Introduction
The main pathological changes in acute respiratory
distress syndrome (ARDS) are diffuse pulmonary interstitial and
alveolar edema, caused by increased pulmonary microvascular
permeability. The clinical fatality rate of ARDS is as high as
40–70% (1). The underlying
pathogenesis of ARDS is excessively uncontrolled inflammatory
responses that are mediated by various inflammatory cytokines,
however, treatment has limited efficacy (2). Lipopolysaccharide (LPS) is the major
component of the outer membrane of gram-negative bacteria and is a
common trigger of sepsis, which is an important initiating factor
in the activation of nuclear factor (NF)-κB (3). Previous studies have demonstrated
that a large number of polymorphonuclear neutrophils (PMNs)
accumulate in the lung tissue and release inflammatory cytokines,
including interleukin (IL)-1β, IL-8 and tumor necrosis factor
(TNF)-α, when the body fights against infection or trauma, which
plays an important role in initiating and maintaining the
inflammatory response (4,5). Research has often focused on the
activation of monocyte-macrophage systems, which has gradually
gained interest in the study of the pathogenesis of ARDS. PMNs are
important effector cells in ARDS, and the infiltration and
activation of PMNs involve a complex process that includes the
recruitment, adhesion and chemotaxis of PMNs. In addition, the
process is associated with PMN adhesion molecules CD11b/CD18,
pulmonary vascular endothelial intercellular adhesion molecule-1
(ICAM-1) and PMN chemokines, such as IL-8. The expression of
CD11b/CD18 plays an important role in PMN aggregation and
activation (6). CD11b/CD18 is a
heterodimer of the αM (CD11b) and β2 (CD18) subunits, which are key
factors in the inflammatory response, as they combine with ICAM-1
and mediate adhesion between PMNs and microvascular endothelial
cells, which then transmits an intracellular signal (7). CD11b/CD18 may be used as indicators
of cell adhesion function (6).
NF-κB is a critical nuclear transcription factor that activates the
transcription of a number of inflammatory cytokine genes. The
activation of NF-κB is closely associated with the overexpression
of adhesion molecules, chemokines and other cytokines. The
aggregation and migration of PMNs, which participate in the
regulation of inflammation, plays an important role in ARDS
(8).
Pyrrolidine dithiocarbamate (PDTC), an inhibitor of
NF-κB, inhibits the expression of inflammatory cytokines, such as
TNF-α and ILs, at the transcriptional level (9). In addition, PDTC reduces the
expression of adhesion molecules on the surface of PMNs and
endothelial cells and the accumulation of inflammatory cells in
inflammatory sites (10,11). This reduces the release of
myeloperoxidase (MPO) in the inflammatory sites, thus, decreases
the damage to the body (12).
Apoptosis is the main pathway of PMN clearance in the inflammatory
response. PDTC may promote the apoptosis of PMN by inhibiting the
activation of NF-κB (13).
Therefore, regulating the activation of NF-κB and
inhibiting p65 subunit transfer to the nucleus may prevent the
aggregation of PMNs and reduce the incidence of ARDS. The aim of
the present study was to evaluate the direct effects of PDTC on PMN
activity, characterized by the protein expression changes of NF-κB
p65, the infiltration of PMNs and the excessive release of
inflammatory cytokines.
Materials and methods
Animals
BALB/c mice (age, 6–8 weeks; weight, 20±2 g) were
purchased from the Experimental Animal Center of Shandong
University (Jinan, China). The experimental procedures were
approved by the ethics review committee for animal studies at Qilu
Hospital, Shandong University and according to animal welfare and
all the animals experimental guidelines were followed. Mice were
maintained at room temperature (24°C) with a 12:12 h light-dark
cycle and allowed free access to water and standard laboratory
chow.
Survival ratio of the mice and
experimental grouping
To assess the survival ratio, the mice received an
intraperitoneal injection of 20 mg/kg LPS (Escherichia coli
O55:B5; Sigma-Aldrich, St. Louis, MO, USA) with or without various
intraperitoneal doses of PDTC (40, 120 or 160 mg/kg; L04358;
ALIKESI, USA). PDTC was administered 30 min prior to the LPS
challenge. The survival rates of the mice were recorded every 12 h
for three days following LPS administration in each group
(n=10).
To further study the protective effect of PDTC on
mice treated with LPS, the mice were randomly divided into three
groups: Control (20 ml/kg saline, i.p.), LPS (20 mg/kg LPS, i.p.)
and PDTC + LPS (120 mg/kg PDTC, i.p. and 20 mg/kg LPS, i.p.). The
mice were anesthetized by intraperitoneal injection of 10% chloral
hydrate (3.5 ml/kg) and sacrificed using arotic phlebotomy at 4, 12
and 24 h.
Histopathological analysis
The left lung was fixed with 4% paraformaldehyde for
24 h, embedded in paraffin and cut into 4-μm sections. Following
staining with hematoxylin and eosin (Sigma-Aldrich), microscopic
evaluation was performed to characterize the injury status. Lung
injury was scored according to congestion, edema, interstitial
inflammation and the aggregation of inflammatory cells. Each
pathological feature was scored on a scale from 0 (normal) to 4
(severe). The total score was calculated by adding up the
individual scores of each region.
Lung wet to dry weight (W/D) ratio
measurement
The right lung was removed and the wet weight was
measured. Lung tissue was placed in an oven at 80°C for 72 h to
obtain the dry weight. The W/D ratios of the lungs were calculated
to quantify the degree of pulmonary edema.
Lung permeability index (LPI)
The LPI was equal to the total protein in the
bronchoalveolar fluid (BALF) divided by the total protein in the
serum. The protein concentration in the BALF and serum was measured
using the Bradford Assay (Thermo Fisher Scientific, Waltham, MA,
USA) (14).
Preparation of the BALF
BALF was collected and washed three times with 1.5
ml ice-cold phosphate-buffered saline (PBS) in all the groups. In
each mouse, 80% (1.2 ml) of the total volume was recovered
(15). Following centrifugation of
the BALF at 1,700 × g for 7 min at 4°C, the supernatant was stored
at −80°C for subsequent experiments.
The cell pellet in the BALF was resuspended in 1 ml
red blood cell lysis buffer to eliminate the red cells. White cells
in the BALF were then repelleted by centrifugation at 500 × g for
20 min at 4°C. The cell pellet was again suspended in PBS and the
proportion of PMNs and living cells was adjusted to >95% by
trypan blue staining.
Extraction of protein in the cytoplasm
and nucleus (16)
Lung tissue samples, weighing ~100 mg, were
thoroughly washed with 0.01 M PBS, adding 1.5 ml nuclear protein
extract lysis buffer A (BioTeke Corporation, Beijing, China). The
samples were then placed on ice for 15–30 min and homogenized with
an electric homogenizer following the addition of 0.5 ml ice cold
10% NP-40. Next, the samples were vortexed for 10 sec and
centrifuged at 4°C and 12,000 ×g for 30 sec; the supernatant
produced was the cytoplasm protein extract. The precipitate was
washed once with cold PBS and centrifuged at 4°C and 12,000 × g for
30 sec, after which the supernatant was discarded. Next, 1.5 ml
join nucleoprotein extract lysis buffer B (BioTeke Corporation) was
added and the samples were placed on ice for 30 min. The samples
were centrifuged again at 4°C and 12,000 g/min for 2 min to produce
the aspirate supernatant (nucleoprotein). Using Bradford
colorimetric determination, the nuclear protein concentration was
adjusted to 0.5–1.0 μg/μl and the samples were placed and stored at
−70°C.
