Introduction
One of the directions actively pursued in epilepsy
research is the reconstruction of hippocampal structural plasticity
and function; this plasticity mainly manifests in synapses
(1,2). p38 (also known as synaptophysin)
comprises a pair of glycoproteins with a relative molecular weight
of 38,000. p38 is expressed in the presynaptic vesicle membrane and
it is associated with synaptic structure and function. As a
presynaptic terminal-specific marker, p38 is often used to
determine synaptic density and distribution. Variations in p38
expression are used to monitor the formation of synapses and the
functional status of the nervous system. Due to the fact that p38
is not expressed by gliacytes, its expression reflects only
neuronal plasticity. p38 expression has been studied in patients
with epilepsy with the aim of evaluating synapse reconstruction and
synaptic plasticity (3).
Nuclear factor κ B (NF-κB) is a dimer composed of
two Rel family proteins. It is an important transcription factor
that regulates the expression of multiple eukaryotic genes and
influences a variety of cell functions (4). The activated forms of NF-κB are p50
and p65. When cells are in a quiescent condition, NF-κB binds to
the inhibitory factor IκB, and the resultant protein complex
remains in the cytoplasm. Following various cell stimulatory
signals, NF-κB is activated by the phosphorylation and degradation
of IκB or the phosphorylation of NF-κB through other
IκB-independent pathways (5).
These processes result in translocation of NF-κB to the nucleus,
leading to regulation of the expression of downstream genes,
including the immediate-early genes c-fos, c-myc and p53. Abnormal
expression levels of c-fos, c-myc and p53 are associated with
epileptic seizures (6,7). Blondeau et al (6) found that kainic acid was capable of
facilitating the attachment of NF-κB to DNA, enabling one of the
subunits of NF-κB to translocate to the nucleus. NF-κB activation
is considered to be a key step in epileptic pathogenesis, and the
role of NF-κB in epilepsy is presently the focus of numerous
studies (8). It has been indicated
that prior to pentylenetetrazol (PTZ) kindling or administration of
a PTZ sub-dose to chronically stimulate epileptic seizures, NF-κB
is activated to play an important role in epileptic plasticity
(9). As a transcription factor,
NF-κB participates in variations in epileptic plasticity by
regulating the expression of multiple genes; such variations in
epileptogenesis may be used to study the target genes of NF-κB. At
present, whether NF-κB regulates p38 has yet to be elucidated.
PTZ is a central nervous system stimulant that
induces acute and chronic kindling models of epilepsy, which may be
used as model systems to investigate epileptic pathogenesis. The
PC12 cell line is derived from rat adrenal phaeochromocytoma cells
that are cultured in the presence of nerve growth factor to
stimulate differentiation into neuron-like cells. Therefore, this
cell line closely resembles neural cells in terms of morphology as
well as physiological and biochemical functioning (3). The PC12 cell line is widely used as a
model for physiological and pathological studies of neurons since
neurons are difficult to culture in vitro. The aim of this
study was to investigate the pathogenesis of epilepsy by exploring
the molecular basis of variations in epileptic plasticity. In
particular, the effects of PTZ and NF-κB decoy
oligodeoxynucleotides (ODNs) on neuron-like PC12 cells and p38
expression were determined.
Materials and methods
Cell line
Rat phaeochromocytoma PC12 cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal
bovine serum and 10% horse serum. The cells were cultured in an
incubator containing 5% CO2 at 37°C with saturated
humidity. The culture medium was replaced every two to three days.
Neuron-like PC12 cells were prepared by exposure to nerve growth
factor. The study was approved by the ethics committee of the
Tongji Hospital Affiliated to Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China). All reagents
were purchased from Genemail Biotech Co., Ltd. (Xi’an, China).
Grouping and cell treatment
Cells in the logarithmic growth phase were
collected, adjusted to appropriate seeding concentrations with DMEM
and inoculated into various culture plates (containing slides
coated with polylysine). When the cells reached 70% confluence,
transfection was conducted using Lipofectamine 2000, followed by a
further 24 h of cell culture. The cells were divided into control
(DMEM) and PTZ (final concentration, 10 mmol/l) groups. The PTZ
group was subdivided into three groups: blank (without ODNs),
missense NF-κB decoy ODNs and NF-κB decoy ODNs. The cells were then
continuously cultured. NF-κB activity was determined using confocal
laser scanning microscopy (CLSM) after 2 h of culture. In addition,
MTT spectrophotometry was performed after 2 and 24 h of culture.
