Introduction
Arginase enzymes play a major role in the
biosynthesis of polyamines and amino acids, including ornithine,
proline and glutamate (1,2). Arginase has two distinct isoforms,
arginase I and II, which differ in subcellular localization.
Arginase I is predominantly localized in the cytosol of hepatic
cells and is a key enzyme in the urea cycle. Arginase II is
expressed in the mitochondria of extrahepatic cells and is encoded
by a different gene (3). The two
arginases are constitutively expressed in cells and tissues, and
indirectly regulate nitric oxide (NO) generation from nitric oxide
synthase (NOS) by competition for a common enzyme substrate
(4,5). Therefore, the induction of NOS
arginase has been investigated in the inflammatory cells of
asthmatic lungs as pathophysiological evidence that the consumption
of L-arginine by arginase may lead to the depletion of NO
production and endothelial dysfunction; thus, the enlargement of
bronchial smooth muscle associated with airway hyperresponsiveness
(6–8). Furthermore, following catabolism by
arginase, arginine is no longer available to NOS; thus, subsequent
NO synthesis is diminished (9).
Diabetes mellitus (DM) is a worldwide disease that
is frequently associated with a high risk of atherosclerosis and
renal, nervous system and ocular damage (10). Oxidative damage is involved in DM
and the associated complications (11), with reactive oxygen species (ROS)
being implicated in the pathogenesis of DM (12). Patients with type 2 DM frequently
exhibit vascular endothelium dysfunction associated with
hypercholesterolemia, and NO deficiency is a major factor
contributing to endothelial dysfunction, which has been
demonstrated in hypertension, tobacco smoking and malaria (13). Furthermore, an increased production
of ROS has been shown to be associated with protein glycation
and/or glucose auto-oxidation in patients with DM (14).
Serum arginase levels have been analyzed in a number
of diseases using activity assays and enzyme-linked immunosorbent
assay (ELISA) methods. For patients with DM, although serum
arginase activity levels remain controversial (15), serum arginase I levels have been
shown to be elevated using ELISA (16). In other diseases, including sickle
cell disease (17), retinopathy
(18) and cardiovascular disease
(19), changes in arginase I
levels have also been observed. However, little is known with
regard to the expression levels of arginase I in patients with type
2 DM, and the association with DM remains elusive.
Therefore, in the present study, the serum levels of
arginase I in patients with DM were determined. In addition, the
use of arginase I as a diagnostic biomarker for type 2 DM was
investigated.
Material and methods
Animals and model establishment
All experiments were performed in accordance with
the institutional animal care and use guidelines of the Ethical
Committee of the Henan Nanyang Central Hospital (Nanyang, China).
Male Wistar rats (age, ~10 weeks; weight, ~200 g) were used in the
study and housed in standard light/dark cycles, with access to a
standard rat diet and water ad libitum.
The animals were randomly divided into three groups,
including control, diabetic model and citrulline-treated diabetic
groups. DM was induced by a single intraperitoneal injection of 50
mg/kg streptozotocin (STZ; (Beijing, China) and treated with
citrulline in distilled water by orogastric gavage for six weeks of
study, while the control and diabetic groups received water as a
vehicle. The doses of citrulline were selected based on the
reported arginase inhibiting activity (20) and confirmed by arginase activity
measurement.
Serum blood collection
The rats were fully anesthetized with 3%
pentobarbital sodium, and the chest was opened. A needle was
inserted through the diaphragm and into the heart. Negative
pressure was gently applied once the heart had been punctured, and
the needle was repositioned as required until blood flowed into the
syringe. The blood collected from the rats was allowed to clot at
room temperature. The serum was separated by centrifugation at
5,000 g for 10 min, and aliquots of serum were stored at −80°C
until required for further analysis.
Arginase I activity
Arginase activity was analyzed as previously
described (21). Briefly, 100 μl
serum was incubated with equal volumes of 10 mM manganese chloride
in 50 mM Tris-HCl (pH 7.4) (Beijing, China) at 55°C for 10 min to
activate the enzyme. All the experimental procedures were performed
according to the methods previously described by Giri et al
(22). The samples were then
transferred to a 96-well plate for ELISA analysis at 540 nm.