Measurement of NF-κB p65 protein
expression by western blot analysis
Total protein was quantitated using the Pierce BCA
Protein Assay kit (Thermo Fisher Scientific). Equal quantities of
total protein (20 μg) were separated on 10% Bis-Tris gels in MOPS
SDS Running Buffer (Invitrogen Life Technologies, Carlsbad, CA,
USA) and transferred to polyvinylidene difluoride (PVDF) membranes
(Immobilon; Millipore Corporation, Bedford, MA, USA). The PVDF
membranes were blocked with 5% skim milk in Tris-buffered saline
[TBS; 50 mM Tris-Cl (pH 7.5), 150 mM NaCl] for 60 min at room
temperature and then incubated overnight with anti-pNF-κB p65
(1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
anti-β-actin antibodies (1:500; Santa Cruz Biotechnology, Inc.) in
TBS-Tween-20 (TBST; 0.1% Tween-20). The membranes were washed three
times with 1X TBST for 5 min, and then incubated for 1 h with
secondary antibodies conjugated to horseradish peroxidase (HRP) at
room temperature. The membrane was exposed to high performance
autoradiography film (Fuji XR film; Fujifilm Corporation, Tokyo,
Japan) and visualized using an enhanced chemiluminescence system
(Santa Cruz Biotechnology, Inc.).
Integrated density values of the band intensities
from the films were analyzed by ImageQuant 5.2 software (Molecular
Dynamics, Sunnyvale, CA, USA).
Measurement of the PMN apoptosis rate in
the BALF by flow cytometry
Freshly isolated PMNs were suspended in 100 μl
incubation buffer (10 mmol HEPES, 140 mmol NaCl, 5 mmol
CaCl2 at pH 7.14), 2 μl Annexin-V-FLUOS and 2 μl
propidium iodide (PI; 50 mg/l) at room temperature for 10–15 min.
Subsequently, 5,000 PMNs were analyzed using a flow cytometer
(Bio-Rad, Hercules, CA, USA). If positive for Annexin-V, the cells
were early apoptotic cells, however, if the cells were positive for
PI, the cells were necrotic. When the cells were Annexin-V- and
PI-positive, the PMNs were late apoptotic necrotic cells.
Immunohistochemistal analysis of the
expression of ICAM-1 and CD11b/CD18
Mice were sacrificed and the lung lobes were
dissected, fixed in 10% formaldehyde and processed for
immunohistochemistry. Sections (4 μm thick) were dewaxed,
rehydrated and antigen retrieval was performed with 10 mM sodium
citrate (pH 6.1). The sections were then blocked with 5% bovine
serum albumin (Sigma-Aldrich) for 60 min at room temperature and
incubated with anti-ICAM-1 (1:200; Santa Cruz Biotechnology, Inc.)
and anti-CD11b/CD18 antibodies (1:300; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. Subsequently, the samples were incubated
with polyclonal HRP-conjugated secondary antibodies (1:100; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. The nuclei
were counterstained with hematoxylin (Sigma-Aldrich) and the
control slides were incubated with the same antibodies. Cover slips
were mounted with 80% glycerol (ZsBio, Beijing, China).
Samples were examined using a microscope equipped
with a digital camera (Hitachi, Ltd., Tokyo, Japan). ICAM-1 and
CD11b/CD18 positive expression areas were quantified by
densitometry using Image-Pro Plus software (Media Cybernetics,
Inc., Rockville, MD, USA).
Measurement of TNF-α, IL-1β and IL-8
expression in the BALF by ELISA
Levels of TNF-α, IL-1β and IL-8 in the BALF were
determined using commercially available ELISA kits, according to
the manufacturer’s instructions.
MPO activity
The left lobe specimens of the mice were removed and
preserved at −80°C. The specimens were placed in an eppendorf tube
and media was added at the ratio of 1:19, according to the
volume and weight. Samples were then centrifuged at 5,000 × g for
15 min. MPO colorimetric absorbance was measured at 460 nm,
according to the manufacturer’s instructions (Wuhan Amyjet
Scientific Co., Ltd., Wuhan, China), and the activity was
calculated as described previously (17).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analysis was performed using analysis of
variance and Tukey’s test was used for comparisons among groups.
P<0.05 was considered to indicate a statistically significant
difference. Survival data are presented using the Kaplan-Meier
method and statistical analysis was conducted with SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA).
Results
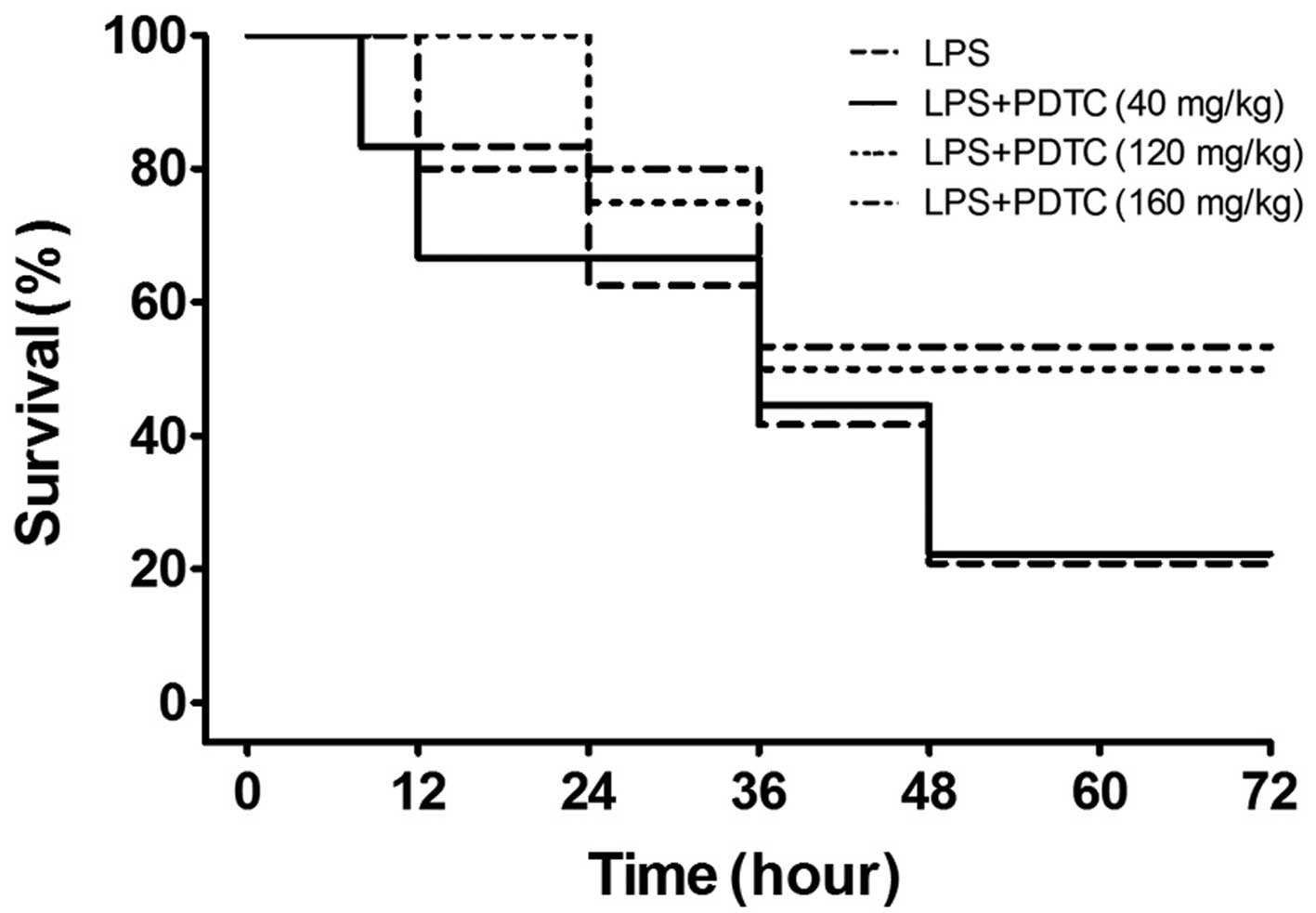
Effect of PDTC on LPS-induced mortality
in mice
Shortness of breath, oral cyanosis and blood-like
secretions in the nose were observed in the mice following
treatment with LPS for 4 h, and the manifestations were progressive
over time. The survival rate at 12, 24, 36, 48, 60 and 72 h were
100, 70, 50, 20, 20 and 20%, respectively. As shown in Fig. 1, the accumulative mortality rates
during the 72 h in the 120 and 160 mg/kg PDTC treatment groups were
50 and 48%, respectively, which was significantly lower than the
LPS group (80%; P<0.01). However, 40 mg/kg PDTC failed to
protect against mortality (P>0.05). Therefore, 120 mg/kg PDTC
was used in the further experiments.