The extent of cellular apoptosis was determined by flow cytometry
and western blotting was conducted at 24 h.
ODN sequences and transfection
The decoy ODNs (sequences: 5′-GAGGGGACTTTCCCT-3′ and
3′-CTCCCC TGAAA-GGGA-5′) were designed according to the sequence of
NF-κB (4). A missense ODN
(5′-GATGCGTCTGTCGCA-3′) and a control ODN (3′-CTACGCAGACAGCGT-5′)
were also synthesized. Using these ODNs, transfection was conducted
according to the manufacturer’s instructions.
MTT assay
Cells were inoculated into a 96-well plate with a
seeding density of 1×105 cells/ml, prior to being
divided and processed into the control and PTZ groups. The setup
was performed in triplicate and a blank well was used as one of the
controls. A total of 20 μl MTT solution (5 mg/ml) was added to each
well and incubated for 4 h. Subsequently, the supernatant was
removed and 100 μl dimethyl sulphoxide was added to each well. The
cells were incubated for 20 h at 37°C. A Multiskan MK3 model
automatic enzyme-labelled instrument was used to detect the
absorbance values for each well. These absorbance values were
indicative of the cell survival rate.
Apoptosis assay
Cells were inoculated into a 96-well plate with a
seeding density of 1×105 cells/ml. The cells were
divided and processed to form the control and PTZ groups, and the
setup was performed in triplicate. The cells were washed twice with
pre-cooled phosphate-buffered saline (PBS) and digested with 0.25%
pancreatin to form a monoplast suspension. The monoplast suspension
was centrifuged at 15,000 × g, fixed with 70% ethanol and washed
with PBS to remove the fixative. The residue was reacted with RNase
overnight. The resultant solution was mixed with propidium iodide
(PI) liquid. DNA analysis was conducted using a flow cytometer.
CLSM assay
NF-κB protein and a nuclear DNA fluorescent marker
were inoculated into a 96-well plate containing 1×105
cells/ml. The cells were grouped and processed to form the control
and PTZ experimental groups. The cell slides were fixed with 4%
paraformaldehyde, washed with PBS and sealed. p65 rabbit anti-mouse
antibody (1:250 dilution) was added, and the cells were incubated
at 4°C overnight prior to being washed with PBS. Fluorescein
isothiocyanate (FITC)-labelled goat anti-rabbit antibody (1:50
dilution) was added, followed by incubation at 37°C for 30 min and
the addition of 5 μg/ml PI plus 100 μg/ml RNaseA enzyme. The cell
slides were protected from light for 25 min and mounted with
glycerol for NF-κB detection.
The cell slides were viewed at 40× objective
magnification. An argon ion laser was used as the light source and
the excitation wavelength was 488 nm. The photomultiplier tube
(PMT) 1 580 long pass emission filter was used to observe PI red
fluorescence, and the PMT 2 522/DF35 emission filter was used to
visualize FITC green fluorescence. The capacity factor was 30% and
the scanning speed was slow. WinView/32 software was used to
analyse the ratio of green fluorescence in the nuclear area (Fn) to
red fluorescence in the cytoplasmic area (Fc). The Fn/Fc values of
20 cell samples were calculated.
Western blotting
Cells were inoculated into a 96-well plate with a
seeding density of 1×105 cells/ml. The cells were
grouped and processed to form the control and PTZ groups. Following
culturing for 2 h, the cells were washed with ice-cold PBS and 50
μl cell lysis buffer was added. The resultant mixture was processed
by ultrasonic wave four times (5 sec/round) and centrifuged for 15
min at 4°C and 12,000 r/min. The supernatant was recovered and the
total protein concentration was determined using the Bradford
method. The supernatant was stored at −70°C until further use. SDS
loading buffer was mixed with the protein sample. The mixture was
boiled in a water bath for 5 min and then stored at −20°C until
further use.
