Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
autoimmune disease, characterized by joint synovial inflammation
and destruction of cartilage and bone. In the abnormal immune
response associated with RA, the role of T cells, particularly
cluster of differentiation (CD)4+ T cells, has always
been of interest (1). T helper 1
(Th1) cells, as an important subtype of CD4+ T cells,
have been previously recognized in the pathogenesis of RA. Previous
studies have also shown that Th1 cells and the cytokine interferon
(IFN)-γ play an important role in promoting inflammation in RA
(2,3) and that inhibiting Th1 responses were
testified to be valid in the patients with RA (4). IFN-γ is the hallmark cytokine of Th1
cells (5). In humans, Th1 cells
can be differentiated from CD4+ T cells by activation of
the Th1 cell-specific T-box transcription factor (TBX21) and IFN-γ
genes under the stimulation of interleukin (IL)-12 and anti-IL-4
antibodies (6).
The vagus nerve can limit inflammation via the α7
nicotinic acetylcholine receptor (α7nAChR). GTS-21, also known as
DMBX-anabaseine, is a selective α7nAChR agonist that has been
demonstrated to inhibit serum tumor necrosis factor (TNF) and
high-mobility group box 1 in a dose-dependent manner in mice with
lethal endotoxemia and sepsis (7,8) and
collagen-induced arthritis (9).
Ex vivo, GTS-21 is able to reduce TNF production in RA whole
blood cultures stimulated by endotoxin (10). To date, GTS-21 is one of the most
well-characterized α7nAChR-specific agonists (11). Administration with GTS-21 in
clinical trials was tolerated well by healthy volunteers and
patients (12,13). GTS-21 exerts its effects by
interacting directly with nAChRs. The α7nAChR, which is expressed
in neurons and immune cells, has been proposed to have a role in
anti-inflammatory and neuron-protective effects (9). Although GTS-21 has a protective
effect in RA, the specific mechanism is not yet fully understood.
It has been shown that α7nAChR is also located on the surface of T
cells (14). To date, little
attention has been focused on the effects of GTS-21 on Th1 cells in
RA. Therefore, in the present study the effects of GTS-21 on Th1
cells from patients with RA were examined for the first time, to
the best of our knowledge, and the preliminary molecular mechanism
underlying the effects of GTS-21 was studied from the level of the
α7nAchR.
Materials and methods
Patients
A total of 12 healthy volunteers and 10 patients who
fulfilled the 2009 American College of Rheumatology revised
criteria for RA (Tables I and
II) were studied. A 28-joint
Disease Activity Score (DAS28) was used to analyze the RA activity.
(DAS28 = 0.56 × sqrt (number of tenderness joints) + 0.28 × sqrt
(number of swollen joints) + 0.70 × Ln (ESR) + 0.014 × VAS (visual
analogue scale), DAS28<2.6 remission; DAS28 2.6–3.2 low disease
activity; DAS28 3.2–5.1 moderate disease activity; DAS28>5.1
high disease activity). ESR and CRP reflect the activity of RA. RF
and anti-CCP antibodies are the relatively specific autoantibodies
of RA. All of them are the items of the 2009 American College of
Rheumatology revised criteria for RA. All of the subjects were
treatment-naïve patients with RA and non-smokers. Patients who had
complications were excluded. The study was approved by the Medical
Ethics Committee of the Xiangya Hospital of Central South
University (Changsha, China). All subjects signed the informed
consent approved by the ethics committee.
 | Table IBasic demographic data of patients
with RA and controls. |
Table I
Basic demographic data of patients
with RA and controls.
| Characteristic | Patients with RA | Controls |
|---|
| No. of subjects,
n | 10 | 12 |
| Age, mean (SD) | 42.3 (9.3) | 39.1 (9.7) |
| Male/female, n/n | 2/8 | 4/8 |
 | Table IIClinical characteristics of patients
with rheumatoid arthritis. |
Table II
Clinical characteristics of patients
with rheumatoid arthritis.