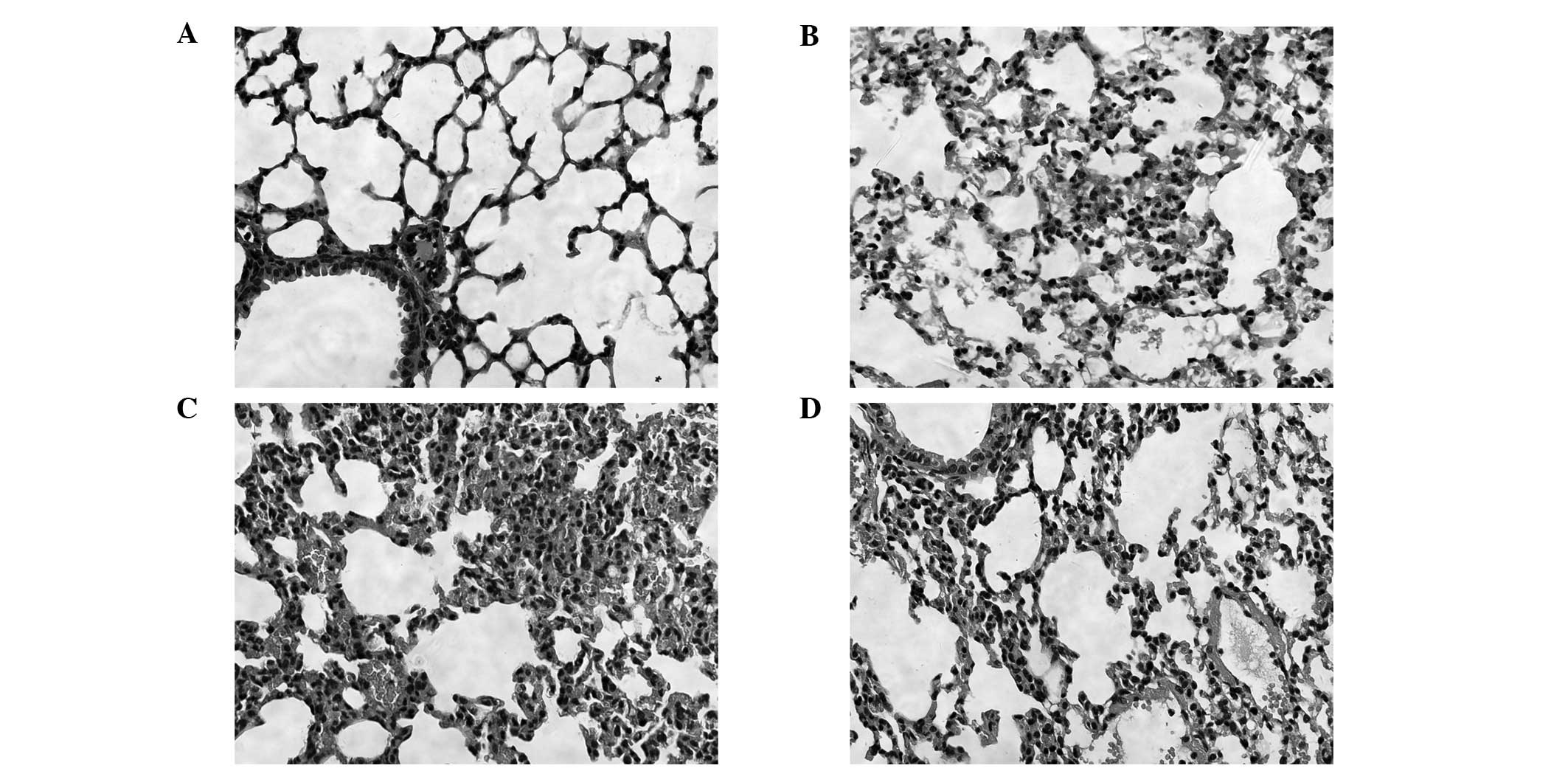
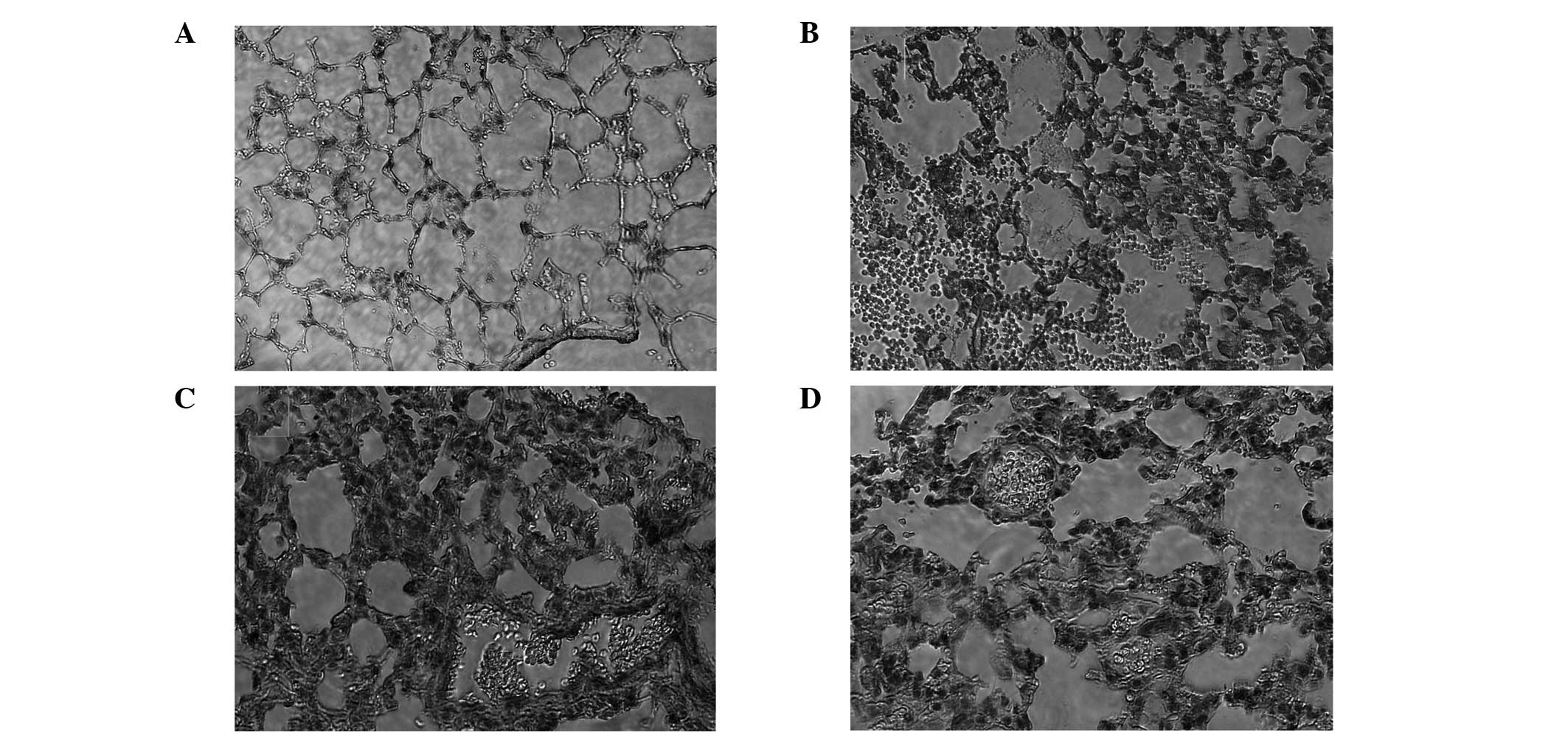
Pathology results of the lung tissue
Effects of PDTC on lung histopathological changes
were analyzed in the mice challenged with LPS. In the control
group, the structure of the lung tissue was integrated and there
were no inflammatory cells. In the 4 and 24 h LPS groups, widened
lung intervals, highly congested pulmonary interstitial space,
fractured alveolar walls and a large number of infiltrative
inflammatory cells were observed. In addition, inflammatory cell
infiltration increased and lung tissue damage became aggravated as
the time progressed. In the 24 h PDTC + LPS group, there were
similar symptoms to the LPS groups, however, the lung tissue injury
was milder than that observed in the LPS groups (Fig. 2).
Determination of the W/D ratio and LPI by
assessing the pulmonary vascular permeability change
Lung W/D ratio values significantly increased in the
mice that had received an intraperitoneal injection of LPS compared
with the control group at 4, 12 and 24 h (P<0.05). In addition,
the W/D ratio values gradually increased as the time extended.
Compared with the LPS group, the W/D ratios significantly decreased
in the mice that had received an intraperitoneal injection of PDTC,