SDS-PAGE was conducted at 8 V/cm and 360 mA for 90
min to determine the presence of p38. The protein was subsequently
transferred onto a polyvinylidene fluoride film and sealed. Rabbit
anti-human monoclonal p38 antibody (1:250 dilution) was added,
prior to the mixture being incubated at 4°C for 16 h and washed
with Tris-buffered saline with Tween 20. A horseradish
peroxidase-labelled anti-rabbit immunoglobulin G secondary antibody
(1:1,000) was added. After 1 h of incubation at room temperature,
diaminobenzidine was added for development. Pan-actin was used as
an internal reference. A gel imaging analysis system was used to
document and analyse the gel data, and the p38/pan-actin absorbance
ratio was calculated.
Statistical analysis
All the experiments were performed in triplicate.
Data are expressed as the mean ± standard deviation and analysis
was performed using SPSS 12.0 software (SPSS, Inc., Chicago, IL,
USA). Analysis of variance was used to compare the mean values.
Results
Cell survival rate
At 2 and 24 h, no significant differences were
observed in the survival rate of neuron-like PC12 cells exposed to
PTZ, missense NF-κB decoy ODNs or NF-κB decoy ODNs (pairwise
comparisons, all P>0.05; Table
I).
 | Table IInfluences of PTZ, missense NF-κB
decoy ODN and NF-κB decoy ODN on the cell survival rate. |
Table I
Influences of PTZ, missense NF-κB
decoy ODN and NF-κB decoy ODN on the cell survival rate.
| Survival rate
(A) |
|---|
|
|
|---|
| 2 h | 24 h |
|---|
|
|
|
|---|
| Groups | Control group | PTZ group | Control group | PTZ group |
|---|
| Blank | 0.402±0.074 | 0.426±0.053 | 0.411±0.067 | 0.420±0.042 |
| Missense NF-κB decoy