Quantitative polymerase chain reaction
(PCR)
Total RNA was isolated from the collected blood
using TRIzol reagent (Sigma-Aldrich, Beijing, China), according to
the manufacturer’s instructions. Following DNAse treatment, total
RNA (1 μg) was reverse transcribed using Moloney Murine Leukemia
Virus Reverse Transriptase and oligo(dT) 12–18 primers. A reaction
performed in the absence of the reverse transcriptase enzyme
functioned as a negative control. Amplified cDNA was subjected to
PCR using specific gene primers that are listed in Table I, according to the methods
previously described by Giri et al (22). GADPH was used as an internal
control.
 | Table ISequences of primers used for arginase
I PCR. |
Table I
Sequences of primers used for arginase
I PCR.
| Genes | Primer sequences | Product length
(bp) |
|---|
| Arginase I | 5′
ATGTCCCTAAGGGGCAGCCTCTCGCGT 3′
5′ CACAGCTGTAGCCATCTGACACAGCTC 3′ | 340 |
| GAPDH | 5′
TGCCTCCTGCACCACCAACTGC 3′
5′ AATGCCAGCCCCAGCGTCAAAG 3′ | 456 |
Western blot analysis
All lysates extracted from the blood were separated
by 15% SDS-PAGE and electrotransferred onto nitrocellulose
membranes. The membranes were blocked with 5% defatted milk in
phosphate-buffered saline (PBS) overnight at 4°C, and then
incubated with monoclonal antibodies against arginase I (1:2,000),
mouse anti-Tie 2 (1:3,000) and anti-human β-actin (1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 2 h at room
temperature. Next, the membranes were incubated with a horseradish
peroxidase-conjugated anti-mouse (1:3,000) secondary antibody