but were higher compared with the control group (P<0.05;
Fig. 3).
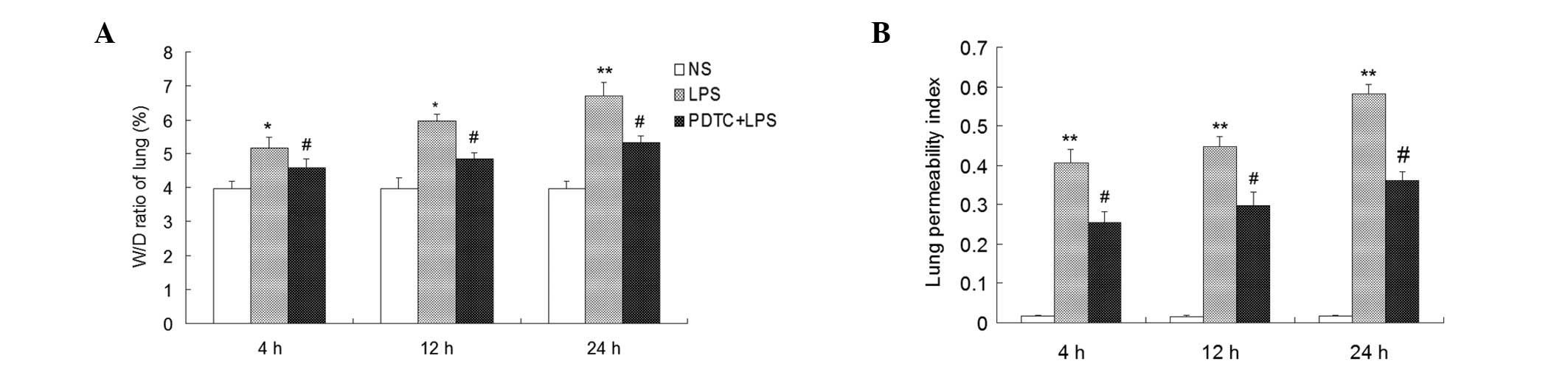 | Figure 3Effect of PDTC on the W/D ratio of
lung tissue and protein leakage in BALF samples of LPS-induced ARDS
mice. PDTC (120 mg/kg, i.p.) was administered 30 min prior to LPS
administration (20 mg/kg, i.p.). Mice were anesthetized at 4, 12
and 24 h following the saline or LPS challenge. Lung tissue
samples, BALF and serum were then collected immediately for (A)
lung W/D ratio and (B) lung permeability index assays. Values are
presented as the mean ± standard deviation. *P<0.05
and **P<0.01, vs. control; #P<0.05, vs.
LPS group (n=10). NS, normal saline; PDTC, pyrrolidine
dithiocarbamate; LPS, lipopolysaccharide; W/D, wet to dry weight;
BALF, bronchoalveolar lavage fluid; ARDS, acute respiratory
distress syndrome. |
LPI values were significantly higher in the LPS
group compared with the control group (P<0.01), and PDTC was
shown to inhibit the LPI increase (P<0.05; Fig 3).
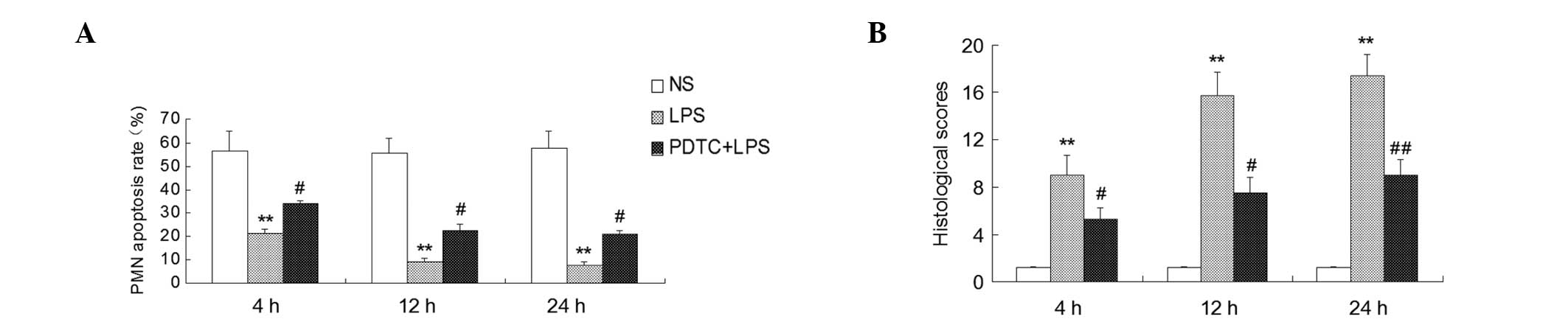
Apoptosis rates and histological scores
of the PMNs
As determined by flow cytometry, the PMN apoptosis
rates were lower in the BALF of the LPS group compared with the
control group at 4, 12 and 24 h following treatment (P<0.01).