ODN | 0.427±0.046 | 0.395±0.031 | 0.427±0.046 | 0.395±0.031 |
| NF-κB decoy ODN | 0.423±0.028 | 0.414±0.020 | 0.403±0.027 | 0.415±0.050 |
Cellular apoptosis
The neuron-like PC12 cells in various groups
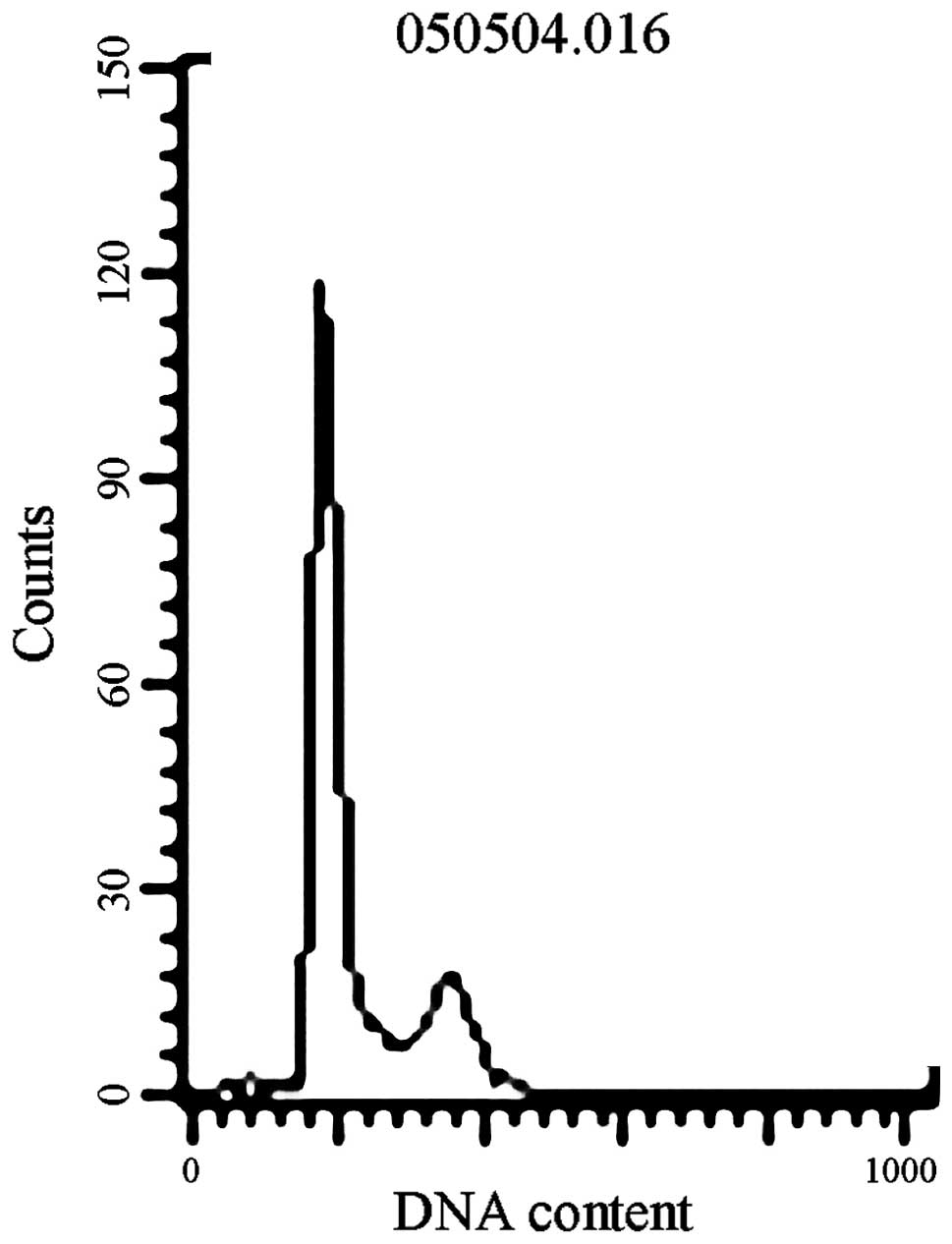
exhibited no cellular apoptosis peak at 2 and 24 h (Fig. 1).
NF-κB activity
PMT 1 showed that the neuron-like PC12 cells
exhibited round, red fluorescent areas (Fig. 2A–C), whereas PMT 2 showed that the
neuron-like PC12 cells exhibited green fluorescence, representing
the NF-κB p65 subunit (Fig. 2D–F).
Once the two images were overlaid, a complete cell section was
visible, with the nucleus in red and the cytoplasm in green
(Fig. 2G–I). In the control group,
PMT 2 showed that green fluorescence was distributed mainly in the
cytoplasm (Fig. 2B). In the PTZ
group, PMT 2 showed that the entire cell exhibited green
fluorescence, which was more intense in the nucleus than in the
cytoplasm (Fig. 2F and H). The
overlaid images of the stained cells showed that the intensity of
green fluorescence in the nucleus of the PTZ group was
significantly higher than that in the control group (Fig. 2C, F and I). Statistical analysis
revealed that the Fn/Fc ratio of the PTZ group was significantly
higher than that of the control group (P<0.01). In the PTZ
group, the Fn/Fc ratio of the NF-κB decoy ODN group was
significantly lower than that of the blank and the missense NF-κB
decoy ODN groups (both P<0.05). No significant difference in the
Fn/Fc ratio was observed between the blank and the missense NF-κB
decoy ODN groups (both P>0.05; Table II).
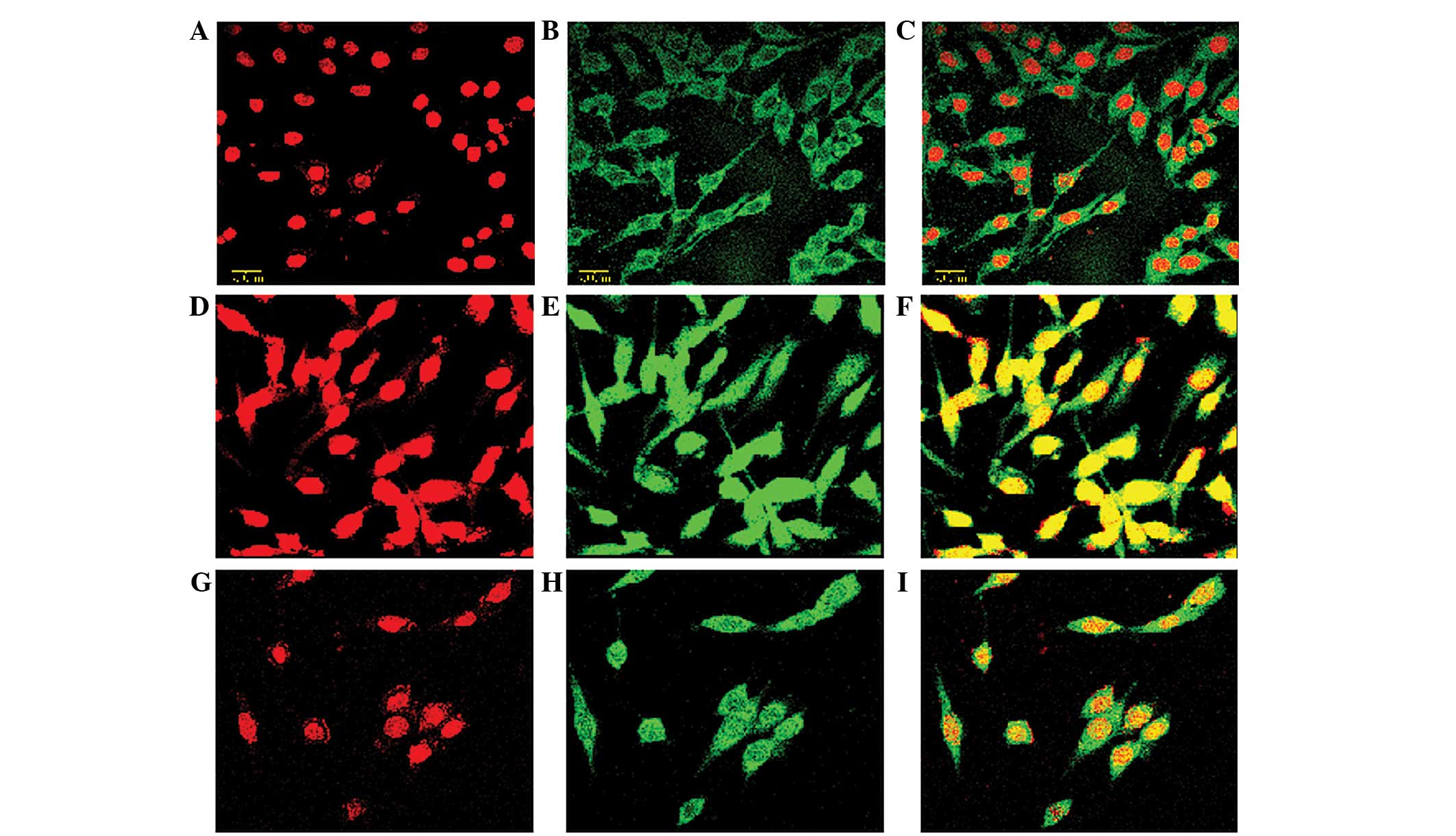 | Figure 2Neuron-like PC12 cells exhibited (A-C)