(Santa Cruz Biotechnology). Reactive signals were visualized using
an enhanced chemiluminescence kit (PE Applied Biosystems, Foster
City, CA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Mean values were compared between groups using the
unpaired Student’s t-test for normally distributed variables, while
Pearson’s analysis was computed to determine the correlations
between variables. P<0.05 was considered to indicate a
statistically significant difference. If variables were not
normally distributed, data were log-transformed prior to
correlation analysis. Concordance analysis was performed using
Cohen’s test.
Results
Blood and serum parameters
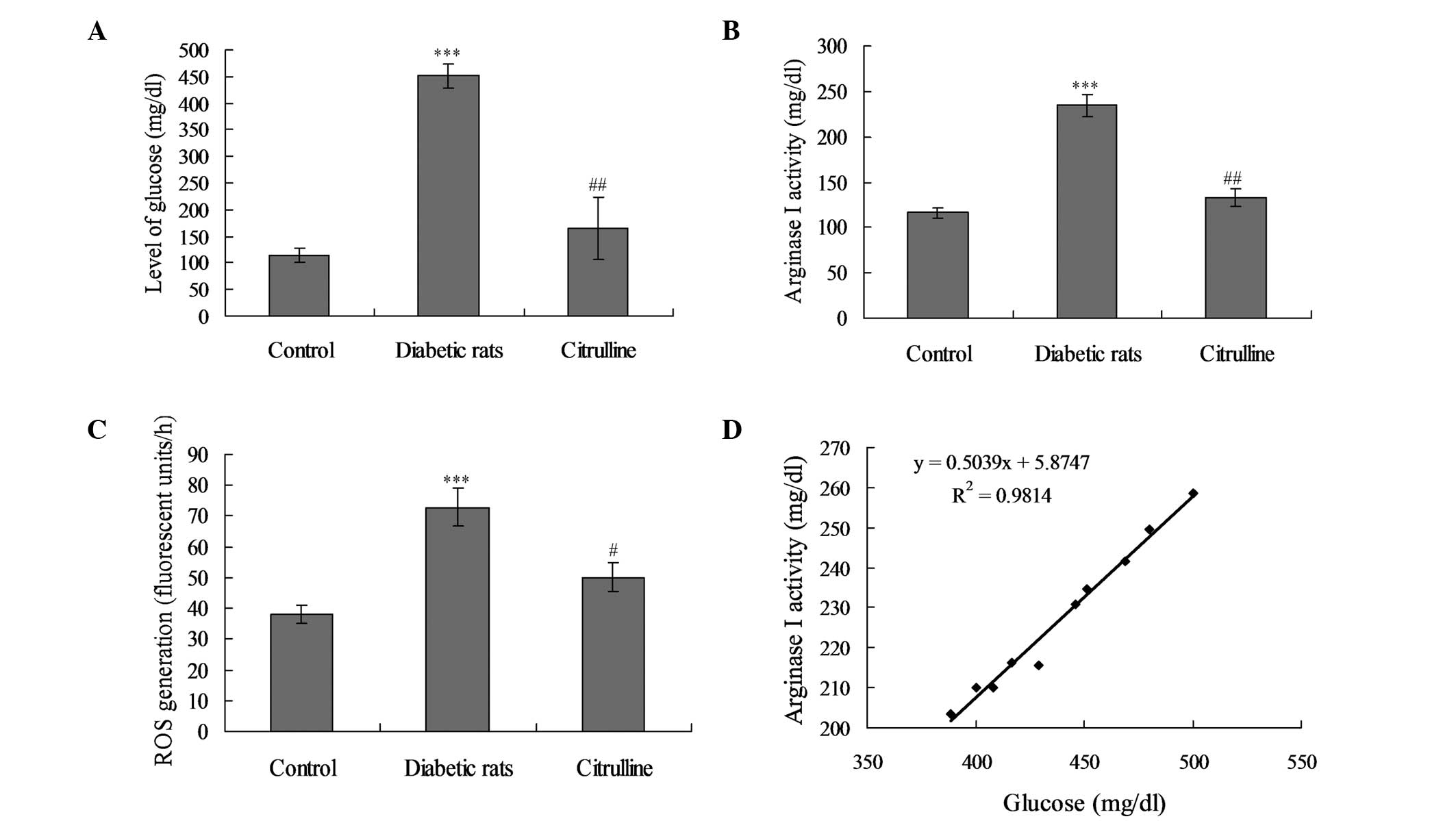
Treatment with 50 mg/kg STZ triggered a significant
increase in the blood glucose levels when compared with the control
rats (Fig. 1A; P<0.001).
However, arginase inhibition by citrulline did not exhibit any
significant effect on the glucose levels.
Serum arginase I activity levels in diabetic rats
treated with STZ were significantly elevated when compared with the
control group (Fig. 1B;
P<0.001). However, activation of arginase I was significantly
inhibited following the administration of an arginase inhibitor
(P<0.05). With regard to ROS generation, ROS levels were
significantly increased in the diabetic rats when compared with the
control and citrullin-treated rats (Fig. 1C; P<0.001). Furthermore,
arginase I activity levels significantly correlated with the blood
glucose levels in the diabetic rats (Fig. 1D; P<0.01, r=0.8672), but not in
the arginase inhibitor group (data not shown).