Compared with the LPS group, the PMN apoptosis rate significantly
increased in the PDTC + LPS group (P<0.05; Fig. 4). Histological scores of the lung
tissue were significantly increased in the mice challenged with LPS
as compared with the control group (P<0.01). In addition, the
histological scores were lower in the mice that had received an
intraperitoneal injection of PDTC than in the LPS group at 4 and 12
h (P<0.05, P<0.01; Fig. 4),
at 24 h (P<0.01; Fig. 4).
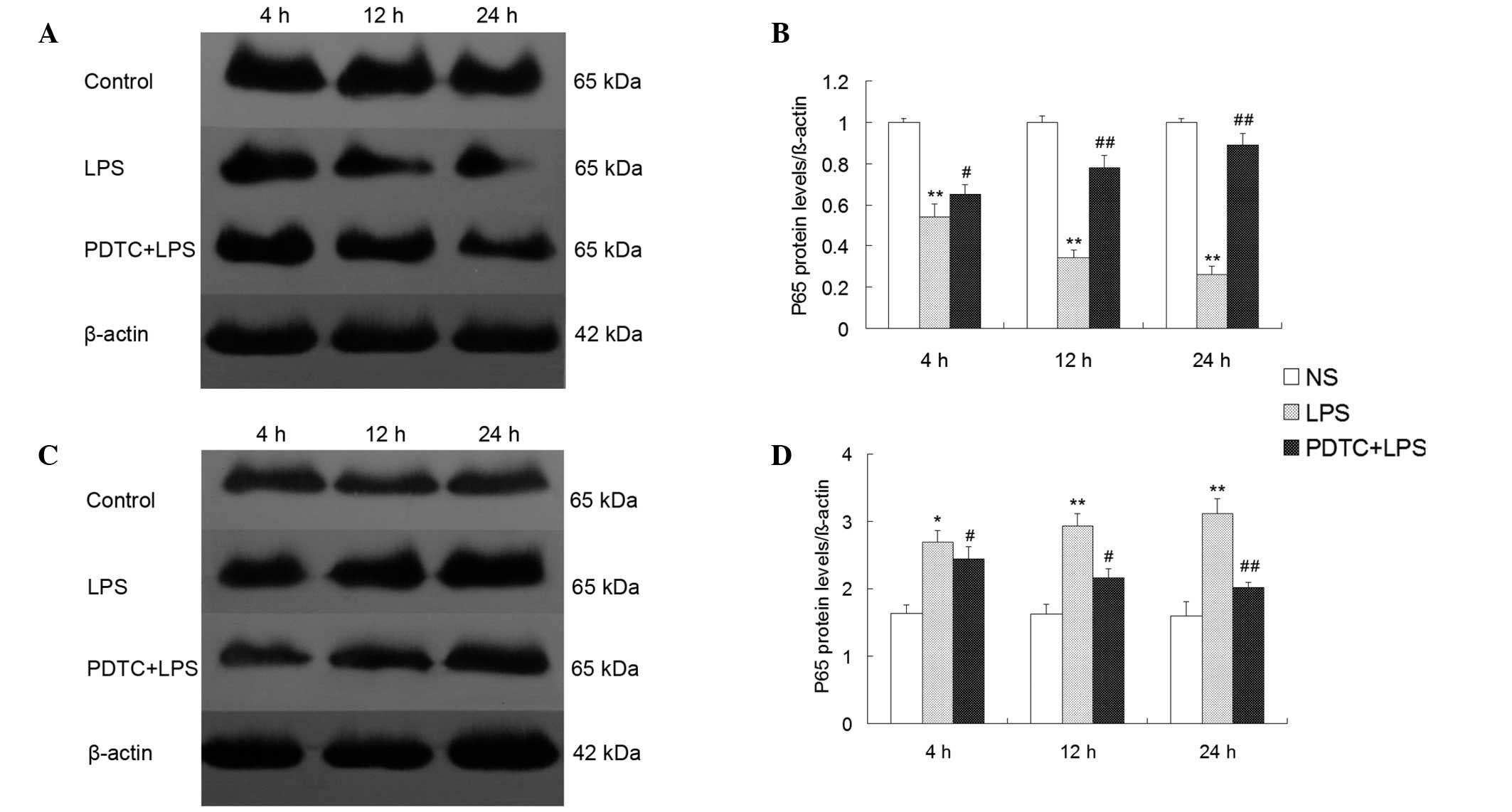
NF-κB p65 protein expression in the
cytoplasm and nuclei of lung tissue
NF-κB p65 protein expression was significantly lower
in the cytoplasm of the LPS group compared with the control group
(P<0.01), however, p65 protein expression was significantly
higher in the pulmonary nuclei of the LPS group compared with the
control group (P<0.01). The p65 protein expression intensity in
the cytoplasm of the lung tissue was increased in the PDTC + LPS
group at 4 h (P<0.05; Fig. 5),
at 12 and 24 h (P<0.01; Fig.
5), while p65 protein expression was decreased in the nuclei of
the PDTC + LPS groupat 4 and 12 h (P<0.05; Fig. 5), at 24 h (P<0.01; Fig. 5).
Expression of TNF-α, IL-1β and IL-8 in
the BALF
The protective effect of PDTC on the overproduction
of proinflammatory cytokines induced by LPS was observed. TNF-α,
IL-1β and IL-8 expression levels in the BALF of the LPS group were
markedly higher compared with the control group (P<0.01).
However, in the group treated with 120 mg/kg PDTC prior to
treatment with LPS, the expression levels of TNF-α was
significantly decreased compared with the LPS group at 4 h
(P<0.05; Fig. 6), at 12 and 24
h (P<0.01; Fig. 6); the
expression levels of IL-1β and IL-8 were markedly decreased
compared with the LPS group at 4 h (P<0.01; Fig. 6).