| Characteristic | Result |
|---|
| DAS28, mean (SD) | 6.51 (1.2) |
| Anti-CCP-positive, n
(%) | 7 (70) |
| RF-positive, n
(%) | 8 (80) |
| ESR in mm/h, mean
(SD) | 46.5 (33.7) |
| CRP in mg/l, mean
(SD) | 45.3 (31.1) |
Cell preparation
Peripheral blood samples were obtained from patients
with RA and healthy controls. The peripheral blood mononuclear
cells (PBMCs) were separated from heparinized blood by density
gradient centrifugation over Ficoll-Hypaque PLUS (GE Healthcare,
Piscataway, NJ, USA). The CD4+ T cells were purified
(>96%) from PBMCs using CD4+ T-cell Isolation Kit
MicroBeads (Miltenyi Biotec, Bergisch-Gladbach, Germany), according
to the manufacturer’s instructions.
Cell culture and stimulation
Cells were suspended in RPMI-1640 medium
supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin
and 100 g/ml streptomycin at 37°C in a 5% CO2
atmosphere. The PBMCs (1×106 cells/ml) were cultured for
72 h in 24-well plates and subsequently stimulated with anti-CD3 (3
μg/ml, clone HIT3a; BD Biosciences, Franklin Lakes, NJ, USA) and
anti-CD28 (3 μg/ml, clone CD28.2; BD Biosciences) antibodies in the
presence or absence of different concentrations (0.01, 0.1 or 1
μmol/l) of GTS-21 (Abcam, Cambridge, MA, USA). Purified
CD4+ T cells (1×106 cells/ml) were stimulated
using anti-CD3-coated 96-well plates (BioCoat™ anti-human CD3
T-cell activation plates; BD Biosciences) plus anti-CD28 antibodies
(3 μg/ml), in the presence of IL-12 (15 ng/ml, Peprotech, Inc.,
Rocky Hill, NJ, USA) and anti-IL-4 antibodies (4 μg/ml, Peprotech,
Inc.) for Th1 differentiation for 72 h with GTS-21 (1 μmol/l) alone
or combined with α-bungarotoxin (αBgt, 1 μmol/l; Sigma, St. Louis,
MO, USA).
Cell proliferation/viability
analysis
The proliferation and viability of PBMCs and
CD4+ T cells were analyzed with AlamarBlue®
assays (AbD Serotec, Kidlington, UK) (15,16).
PBMCs from patients with RA were stimulated with anti-CD3/-CD28
antibodies and CD4+ T cells, which were cultured under
Th1-promoting conditions, together with GTS-21 and/or αBgt at the
indicated concentrations. After three days, cell
proliferation/viability was monitored by AlamarBlue. A 100-μl/well
sample of the cell suspension at a final concentration of
1×106 cells/ml was seeded in triplicate in a 96-well
microtiter plate, and 10 μl AlamarBlue was added to each well. The
plates were then incubated at 37°C in a humidified 5%
CO2 incubator for 8 h. AlamarBlue fluorescence was
measured at an excitation wavelength of 544 nm and an emission
wavelength of 590 mm (FLUOstar BMG Lab Technologies, Offenburg,
Germany).
Flow cytometric analysis
To enable the intracellular detection of cytokines,
the Th1-differentiated CD4+ T cells were harvested and
stimulated for 5 h with Leukocyte Activation Cocktail and BD
GolgiPlug (containing PMA and ionomycinin; BD Biosciences). Cells
were then stained extracellularly with phycoerythrin (PE)
Cy5-conjugated CD3 monoclonal antibody (mAb) (eBiosciences, San
Diego, CA, USA) and fluorescein isothiocyanate-conjugated CD8 mAb
(eBiosciences), and subsequently fixed and permeabilized using a
Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences).
Intracellular staining with PE-conjugated IFN-γ mAb (eBiosciences)
was then performed. Cell samples were acquired on a FACSCalibur
flow cytometer and analyzed using CellQuest™ software (BD
Biosciences).