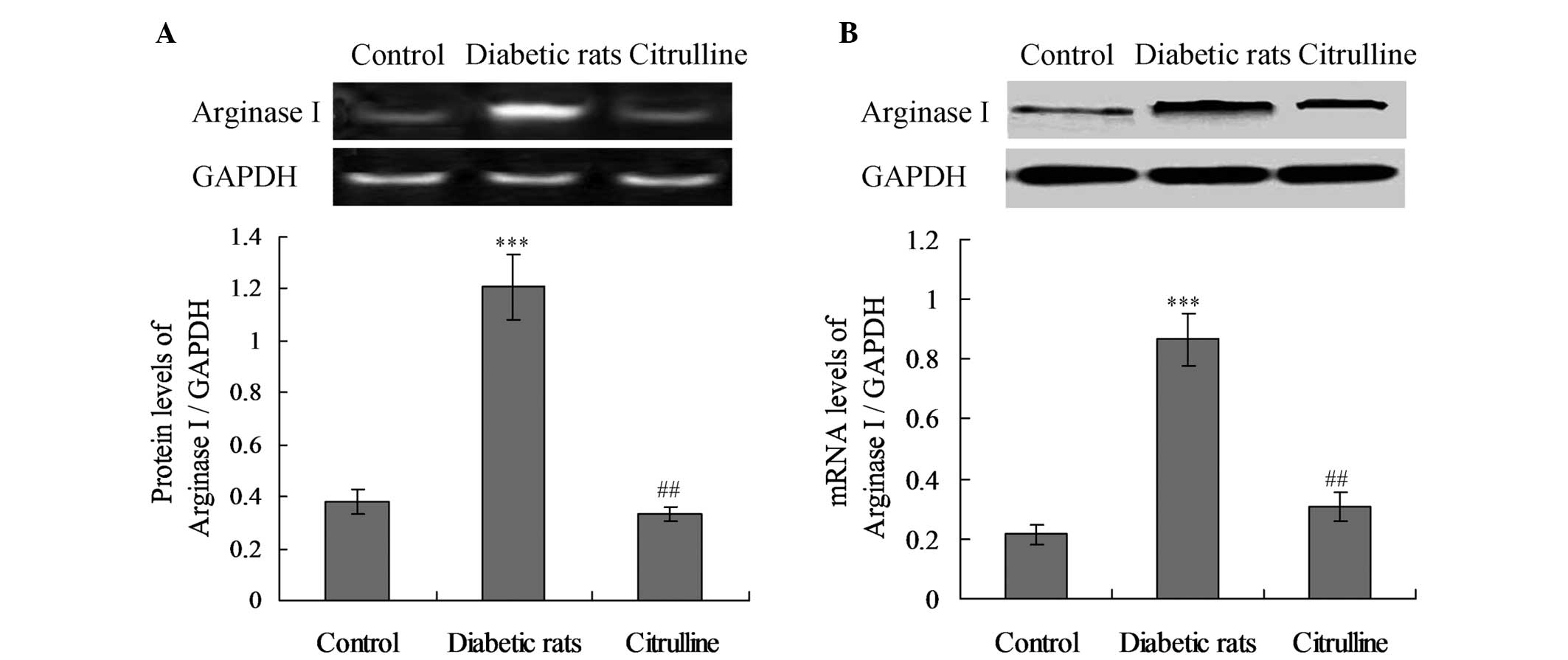
mRNA and protein expression levels of
arginase I are enhanced in diabetic rats
To further evaluate the effect of STZ-induced DM on
the circulating levels of arginase I, the mRNA and protein
expression levels of arginase I were analyzed in the blood serum.
As shown in Fig. 2, no
statistically significant difference in the mRNA expression levels
of arginase I was observed between the control and citrulline
groups (Fig. 2B; P>0.05).
However, the mRNA expression levels of arginase I in the diabetic
rats were significantly increased compared with the control group
(Fig. 2B; P<0.001). Similarly,
the arginase I protein expression levels were enhanced in the
diabetic rats when compared with the control rats (Fig. 2A; P<0.001).
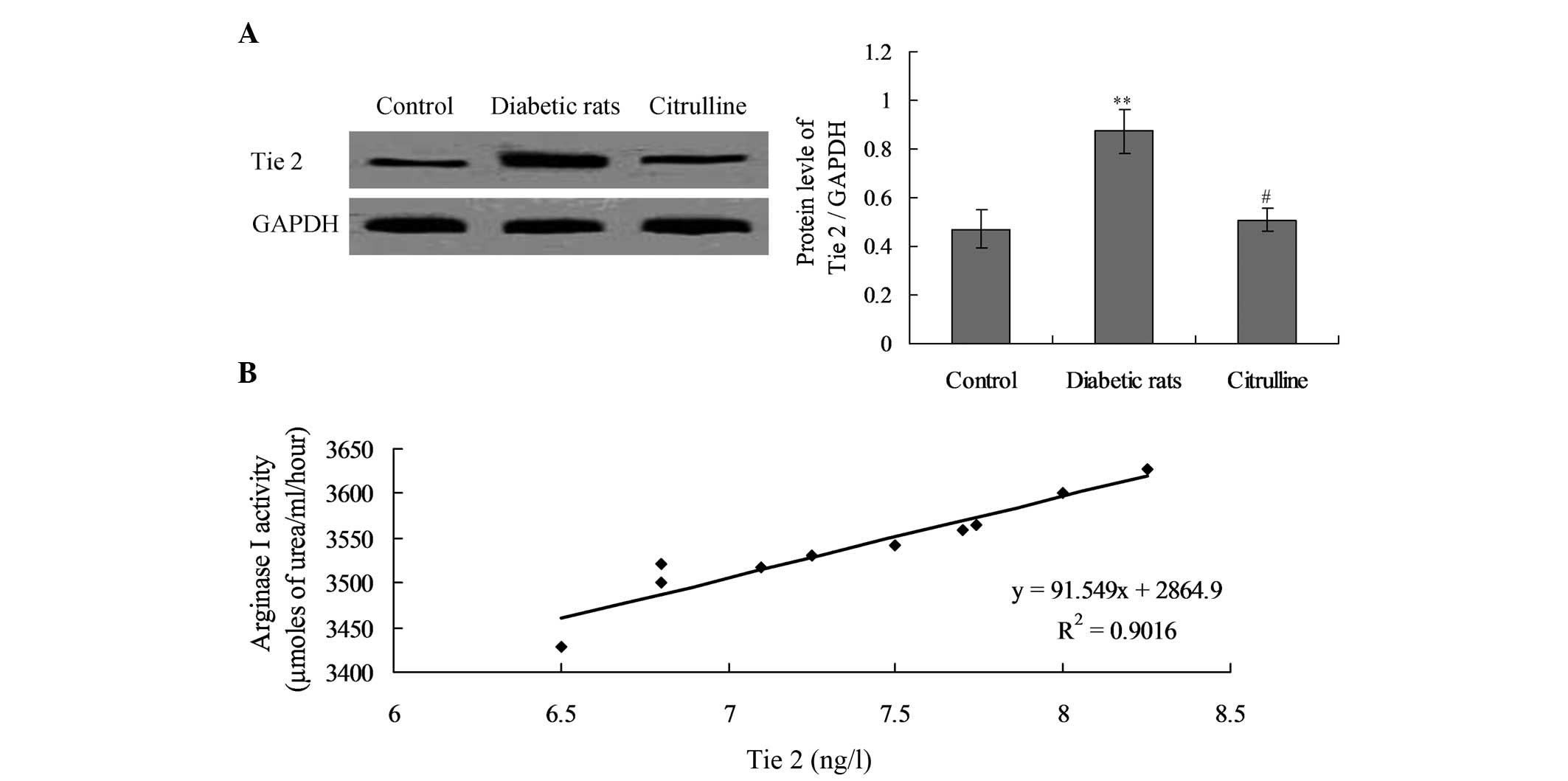
Expression levels of Tie 2 are increased
and correlate with arginase I activity in diabetic rats
In order to investigate the cause of arginase I
activity, the expression levels of the arginase I-associated
receptor, Tie 2, were detected in diabetic rats. The results
indicated that Tie 2 expression in diabetic rats was significantly
increased compared with the control group (Fig. 3; P<0.01). However, citrulline
treatment was found to significantly decrease Tie 2 expression in
the diabetic rats (Fig. 3;
P<0.05).
Furthermore, the correlation between arginase I
activity and Tie 2 expression levels was analyzed by correlation
analysis. The results revealed that arginase I activity positively
correlated with Tie 2 receptor expression in the diabetic rats
(Fig. 3B; P<0.01,
r=0.9013).
Arginase I activity reflects glucose
intolerance and post-load glucose levels
In order to investigate the associations between
arginase I activity and other blood parameters, Spearman’s
correlation analysis of arginase I activity with other blood
parameters was performed. As shown in Table II, arginase I activity exhibited a
significant positive correlation with glucose intolerance and
post-load glucose values in the diabetic rats.
 | Table IISpearman’s correlation analysis of
arginase I activity with other parameters. |
Table II
Spearman’s correlation analysis of
arginase I activity with other parameters.