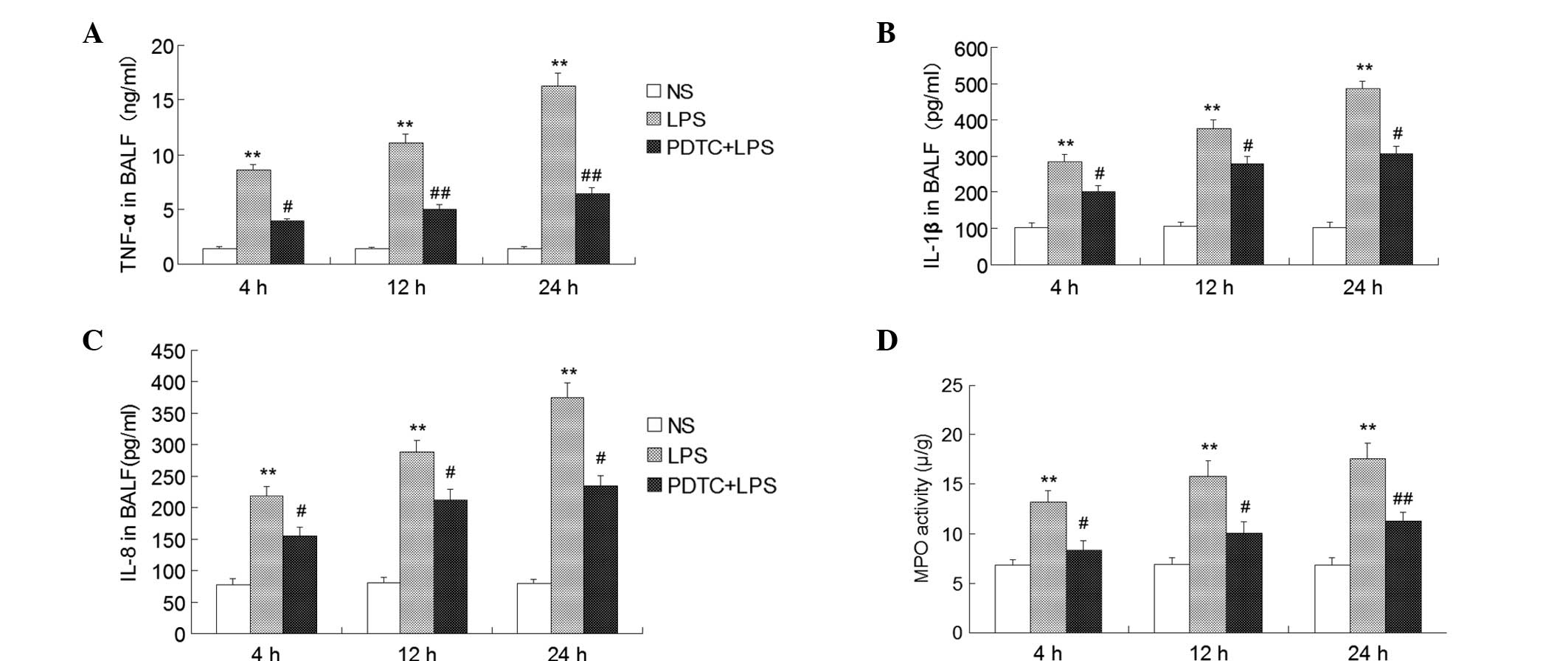 | Figure 6Effect of PDTC on the expression of
inflammatory cytokines, including (A) TNF-α, (B) IL-1β and (C)
IL-8, in the BALF and the (D) activity of MPO in the lung tissues.
Values are presented as the mean ± standard deviation.
**P<0.01, vs. control; #P<0.05 and
##P<0.01, vs. LPS group (n=10). NS, normal saline;
PDTC, pyrrolidine dithiocarbamate; BALF, bronchoalveolar lavage
fluid; MPO, myeloperoxidase; TNF, tumor necrosis factor; IL,
interleukin. |
Effect of PDTC on MPO activity in ARDS
mice
MPO activity is an important index that can be used
to evaluate the accumulation of neutrophils in lung tissue. The
activity of MPO in the LPS group was markedly increased compared
with the control group (P<0.01). However, this change was
blocked significantly in the group treated with PDTC prior to being
challenged with LPS at 4 and 12 h (P<0.05; Fig. 6), at 24 h (P<0.01; Fig. 6).
Expression levels of CD11b/CD18 and
ICAM-1
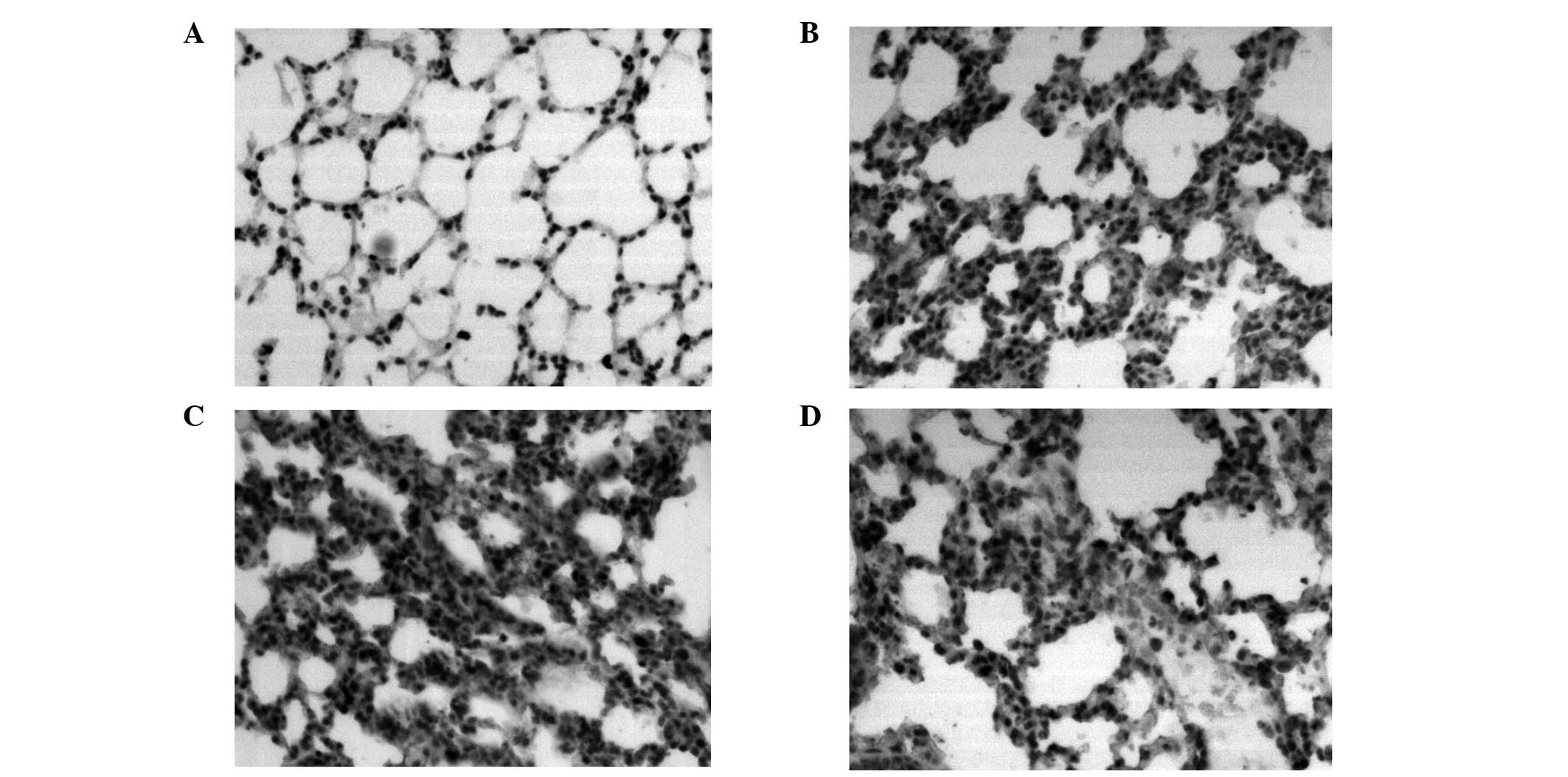
Immunohistochemistry results revealed that
CD11b/CD18 (Fig. 7) and the
ligand, ICAM-1 (Fig. 8), were
highly expressed in alveolar epithelial cells and lung perivascular
cells of the mild, moderate and severe LPS-induced ARDS mice. Cell
morphological analysis revealed that PMNs were the predominant
cells in the infiltration. However, in the control group, there was
no expression in the lung tissue of the mice. The expression levels
of CD11b/CD18 and ICAM-1 in the PDTC + LPS group were markedly
lower compared with the LPS group.
