
design, methods and patient baseline characteristics. BJU Int.
100:813–819. 2007. View Article : Google Scholar
|
|
23
|
Black L, Naslund MJ, Gilbert TD Jr, Davis
EA and Ollendorf DA: An examination of treatment patterns and costs
of care among patients with benign prostatic hyperplasia. Am J
Manag Care. 12(4 Suppl): S99–S110. 2006.PubMed/NCBI
|
|
24
|
MacDonald R and Wilt TJ: Alfuzosin for
treatment of lower urinary tract symptoms compatible with benign
prostatic hyperplasia: a systematic review of efficacy and adverse
effects. Urology. 66:780–788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Djavan B and Marberger M: A meta-analysis
on the efficacy and tolerability of alpha1-adrenoceptor antagonists
in patients with lower urinary tract symptoms suggestive of benign
prostatic obstruction. Eur Urol. 36:1–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gormley GJ, Stoner E, Bruskewitz RC, et
al: The effect of finasteride in men with benign prostatic
hyperplasia. The Finasteride Study Group. N Engl J Med.
327:1185–1191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roehrborn CG, Boyle P, Nickel JC, et al;
ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and
safety of a dual inhibitor of 5-alpha-reductase types 1 and 2
(dutasteride) in men with benign prostatic hyperplasia. Urology.
60:434–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boyle P, Robertson C, Lowe F and Roehrborn
C: Meta-analysis of clinical trials of permixon in the treatment of
symptomatic benign prostatic hyperplasia. Urology. 55:533–539.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilt T, Ishani A, MacDonald R, Stark G,
Mulrow C and Lau J: Beta-sitosterols for benign prostatic
hyperplasia. Cochrane Database Syst Rev. CD0010432000.PubMed/NCBI
|
|
30
|
Wilt T, Ishani A, Mac Donald R, Rutks I
and Stark G: Pygeum africanum for benign prostatic hyperplasia.
Cochrane Database Syst Rev. CD0010442002.PubMed/NCBI
|
|
31
|
Lin JM, Huang YP, Zhou Jh and Hong ZF:
Therapeutic efficacy of Qianliening capsules in the treatment of
benign prostatic hyperplasia. Yatai Chuantong Yiyao. 9:140–143.
2013.(In Chinese).
|
|
32
|
Zhou J, Lin J, Xu W, Zhong X, Zheng Y,
Hong Z and Peng J: Qianliening capsule treats benign prostatic
hyperplasia through regulating the expression of sex hormones,
estrogen receptor and androgen receptor. Afr J Pharm and Pharmacol.
6:173–180. 2012. View Article : Google Scholar
|
|
33
|
Zhong X, Lin J, Zhou J, Xu W, Hong Z and
Peng J: Qianliening capsule treats benign prostatic hyperplasia
(BPH) by down-regulating the expression of PCNA, CyclinD1 and CDK4.
Afr J Biotechnol. 11:7731–7737. 2012.
|
|
34
|
Zheng H, Xu W, Lin J, Peng J and Hong ZF:
Qianliening capsule treats benign prostatic hyperplasia via
induction of prostatic cell apoptosis. Mol Med Rep. 7:848–854.
2013.PubMed/NCBI
|
|
35
|
Lin J, Zhou J, Xu W, Zhong X, Hong Z and
Peng J: Qianliening capsule treats benign prostatic hyperplasia via
suppression of the EGF/STAT3 signaling pathway. Exp Ther Med.
5:1293–1300. 2013.PubMed/NCBI
|
|
36
|
Hong ZF, Lin JM, Zhong XY, et al:
Qianliening capsule inhibits human prostate cell growth via
induction of mitochondrion-dependent cell apoptosis. Chin J Integr
Med. 18:824–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng HY, Xu W, Lin JM, Li H, Zhou JH and
Hong ZF: Toxicological studies on qianliening capsule. Zhe Jiang
Zhong Yi Yao Da Xue Xue Bao Bian Ji Bu. 35:63–65. 462011.(In
Chinese).
|
|
38
|
Tang J and Yang J: Etiopathogenesis of
benign prostatic hyperplasia. Indian J Urol. 25:312–317. 2009.
View Article : Google Scholar
|
|
39
|
Bosch RJ: Pathogenesis of benign prostatic
hyperplasia. Eur Urol. 20(Suppl 1): 27–30. 1991.
|
|
40
|
Huang YP, Du J, Hong ZF, Chen ZQ, Wu JF
and Zhao JY: Effects of Kangquan Recipe on sex steroids and cell
proliferation in rats with benign prostatic hyperplasia. Chin J
Integr Med. 15:289–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29(6 Suppl 16): 10–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|