| Parameters | Correlation
coefficient (′) | P-value |
|---|
| Glucose
intolerance | 0.704 | 0.002 |
| Post-load
glucose | 0.497 | 0.047 |
| Fasting glucose | 0.212 | 0.083 |
| Angiopoietin 1 | 0.198 | 0.103 |
| Angiopoietin 2 | 0.183 | 0.116 |
Concordance analysis
Arginase I activity DM (+) and DM (−)
characteristics were used to analyze the concordance with the
clinical diagnosis of diabetic rats (Table III). The results demonstrated
that eight diabetic rats (DM+) analyzed with arginase I activity
were consistent with the clinical diagnosis, and the sensitivity
was 80.0% (8/10). According to Kappa analysis, the concordance
value between arginase I activity and the clinical diagnosis for DM
was 0.876 (κ=0.876; P<0.001; Table III).
 | Table IIIConcordance between arginase I
activity and clinical diagnosis in diabetic rats. |
Table III
Concordance between arginase I
activity and clinical diagnosis in diabetic rats.
| Arginase I
activity | |
|---|
|
| |
|---|
| Clinical
diagnosis | DM (+) | DM (−) | Total |
|---|
| DM (+) | 8 | 1 | 9 |
| DM (−) | 1 | 0 | 1 |
| Total | 9 | 1 | 10 |
Discussion
Recently, the potential role of arginase (including
arginase I and II) in the pathogenesis of DM has been investigated
(23,24). However, to the best of our
knowledge, the present study is the first study investigating the
potential role of arginase I as a diagnostic or prognostic marker
for type 2 DM. In the present study, diabetic rats exhibited
increased levels of arginase I, which correlated with the blood
glucose level; thus, may contribute to the severity of DM in rats.
Decreasing the arginase I activity in diabetic rats may potentially
provide a therapeutic method for type 2 DM (23).
In the present study, the increased expression of
ROS was found to be involved in the pathogenesis of DM in rats. The
production of ROS has been attributed to protein glycation; thus,
increased levels of ROS by-products may result in changes in energy
metabolism and the antioxidant defense status, participating in
vascular complications in patients with DM (25,26).
Regulation of arginase activity in diabetic rats may aid ROS
metabolism. ROS affect amino acid and cation transport through the
blood cell membranes; for example, cystine transport when blood
cells are exposed to oxidative stress. Therefore, adjusting
arginase activity may be a potential therapeutic method for
improving the symptoms of DM in rats. In the present study,
citrulline was used to inhibit arginase I activity. The results
demonstrated that the mRNA and protein expression levels of
arginase I were increased in the diabetic rats when compared with
control group, but not in the citrulline treatment group. This
observation indicates that arginase I protein has an important role
in the pathogenesis of DM, which is consistent with the results of
previous studies (27,28).
In the present study, a significant increase in Tie
2 receptor expression and arginase activity levels was observed in
the blood serum of the diabetic rats. The increase in Tie 2
expression in the blood serum may be due to the shredding of
extracellular activity of matrix metalloproteinases (29,30),
or due to the Golgi-mediated release of the stored pool of Tie 2
(31). In addition, the changes in
arginase I expression levels may be associated with the changes in
Tie 2 receptor expression in the diabetic rats. Although the exact
effects of high expression levels of Tie 2 in the blood remain
unclear, the effects are associated with arginase I activity.
Increased expression and activity levels of arginase
I in the blood have been investigated in rats with type 2 DM, and
inhibition of arginase I has been shown to exhibit a protective
effect in DM rat models (32). The
results of the present study indicated that the arginase I
inhibitor, citrulline, significantly decreased the activity of
arginase I, as well as the blood glucose levels of diabetic rats.
These observations confirm that arginase I activity may reflect the
severity of DM.
In order to evaluate the possible or potential role
of arginase I as a prognostic marker for DM, concordance analysis
was performed. The results indicated that the sensitivity for
arginase I was 80.0% (8/10). According to Kappa analysis, the
concordance value between arginase I activity and the clinical
diagnosis of DM was 0.876 (κ=0.876; P<0.001). A κ-value of
>0.75 is considered to exhibit a better concordance. Therefore,
arginase I diagnostic analysis exhibited a better concordance with
the clinical diagnosis of DM in the present study.