round, red fluorescent areas (as shown by PMT1) and (D-F) green
fluorescence representing p65 protein (as shown by PMT2). (G-I)
Overlay of the PMT 1 and 2 images showed complete cell sections
exhibiting a red nucleus and a green cytoplasm. (D) In the PTZ
group, including the blank, missense NF-κB decoy ODN and NF-κB
decoy ODN subgroups, PMT 2 showed that the whole cell exhibited
green fluorescence and the intensity of green fluorescence was
higher in the nucleus compared with the cytoplasm; (E) in the
control group, including the blank, missense NF-κB decoy ODN and
NF-κB decoy ODN subgroups, PMT 2 showed that green fluorescence was
mainly distributed in the cytoplasm; (G-I) overlay of the cell
images showed that the intensity of green fluorescence in the red
fluorescent nuclear area of the PTZ group was significantly higher
than that in the control group. PTZ, pentylenetetrazol; NF-κB,
nuclear factor κ B; ODN, oligodeoxynucleotide, PMT, photomultiplier
tube. |
 | Table IIEffects of PTZ, missense NF-κB decoy
ODN and NF-κB decoy ODN on NF-κB activity. |
Table II
Effects of PTZ, missense NF-κB decoy
ODN and NF-κB decoy ODN on NF-κB activity.
| Fn/ Fc |
|---|
|
|
|---|
| Groups | Control group | PTZ group |
|---|
| Blank | 0.2161±0.0267 | 1.1063±0.1205a,b |
| Missense NF-κB decoy
ODN | 0.2312±0.0620 | 1.1067±0.1054a,b |
| NF-κB decoy ODN | 0.2532±0.0527 | 0.7753±0.0774a |
p38 expression
In the PTZ group, p38 expression was significantly
higher than that in the control group (P<0.01). However, within
the PTZ and control groups, no significant differences were
observed among the blank, missense NF-κB decoy ODN and the NF-κB
decoy ODN subgroups (all P>0.05; Table III; Fig. 3).