Western blot analysis
Purified CD4+ T cells (1×106
cells/ml) were stimulated using anti-CD3-coated 96-well plates, in
the presence of IL-12 (15 ng/ml) and anti-IL-4 antibody (4 μg/ml)
for Th1 differentiation for 48 h with GTS-21 (1 μmol/l) alone or in
combination with αBgt (1 μmol/l). Whole cell lysates were obtained
from Th1-differentiated CD4+ T cells cultured in
RPMI-1640 with 10% FCS, as described previously. Whole cell lysates
were heated for 5 min at 90°C in 5× sodium dodecyl sulfate (SDS)
loading buffer, fractionated by electrophoresis on
SDS-polyacrylamide gels and transferred to polyvinylidene fluoride
membranes. The membranes were blocked for 1 h at room temperature
with 5% skimmed milk in 0.05% Tween 20/Tris buffered saline,
followed by overnight incubation at 4°C with primary antibody
(anti-TBX21 antibody, Abcam; anti-GAPDH antibody, Cell Signaling
Technology, Inc., Danvers, MA USA). The blots were subsequently
incubated with secondary goat anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G for 2 h at room temperature.
The reaction was visualized by chemiluminescence detection. The
bands of interest were quantified with Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) software.
ELISA
The IFN-γ levels in the culture supernatants were
measured using a human IFN-γ immunoassay quantikine ELISA (R&D
Systems, Minneapolis, MN, USA). The cytokine concentration in the
samples was calculated in pg/ml using recombinant human IFN-γ
(R&D Systems) as a standard. Absorbance was measured at 450 nm
with an ELISA plate reader, and the reading at 540 nm was
subtracted to correct for optical imperfections in the plate.
Statistics analysis
Data were analyzed with SPSS 17.0 statistical
software, (SPSS, Inc., Chicago, IL, USA) and are expressed as the
mean ± standard deviation. The significance of differences between
the groups was determined with single-factor variance (one-way
analysis of variance) followed by a multiple comparison test