In conclusion, arginase I activity and expression
levels were significantly higher in the diabetic rats when compared
with the control rats, and were shown to positively correlate with
the blood glucose levels of the control rats. These observations
indicate that arginase I may be used as a prognostic or diagnostic
marker for patients with DM. Thus, arginase I may be considered as
a potential method to diagnose DM in clinical practice.
References
|
1
|
Shen K, Ji Y, Chen GQ, Huang B, Zhang X,
Wu S, Yu GP and Wang XC: Expression and clinical significance of
the NDA repair enzyme MYH in esophageal squamous cell carcinoma.
Exp Ther Med. 2:1117–1120. 2011.PubMed/NCBI
|
|
2
|
Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo
P and Iyer RK: Arginases I and II: do their functions overlap? Mol
Genet Metab. 81(Suppl 1): S38–S44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jenkinson CP, Grody WW and Cederbaum SD:
Comparative properties of arginase. Comp Biochem Physiol B Biochem
Mol Biol. 114:107–132. 1996. View Article : Google Scholar
|
|
4
|
Na S, Kim OS, Ryoo S, Kweon TD, Choi YS,
Shim HS and Oh YJ: Cervical ganglion block attenuates the
progression of pulmonary hypertension via nitric oxide and arginase
pathways. Hypertension. 63:309–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J, Gonon AT, Sjöquist PO, Lundberg JO
and Pernow J: Arginase regulates red blood cell nitric oxide
synthase and export of cardioprotective nitric oxide bioactivity.
Proc Natl Acad Sci USA. 110:15049–15054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maarsingh H, Zaagsma J and Meurs H:
Arginase: a key enzyme in the pathophysiology of allergic asthma
opening novel therapeutic perspectives. Br J Pharmacol.
158:652–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munder M: Arginase: an emerging key player
in the mammalian immune system. Br J Pharmacol. 158:638–651. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu JF, Du ZD, Chen Z, Han ZC and He ZX:
Granulocyte colony-stimulating factor attenuates
monocrotaline-induced pulmonary hypertension by upregulating
endothelial progenitor cells via the nitric oxide system. Exp Ther
Med. 6:1402–1408. 2013.
|
|
9
|
Bekpinar S, Gurdol F, Unlucerci Y, Develi
S and Yilmaz A: Serum levels of arginase I are associated with left
ventricular function after myocardial infarction. Clin Biochem.
44:1090–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zimmet P, Alberti KG and Shaw J: Global
and societal implications of the diabetes epidemic. Nature.
414:782–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoeldtke RD, Bryner KD, McNeill DR,
Warehime SS, Van Dyke K and Hobbs G: Oxidative stress and insulin
requirements in patients with recent-onset type I diabetes. J Clin
Endocrinol Metab. 88:1624–1628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taysi S, Polat F, Gul M, Sari RA and Bakan
E: Lipid peroxidation, some extracullar antioxidant enzymes in
serum of patients with rheumatoid arthritis. Rheumatol Int.
21:200–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu G and Meininger CJ: Arginine nutrition
and cardiovascular function. J Nutr. 130:2626–2629. 2000.PubMed/NCBI
|
|
14
|
Fiorentino TV, Prioletta A, Zuo P and
Folli F: Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases. Curr Pharm Des.
19:5695–5703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bjelakovic G, Sokolovic D, Ljiljana S,
Kocic G, Jevtovic T, Stojanovic I, IIic M, Bjelakovic LJ, Zivic S,
Pavlovic D, Nikolić J and Basic J: Arginase activity and magnesium
levels in blood of children with diabetes mellitus. J Basic Clin
Physiol Pharmacol. 20:319–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teerlink T: Determination of the
endogenous nitric oxide synthase inhibitor asymmetric
dimethylarginine in biological sample by HPLC. Methods Mol Med.
108:263–274. 2005.PubMed/NCBI
|
|
17
|
Newaskar M, Hardy KA and Morris CR: Asthma
in sickle cell disease. Scientific World Journal. 11:1138–1152.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narayanan SP, Rojas M, Suwanpradid J,
Toque HA, Caldwell RW and Caldwell RB: Arginase in retinopathy.