Discussion
Significant pathological characteristics of ARDS
include the aggregation of a large number of activated neutrophils
in the pulmonary vascular and mesenchyme (2). The present study systematically
interpreted the PMN aggregation mechanism in the lungs. PMNs are
important effector cells in an inflammatory reaction and play an
important role in the occurrence and outcome of ARDS (18). Under normal conditions, circulating
PMNs are not activated. PMNs exert a biological function lies in
the transfer through the capillary walls into the tissue interval
and the activation of PMN. IL-8 plays an important role in the
migration of PMNs between the blood circulation and the blood
vessels (19). CD11b/CD18, the
surface adhesion molecule of PMN, is a member of the β2-integrin
family and plays an important role in mediating PMN adhesion and
transfer to the endothelial cells, participating in the
inflammatory response (20). Under
the stimulation of activated factors, including LPS, TNF-α and
IL-8, CD11b/CD18 interacts with its ligand ICAM-1 on pulmonary
vascular endothelial cell surfaces, activating PMNs (19) and firmly adhering to the
endothelial cells. In vitro studies have confirmed that
CD11b/CD18 is the high affinity receptor of LPS, and LPS can
directly induce the expression of CD11b/CD18 (20). Furthermore, under the action of
chemokines, PMNs migrate to the alveoli and interstitial space
(21). Following the activation of
PMNs, the cells release a large number of proinflammatory
cytokines, MPO, elastase and oxygen free radicals, which results in
damage to the pulmonary vascular endothelial cells and alveolar
epithelial cells. In addition, an increase in pulmonary capillary
membrane permeability and pulmonary interstitial edema is observed
(22). The activation process is
extremely crucial for PMNs to mediate lung tissue damage.
CD11b/CD18 was used as the indicator of PMN
activation in the present study. There was only a small amount of
CD11b/CD18 expression on the surface of the PMNs and the majority
of CD11b/CD18 molecules were stored in intracellular particles at
resting state (23). The
expression of CD11b/CD18 on the surface of the PMNs was
significantly increased in the mouse lung tissue with ARDS, and the
expression was sustained at a high level (24). In addition, levels of CD11b/CD18
and ICAM-1 expression, as well as MPO activity, increased and were
maintained over time, with a large number of PMNs infiltrating into
the alveolar. The results indicated that expression levels of
CD11b/CD18 and ICAM-1 were increased, and the interaction was the
basis of PMN and endothelial cell adhesion. Thus, the interaction
may play a key role in the accumulation and activation of PMNs in
the lung tissue. Furthermore, this observation confirmed that the
adhesion of inflammatory cells was an important mechanism in ARDS
(25). IL-8 plays an important
role in the process of PMN migration between the circulating blood
and the extravascular (26). The
present study identified that the expression of IL-8 was
continuously high along with the severity of lung injury, and
peaked following injection of LPS for 4 h. The results also
indicated that increased IL-8 expression may promote the migration
of a large number of PMNs from the peripheral blood to the lung
tissue, and PMNs release toxic substances, such as MPO. In
addition, IL-8 may activate PMNs and directly damage the lung
tissue cells (27).
Previous studies have demonstrated that CD11b/CD18
may increase lung tissue damage by delaying inflammatory cell
apoptosis and inducing the release of inflammatory cytokines from
PMNs, resulting in the inflammatory cascade (14). In the current study, the apoptosis
rate of PMNs was significantly reduced in the BALF of ARDS mice
when lung tissue was damaged over time. In addition, the score of
the damage increased with increasing pulmonary vascular
permeability. The W/D ratio and LPI also increased. The results
confirmed that PMN apoptosis was delayed and the survival time was
prolonged following migration to the lung tissue. These changes of
PMNs are one of the important mechanisms underlying ARDS
inflammation, as during this time, the PMNs are active and have
sustained release of inflammatory mediators and toxic contents,
resulting in lung tissue damage.
As an important nuclear transcription factor, NF-κB
is the intersection of multiple signaling pathways (28). A number of studies have found that
the activation of NF-κB that occurs in ARDS may cause inflammatory
cytokines, including adhesion molecules (CD11b/CD18, ICAM-1),
chemokines (IL-8), TNF-α and IL-1β, to be expressed at the maximum
level, which involves the transcriptional regulation of the
activation of numerous genes (8,29).
NF-κB plays an important role in the inflammatory response,
oxidative stress, apoptosis and other pathological processes.
TNF-α, IL-1β and IL-8, among other proinflammatory cytokines,
further activate NF-κB in the lung tissue, causing the positive and
negative feedback regulation of NF-κB activation to become out of
balance (4). The p50/p65
heterodimer has a major physiological function during inflammation,
with NF-κB p65 being the main subunit (30). The protein exists in an inactive
state in the cytoplasm in the form of a dimer and directly combines
with inhibitory protein IκB to form a trimeric complex. The Rel
protein localization signal is exposed following stimulation with
LPS, causing NF-κB to bind to specific κB sequences of DNA in the
nucleus in order to regulate gene transcription and expression
(9). The western blot analysis
results for NF-κB p65 expression in the mouse lung tissue revealed
that p65 protein expression in the cytoplasm of the lung cells was
significantly decreased in the ARDS group at each time point when
compared with the control group, while the p65 protein expression
in the nuclei was significantly increased. These results indicated
that NF-κB p65 plays an important role in the incidence and
development of ARDS. In addition, NF-κB exhibits a regulatory
function in PMN apoptosis in the lung tissue and inhibits the
apoptosis of PMNs following activation (31).
PDTC, a specific inhibitor of NF-κB, is a
dithiocarbamate of the pyrrole derivatives (32). This molecule can hinder the
dissociation of the inhibitory protein IκB from the NF-κB complex
via antioxidation in order to inhibit the activation of NF-κB
(33). In addition, PDTC impedes
the transfer of p65, an important subunit of NF-κB, to the nucleus
and reduces the expression of p65 in the nucleus significantly,
thus, reducing the expression levels of adhesion molecules, TNF-α,
IL-1β, IL-8 and other inflammatory cytokines at the transcriptional
level (34). PDTC may also
directly reduce the binding ability between NF-κB and DNA, and
obstruct the signaling pathway activated by NF-κB, thus, decreasing
the production of CD11b/CD18, ICAM-1 and chemokines (35). The present study found that
application of PDTC suppressed the activation and transfer of NF-κB
p65 in the lung tissue and increased the expression of cytoplasmic
p65 protein, while decreasing the expression in the nucleus. In
addition, the expression levels of CD11b/CD18, ICAM-1, TNF-α, IL-1β
and IL-8 were significantly reduced in the lung tissue cells, while
the apoptosis rate of the PMNs was significantly increased and the
activity of MPO was less compared with the LPS group. In
combination with the determination of lung tissue pathology and
pulmonary permeability results, PDTC may reduce the expression of
adhesion molecules and chemokines by inhibiting the activation of
NF-κB and the activation of PMNs and causing the release of MPO.
PDTC may also reduce the pulmonary capillary permeability and the
infiltration of inflammatory cells. Therefore, the NF-κB signaling
pathway is hypothesized to be an important intervention in
targeting the regulation of PMN aggregation, and controlling the
activation of NF-κB may become a key strategy for the treatment of
ARDS.
In conclusion, the present study demonstrated that
the transfer and activation of NF-κB from the cytoplasm to the
nucleus in the lung tissue was induced by LPS, which then initiated
the synthesis and release of cell adhesion molecules and
chemokines, causing the accumulation of a large number of PMNs in
the lung tissue. However, pretreatment with PDTC partially reduced
the lethality in LPS-induced mice by attenuating the lung tissue
edema and damage, the production of inflammatory cytokines, the
neutrophil influx to the lung and the overactivation of NF-κB,
promoting PMN apoptosis via the NF-κB pathway. These results
indicate that the NF-κB signaling pathway may be an important
intervention in targeting the regulation of PMN aggregation in the
lungs.