(Student-Newman-Keuls) unless stated otherwise. If the data did not
satisfy the homogeneity of variance, the Kruskal Wallis test was
used instead. The comparison between the cytokine production of
patients with RA and that of healthy volunteers was analyzed using
a nonparametric matched pairs test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical data
Table I shows the
demographic data of patients with RA and healthy volunteers.
Patients and healthy volunteers were similar in age, although the
percentage of females was higher among the patients with RA than
that among the healthy volunteers. Table II shows the clinical and
laboratory findings in treatment-naïve patients with RA. The
patients with RA were suffering from a severely active form of the
disease, as shown via the mean disease activity score in 28 joints
value.
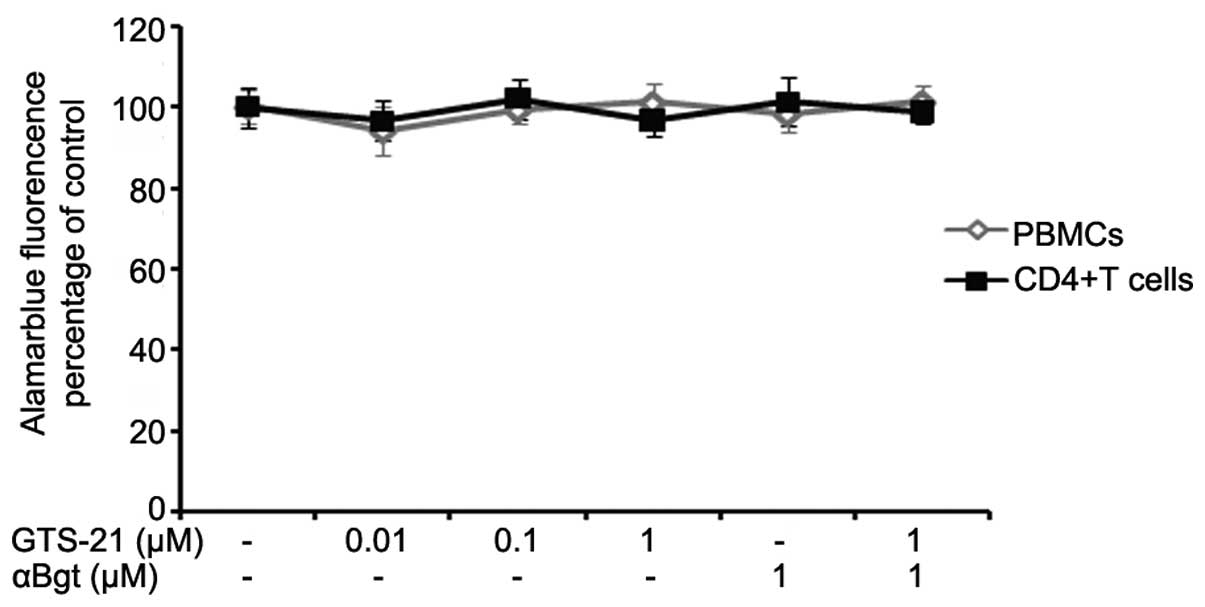
Cell proliferation/viability
analysis
PBMCs from patients with RA were stimulated with
anti-CD3/-CD28 antibodies and CD4+ T cells, which were
cultured under Th1-promoting conditions, together with GTS-21
and/or αBgt at the indicated concentrations. The effects of GTS-21,
αBgt and the combination of the two on cell proliferation and
viability were determined by AlamarBlue assays. Neither GTS-21,
αBgt nor a combination of the two had any effect on the cell
proliferation and viability of anti-CD3/-CD28-stimulated PBMCs or
Th1-differentiated CD4+ T cells (P>0.05, Fig. 1).
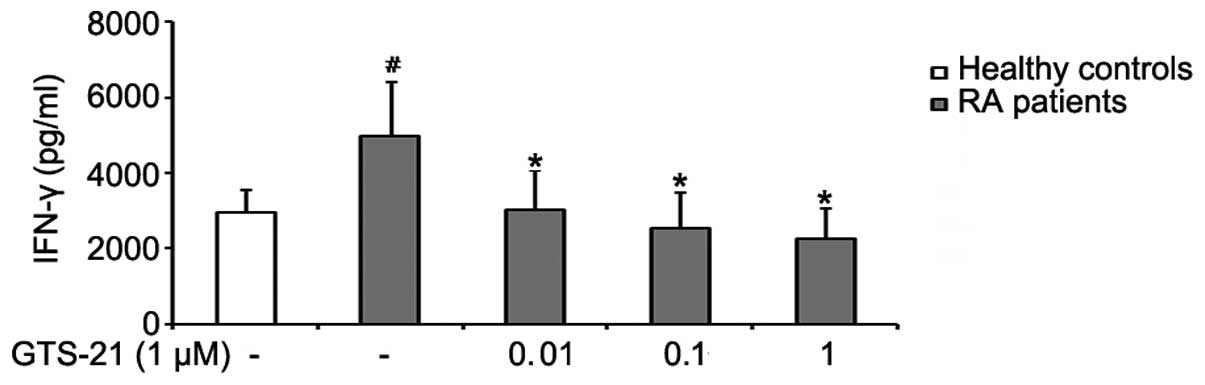
GTS-21 inhibits IFN-γ production by PBMCs
from patients with RA
PBMCs were cultured for 72 h with anti-CD3/-CD28
antibodies alone or with various concentrations of GTS-21.
Anti-CD3/-CD28-stimulated PBMCs from patients with RA produced
significantly more IFN-γ than stimulated PBMCs from healthy
volunteers. GTS-21 inhibited the production of IFN-γ by PBMCs from
patients with RA in a dose-dependent manner and reduced the levels
of IFN-γ to levels similar to, or even below, those found in
healthy volunteers (Fig. 2).