Prog Retin Eye Res. 36:260–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pernow J and Jung C: Arginase as a
potential target in the treatment of cardiovascular disease:
reversal of arginine steal? Cardiovasc Res. 98:334–343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang ES, Ford K, Grokulsky G, Wang YB,
Chiang TM and Acchiardo SR: Normal circulating adult human red
blood cells contain inactive NOS proteins. J Lab Clin Med.
135:444–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris CR, Poljakovic M, Lavrisha L,
Machado L, Kuypers FA and Morris SM Jr: Decreased arginine
bioavailability and increased serum arginase activity in asthma. Am
J Respir Crit Care Med. 170:148–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giri H, Chandel S, Dwarakanath LS,
Sooriyakala S and Dixit M: Increased endothelial inflammation,
sTie-2 and arginase activity in umbilical cords obtained from
gestational diabetic mothers. PLoS One. 8:e845462013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramírez-Zamora S, Méndez-Rodríguez ML,
Olguín-Martínez M, Sánchez-Sevilla L, Quintana-Quintana M,
García-García N and Hernández-Muñoz R: Increased erythrocytes
by-products of arginine catabolism are associated with
hyperglycemia and could be involved in the pathogenesis of type 2
diabetes mellitus. PLoS One. 8:e668232013.PubMed/NCBI
|
|
24
|
Wei J, Tang Q, Liu L and Bin J:
Combination of peroxisome proliferator-activated receptor α/γ
agonists may benefit type 2 diabetes patients with coronary disease
through inhibition of inflammatory cytokine secretion. Exp Ther
Med. 5:783–788. 2013.
|
|
25
|
Griesmacher A, Kindhauser M, Andert SE,
Schreiner W, Toma C, Knoebl P, Pietschmann P, Prager R, Schnack C,
Schernthaner G, et al: Enhanced serum levels of
thiobarbituric-acid-reactive substances in diabetes mellitus. Am J
Med. 98:469–475. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xin G, Du J, Wang YT and Liang TT: Effect
of oxidative stress on heme oxygenase-1 expression in patients with
gestational diabetes mellitus. Exp Ther Med. 7:478–482.
2014.PubMed/NCBI
|
|
27
|
Yao L, Chandra S, Toque HA, Bhatta A,
Rojas M, Caldwell RB and Caldwell RW: Prevention of
diabetes-induced arginase activation and vascular dysfunction by
Rho kinase (ROCK) knockout. Cardiovasc Res. 97:509–519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elms SC, Toque HA, Rojas M, Xu Z, Caldwell
RW and Caldwell RB: The role of arginase I in diabetes-induced
retinal vascular dysfunction in mouse and rat models of diabetes.
Diabetologia. 56:654–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Findley CM, Cudmore MJ, Ahmed A and Kontos
CD: VEGF induces Tie 2 shedding via a phosphoinositide 3-kinase/Akt
dependent pathway to modulate Tie 2 signaling. Arterioscler Thromb
Vasc Biol. 27:2619–2626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onimaru M, Yonemitsu Y, Suzuki H, Fujii T
and Sueishi K: An autocrine linkage between matrix
metalloproteinase-14 and Tie 2 via ectodomain shedding modulates
angiopoietin-1-dependent function in endothelial cells.
Arterioscler Thromb Vasc Biol. 30:818–826. 2010. View Article : Google Scholar
|
|
31
|
Buhimschi CS, Bhandari V, Dulay AT, Thung
S, Rzaeq SS, et al: Amniotic fluid angiopoietin-1, angiopoietin-2,
and soluble receptor tunica interna endothelial cell kinase-2
levels and regulation in normal pregnancy and intraamniotic
inflammation-induced pretern birth. J Clin Endocrinol Metab.
95:3428–3436. 2010. View Article : Google Scholar
|
|
32
|
Sarikaphuti A, Nararatwanchai T,
Hashiguchi T, Ito T, Thaworanunta S, Kikuchi K, Oyama Y, Maruyama I
and Tancharoen S: Preventive effects of Morus alba L.
anthocyanins on diabetes in Zucker diabetic fatty rats. Exp Ther
Med. 6:689–695. 2013.
|