Acknowledgements
The study was supported by grants from the Project
of Science and Technology Department of Liaoning Province (no.
2013225305).
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo MY, Liao MF, Chen FL, et al: Luteolin
attenuates the pulmonary inflammatory response involves abilities
of antioxidation and inhibition of MAPK and NF-κB pathways in mice
with endotoxin-induced acute lung injury. Food Chem Toxicol.
49:2660–2666. 2011.PubMed/NCBI
|
|
4
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giebelen IA, van Westerloo DJ, LaRosa GJ,
et al: Local stimulation of alpha7 cholinergic receptors inhibits
LPS-induced TNF-alpha release in the mouse lung. Shock. 28:700–703.
2007.PubMed/NCBI
|
|
6
|
Lynn WA, Raetz CR, Qureshi N and Golenbock
DT: Lipopolysaccharide-induced stimulation of CD11b/CD18 expression
on neutrophils. Evidence of specific receptor-based response and
inhibition by lipid A-based antagonists. J Immunol. 147:3072–3079.
1991.PubMed/NCBI
|
|
7
|
Smith CW, Marlin SD, Rothlein R, Toman C
and Anderson DC: Cooperative interactions of LFA-1 and Mac-1 with
intercellular adhesion molecule-1 in facilitating adherence and
transendothelial migration of human neutrophils in vitro. J Clin
Invest. 83:2008–2017. 1989. View Article : Google Scholar
|
|
8
|
Everhart MB, Han W, Sherrill TP, et al:
Duration and intensity of NF-kappaB activity determine the severity
of endotoxin-induced acute lung injury. J Immunol. 176:4995–5005.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nathens AB, Bitar R, Davreux C, Bujard M,
et al: Pyrrolidine dithiocarbamate attenuates endotoxin-induced
acute lung injury. Am J Respir Cell Mol Biol. 17:608–616. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindström P, Lerner R, Palmblad J and
Patarroyo M: Rapid adhesive responses of endothelial cells and of
neutrophils induced by leukotriene B4 are mediated by leucocytic
adhesion protein CD18. Scand J Immunol. 31:737–744. 1990.PubMed/NCBI
|
|
11
|
Weber C, Erl W, Pietsch A and Weber PC:
Aspirin inhibits nuclear factor-kappa B mobilization and monocyte
adhesion in stimulated human endothelial cells. Circulation.
91:1914–1917. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borregaard N, Sørensen OE and
Theilgaard-Mönch K: Neutrophil granules: a library of innate
immunity proteins. Trends Immunol. 28:340–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leindler L, Morschl E, László F, et al:
Importance of cytokines, nitric oxide, and apoptosis in the
pathological process of necrotizing pancreatitis in rats. Pancreas.
29:157–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harkin DW, Marron CD, Rother RP, Romaschin
A, et al: C5 complement inhibition attenuates shock and acute lung
injury in an experimental model of ruptured abdominal aortic
aneurysm. Br J Surg. 92:1227–1234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto N, Kawabe T, Imaizumi K, et al:
CD40 plays a crucial role in lipopolysacharide-induced acute lung
injury. Am J Respir Cell Mol Biol. 30:808–815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haddad JJ, Safieh-Garabedian B, Saadé NE
and Lauterbach R: Inhibition of glutathione-related enzymes
augments LPS-mediated cytokine biosynthesis; involvement of an
IkappaB/NF-kappaB-sensitive pathway in the alveolar epithelium. Int
Immunopharmacol. 2:1567–1583. 2002. View Article : Google Scholar
|
|
17
|
Fan J, Marshall JC, Jimenez M, et al:
Hemorrhagic shock primes for increased expression of
cytokine-induced neutrophil chemoattractant in the lung: role in
pulmonary inflammation following lipopolysaccharide. J Immunol.
161:440–447. 1998.
|
|
18
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woodfin A, Voisin MB and Nourshargh S:
Recent developments and complexities in neutrophil transmigration.
Curr Opin Hematol. 17:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Triantafilou M and Triantafilou K:
Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation
cluster. Trends Immunol. 23:301–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meliton AY, Muñoz NM, Meliton LN, Binder
DC, et al: Cytosolic group IVa phospholipase A2 mediates
IL-8/CXCL8-induced transmigration of human polymorphonuclear
leukocytes in vitro. J Inflamm (Lond). 7:142010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wang Y, Zhao X, Andersson R, et
al: Potential effects of peroxisome proliferator-activated receptor
activator on LPS-induced lung injury in rats. Pulm Pharmacol Ther.
22:318–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoshino H, Laan M, Sjöstrand M, et al:
Increased elastase and myeloperoxidase activity associated with
neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin
Immunol. 105:143–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnaout MA: Structure and function of the
leukocyte adhesion molecules CD11/CD18. Blood. 75:1037–1050.
1990.PubMed/NCBI
|
|
25
|
Bhatia RK, Pallister I, Dent C, et al:
Enhanced neutrophil migratory activity following major blunt
trauma. Injury. 36:956–962. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pallister I, Dent C and Topley N:
Increased neutrophil migratory activity after major trauma: a
factor in the etiology of acute respiratory distress syndrome. Crit
Care Med. 30:1717–1721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nooteboom A, van der Linden CJ and
Hendriks T: Modulation of adhesion molecule expression on
endothelial cells after induction by lipopolysaccharide-stimulated
whole blood. Scand J Immunol. 59:440–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Yang X, Liu T, et al: Kaempferol
regulates MAPKs and NF-κB signaling pathways to attenuate
LPS-induced acute lung injury in mice. Int Immunopharmacol.
14:209–216. 2012.PubMed/NCBI
|
|
29
|
Tanaka S, Nishiumi S, Nishida M, Mizushina
Y, et al: Vitamin K3 attenuates lipopolysaccharide-induced acute
lung injury through inhibition of nuclear factor-κB activation.
Clin Exp Immunol. 160:283–292. 2010.PubMed/NCBI
|
|
30
|
Ross SD, Kron IL, Gangemi JJ, et al:
Attenuation of lung reperfusion injury after transplantation using
an inhibitor of nuclear factor-κB. Am J Physiol Lung Cell Mol
Physiol. 297:L528–L536. 2000.PubMed/NCBI
|
|
31
|
Kupfner JG, Arcaroli JJ, Yum HK, et al:
Role of NF-kappaB in endotoxemia-induced alterations of lung
neutrophil apoptosis. J Immunol. 167:7044–7051. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cuzzocrea S, Chatterjee PK, Mazzon E, et
al: Pyrrolidine dithiocarbamate attenuates the development of acute
and chronic inflammation. Br J Pharmacol. 135:496–510. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu SF and Malik AB: NF-kappa B activation
as a pathologic mechanism of septic shock and inflammation. Am J
Physiol Lung Cell Mol Physiol. 290:L622–L645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Németh ZH, Haskó G and Vizi ES:
Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-alpha,
MIP-1alpha, IL-12, and nitric oxide production and protects from
the lethal effect of endotoxin. Shock. 10:49–53. 1998.PubMed/NCBI
|
|
35
|
Roy A, Jana A, Yatish K, et al: Reactive
oxygen species up-regulate CD11b in microglia via nitric oxide:
Implications for neurodegenerative diseases. Free Radic Biol Med.
45:686–699. 2008. View Article : Google Scholar : PubMed/NCBI
|