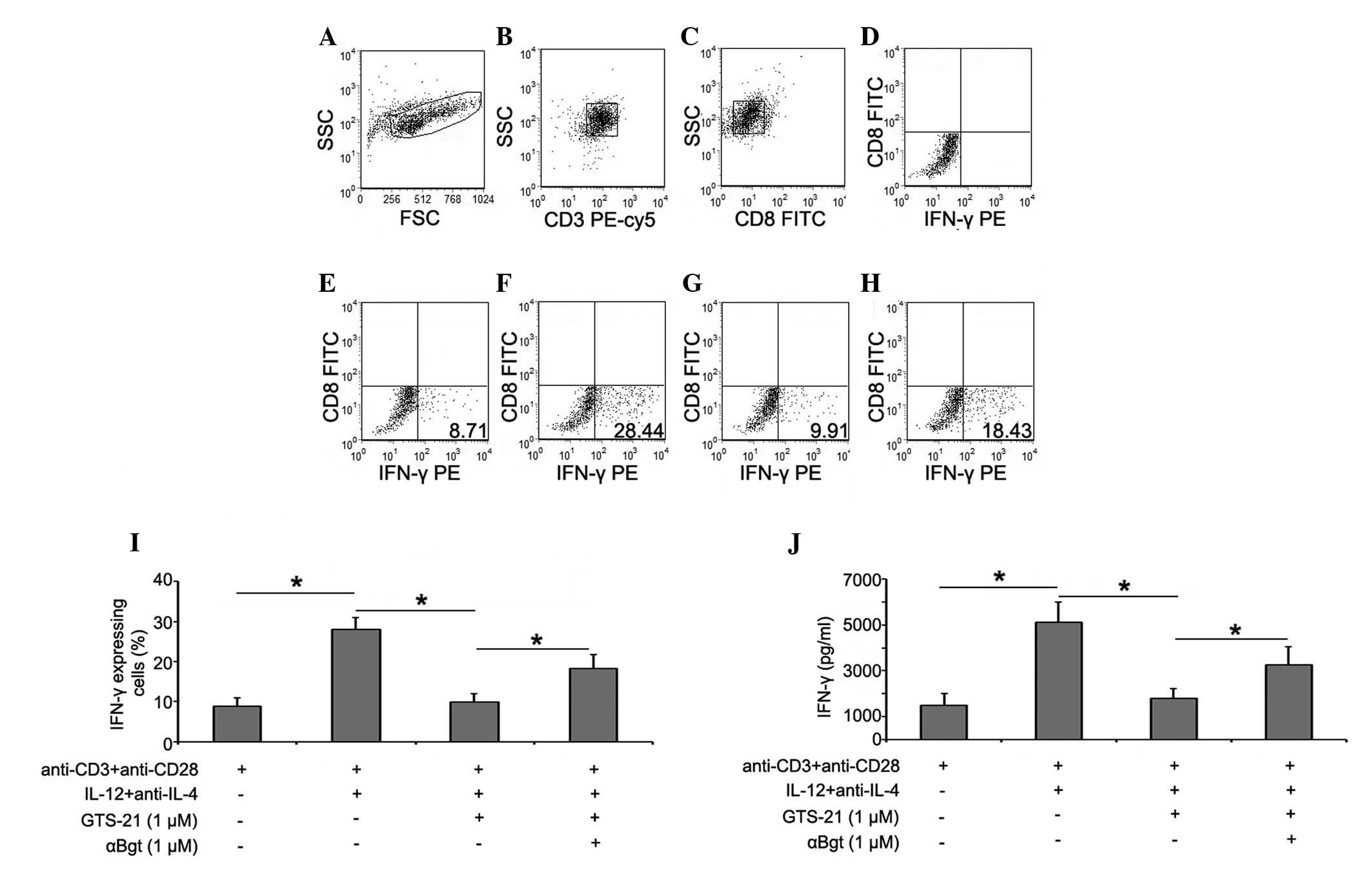
GTS-21 reduces the percentages of
IFN-γ+ T cells in RA CD4+ T cells during Th1
differentiation
To examine the direct effects of GTS-21 on Th1
differentiation, CD4+ T cells were separated from PBMCs
from patients with RA using CD4+ T-cell Isolation Kit
MicroBeads and stimulated subsequently with GTS-21 under
Th1-differentiation conditions. The intracellular expression of
IFN-γ was detected by flow cytometry. Prior to flow cytometric
analysis, it was necessary to augment the intracellular expression
and block the extracellular secretion of IFN-γ using phorbol
myristate acetate (PMA) and ionomycin. However, exposure to PMA
leads to the internalization of membrane CD4 and to the loss of
CD4+ T-cell resolution (17); therefore, the
CD3+CD8− T-cell population was used to
represent the CD4+ T-cell population (18). The expression of IFN-γ in culture
supernatant was detected by ELISA. The results showed that the
percentage of IFN-γ+CD3+CD8− T
cells was increased significantly in the Th1-differentiation group.
Incubation with GTS-21 (1 μmol/l) resulted in a reduction in the
percentage of IFN-γ+CD3+CD8− T
cells. Following prestimulation with αBgt (1 μmol/l) prior to
GTS-21 stimulation, IFN-γ expression during Th1 differentiation was
increased (Fig. 3).
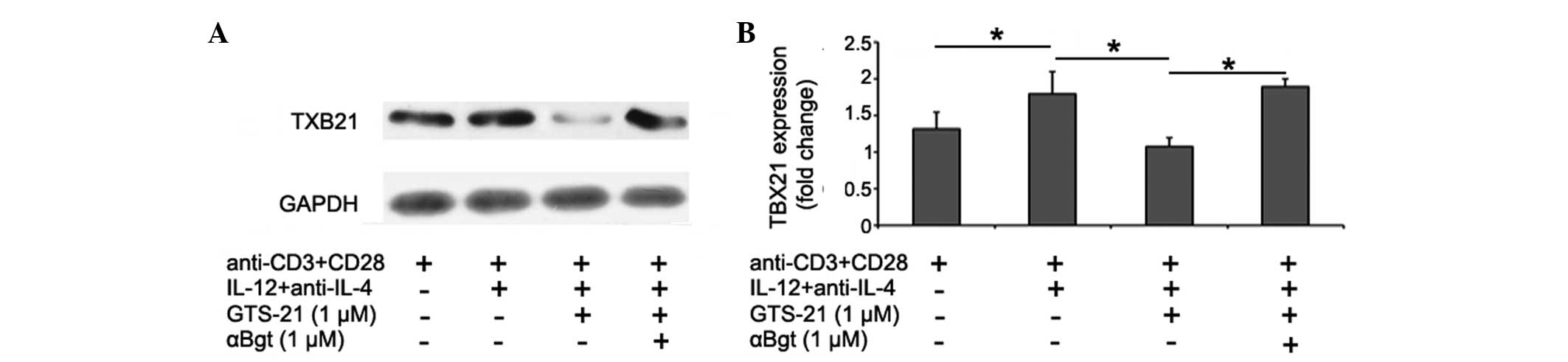
GTS-21 reduces TBX21 levels in
CD4+ T cells from patients with RA during Th1
differentiation
The effects of GTS-21 on the differentiation of Th1
cells from patients with RA were studied at the transcription
factor level. The levels of the Th1 cell-specific transcription
factor TBX21 in CD4+ T cells during Th1 differentiation
were analyzed by western blotting. The data showed that TBX21
levels were increased during Th1 differentiation. GTS-21 (1 μmol/l)
inhibited the expression significantly, and this effect was blocked
by αBgt (1 μmol/l) (Fig. 4).
Discussion
The present study aimed to investigate the effects
of GTS-21 on the differentiation of Th1 cells using CD4+
T cells from patients with RA and to clarify the mechanism by which
GTS-21 modulates the immune response, thus providing a novel basis
for the treatment of RA with GTS-21.
In mammals, IFN-γ is known to be produced primarily
by Th1 cells (5). Given that Th1
cells produce large quantities of IFN-γ, most Th1-mediated effects
are attributed to this cytokine. RA is considered to be a
Th1-driven autoimmune disease (2,3), and
inhibiting Th1 differentiation could be a target in the treatment
of RA to prevent arthritis and bone damage. In the present study,
it was demonstrated that GTS-21 exerts beneficial effects on
production of the Th1 cytokine IFN-γ by PBMCs from treatment-naïve
patients with RA exhibiting high levels of disease activity. GTS-21
inhibits IFN-γ production by PBMCs at low concentrations (0.01, 0.1
and 1 μmol/l) and has its maximal IFN-γ-suppressive effect at a
concentration of 1 μmol/l. AlamarBlue tests revealed that the
inhibitory effect of GTS-21 on IFN-γ production by PBMCs is not
mediated by inhibiting the proliferation/viability of cells. The
results of this study are consistent with those of a previous study
(19) and indicate that GTS-21
strongly downregulates IFN-γ production by PBMCs.
The present study demonstrated that GTS-21 has
inhibitory effects on Th1 differentiation. Th1 cells are
differentiated from CD4+ T cells (6). The Th1-cell differentiation system
used in the present study included anti-CD3 and -CD28 antibodies,
IL-12 and anti-IL-4 antibodies. In this study, it was found that
GTS-21 inhibits Th1 differentiation by reducing the percentage of
IFN-γ+CD3+CD8− T cells and the
levels of IFN-γ in Th1-differentiated CD4+ T-cell
culture supernatant and also the expression of the Th1-specific
transcription factor TBX21. AlamarBlue tests revealed that the
inhibitory effect of GTS-21 on Th1-cell differentiation is not
mediated by inhibiting the proliferation/viability of cells.
αBgt is an nAchR antagonist that binds to the α7 and
α9nAchR, as well as the muscle-type nAchR; previous studies have
demonstrated that T cells do not express the mRNA of α9nAchR and
muscle-type nAchR (20,21). The present study showed that the
effects of GTS-21 on Th1-cell differentiation can be counteracted
by αBgt. This indicates that GTS-21 affects Th1 differentiation by
means of its role in activating the α7nAchR.
Nicotine, a partial α7nAChR agonist, has also been
found to exert anti-inflammatory effects in multiple diseases
(22,23). Notably, nicotine also plays a
protective role in experimental arthritis and RA (24). A previous study showed that
nicotine can reduce the degree of joint inflammation in
collagen-induced arthritic mice and that it is also capable of
reducing the serum levels of IFN-γ and IL-6 secreted from RA
fibroblast-like synoviocytes (25). GTS-21 is a selective α7nAChR
agonist. Due to the obvious limitations of the therapeutic value of
nicotine, as a result of its pharmacological nonspecificity and
toxic side effects, selective α7nAChR agonists may be more
favorable candidates for development as a novel medication for the
treatment of RA.
This is the first study, to the best of our
knowledge, to clarify the inhibitory effects of GTS-21 on Th1-cell
differentiation by CD4+ T cells from patients with RA.
From this novel perspective, the present study has elucidated the
role of GTS-21 in the immune regulation of RA, thus providing a new
basis for the future treatment of RA.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (81102261) and the
Fundamental Research Funds of Central South University
(CX2012B088).
References
|
1
|
Cope AP, Schulze-Koops H and Aringer M:
The central role of T cells in rheumatoid arthritis. Clin Exp
Rheumatol. 25(5 Suppl 46): S4–S11. 2007.PubMed/NCBI
|
|
2
|
Yamada H, Nakashima Y, Okazaki K, et al:
Th1 but not Th17 cells predominate in the joints of patients with
rheumatoid arthritis. Ann Rheum Dis. 67:1299–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cañete JD, Martinez SE, Farrés J, et al:
Differential Th1/Th2 cytokine patterns in chronic arthritis:
interferon gamma is highly expressed in synovium of rheumatoid
arthritis compared with seronegative spondyloarthropathies. Ann
Rheum Dis. 59:263–268. 2000.
|
|
4
|
Nissinen R, Leirisalo-Repo M, Tiittanen M,
et al: CCR3, CCR5, interleukin 4, and interferon-gamma expression
on synovial and peripheral T cells and monocytes in patients with
rheumatoid arthritis. J Rheumatol. 30:1928–1934. 2003.PubMed/NCBI
|
|
5
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang DD, Conze D, Whitmarsh AJ, et al:
Differentiation of CD4+ T cells to Th1 cells requires
MAP kinase JNK2. Immunity. 9:575–585. 1998.
|
|
7
|
Pavlov VA, Ochani M, Yang LH, et al:
Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21
improves survival in murine endotoxemia and severe sepsis. Crit
Care Med. 35:1139–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Liao H, Ochani M, et al:
Cholinergic agonists inhibit HMGB1 release and improve survival in
experimental sepsis. Nat Med. 10:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Yu M, Ochani M, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruchfeld A, Goldstein RS, Chavan S, et
al: Whole blood cytokine attenuation by cholinergic agonists ex
vivo and relationship to vagus nerve activity in rheumatoid
arthritis. J Intern Med. 268:94–101. 2010.PubMed/NCBI
|
|
11
|
de Jonge WJ and Ulloa L: The alpha7
nicotinic acetylcholine receptor as a pharmacological target for
inflammation. Br J Pharmacol. 151:915–929. 2007.PubMed/NCBI
|
|
12
|
Kitagawa H, Takenouchi T, Azuma R, et al:
Safety, pharmacokinetics, and effects on cognitive function of
multiple doses of GTS-21 in healthy, male volunteers.
Neuropsychopharmacol. 28:542–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olincy A, Harris JG, Johnson LL, et al:
Proof-of-concept trial of an alpha7 nicotinic agonist in
schizophrenia. Arch Gen Psychiatry. 63:630–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conti-Fine BM, Navaneetham D, Lei S and
Maus AD: Neuronal nicotinic receptors in non-neuronal cells: new
mediators of tobacco toxicity? Eur J Pharmacol. 393:279–294. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed SA, Gogal RJ Jr and Walsh JE: A new
rapid and simple non-radioactive assay to monitor and determine the
proliferation of lymphocytes: an alternative to [3H]thymidine
incorporation assay. J Immunol Methods. 170:211–224.
1994.PubMed/NCBI
|
|
16
|
O’Brien J, Wilson I, Orton T and Pognan F:
Investigation of the Alamar Blue (resazurin) fluorescent dye for
the assessment of mammalian cell cytotoxicity. Eur J Biochem.
267:5421–5426. 2000.PubMed/NCBI
|
|
17
|
Petersen CM, Christensen EI, Andresen BS
and Møller BK: Internalization, lysosomal degradation and new
synthesis of surface membrane CD4 in phorbol ester-activated
T-lymphocytes and U-937 cells. Exp Cell Res. 201:160–173. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mascher B, Schlenke P and Seyfarth M:
Expression and kinetics of cytokines determined by intracellular
staining using flow cytometry. J Immunol Methods. 223:115–121.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kox M, van Velzen JF, Pompe JC,
Hoedemaekers CW, van der Hoeven JG and Pickkers P: GTS-21 inhibits
pro-inflammatory cytokine release independent of the Toll-like
receptor stimulated via a transcriptional mechanism involving JAK2
activation. Biochem Pharmacol. 78:863–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I
and Brenner T: Anti-inflammatory properties of cholinergic
up-regulation: A new role for acetylcholinesterase inhibitors.
Neuropharmacology. 50:540–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato KZ, Fujii T, Watanabe Y, et al:
Diversity of mRNA expression for muscarinic acetylcholine receptor
subtypes and neuronal nicotinic acetylcholine receptor subunits in
human mononuclear leukocytes and leukemic cell lines. Neurosci
Lett. 266:17–20. 1999. View Article : Google Scholar
|
|
22
|
McGrath J, McDonald JW and Macdonald JK:
Transdermal nicotine for induction of remission in ulcerative
colitis. Cochrane Database Syst Rev. 18:CD0047222004.PubMed/NCBI
|
|
23
|
van Westerloo DJ, Giebelen IA, Florquin S,
et al: The cholinergic anti-inflammatory pathway regulates the host
response during septic peritonitis. J Infect Dis. 191:2138–2148.
2005.PubMed/NCBI
|
|
24
|
van Maanen MA, Lebre MC, van der Poll T,
et al: Stimulation of nicotinic acetylcholine receptors attenuates
collagen-induced arthritis in mice. Arthritis Rheum. 60:114–122.
2009.PubMed/NCBI
|
|
25
|
Li T, Zuo X, Zhou Y, et al: The vagus
nerve and nicotinic receptors involve inhibition of HMGB1 release
and early pro-inflammatory cytokines function in collagen-induced
arthritis. J Clin Immunol. 30:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|