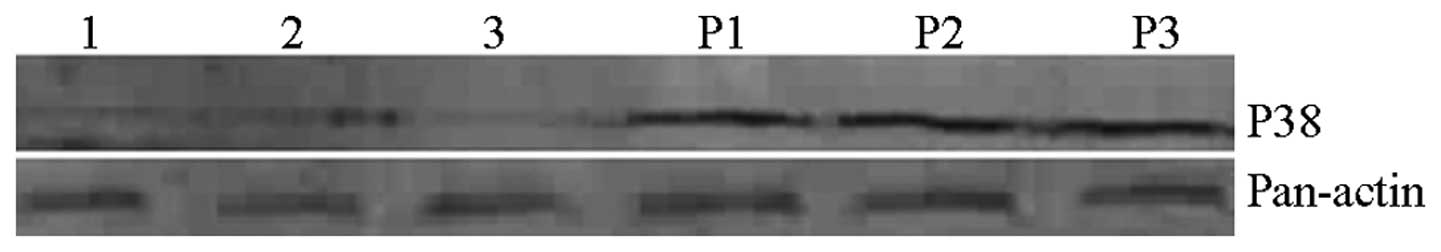 | Figure 3Effects of PTZ, missense NF-κB decoy
ODN and NF-κB decoy ODN on p38 expression. Lane 1, blank/control
group; lane 2, misssense NF-κB decoy ODN/control group; lane 3,
NF-κB decoy ODN/control group; lane P1, blank/PTZ group; lane P2,
missense NF-κB decoy ODN/PTZ group; lane P3, NF-κB decoy ODN/PTZ
group. PTZ, pentylenetetrazol; NF-κB, nuclear factor κ B; ODN,
oligodeoxynucleotide. |
 | Table IIIEffects of PTZ, missense NF-κB decoy
ODN and NF-κB decoy ODN on p38 expression. |
Table III
Effects of PTZ, missense NF-κB decoy
ODN and NF-κB decoy ODN on p38 expression.
| p38/pan-actin |
|---|
|
|
|---|
| Groups | Control group | PTZ group |
|---|
| Blank | 0.2640±0.0496 | 1.0237±0.0724a |
| Missense NF-κB decoy
ODN | 0.2560±0.0614 | 1.0477±0.0705a |
| NF-κB decoy ODN | 0.2493±0.0422 | 1.0390±0.0514a |
Discussion
Biologists and medical scientists agree that the
nervous system is characterised by plasticity. At present, one
direction in epilepsy research is the reconstruction of hippocampal
structural plasticity and function; this plasticity mainly
manifests in synapses. p38 is a glycoprotein that is associated
with synaptic structure and function. Changes in p38 expression are
used to monitor the formation of synapses and the functional status
of the nervous system. p38 is not expressed in gliacytes; thus, the
expression of p38 reflects only neuronal plasticity. p38 expression
has been used to study patients with epilepsy and animal models of
epilepsy to evaluate structural changes due to synaptic plasticity
(2,3).
PTZ is a central nervous system convulsant that acts
a γ-aminobutyric acid receptor antagonist and does not cause
neurotoxic effects. Exposure to successive sub-convulsant doses of
PTZ leads to gradual apoptosis and necrosis of hippocampal
pyramidal cells. Exposure to successive sub-convulsant doses of PTZ
does not lead to apoptosis and necrosis of hippocampal pyramidal
cells. PTZ is also an ideal compound for simulating generalised
tonic-clonic epileptic seizures (10). Whether p38 expression changes prior
to PTZ kindling has not been elucidated. Therefore, considering
that PTZ does not affect the survival rate of neuron-like PC12
cells and does not induce apoptosis, the present study was
conducted to examine the effects of PTZ on p38 expression and
neuronal plasticity in vitro using neuron-like PC12 cells.
No significant differences in the survival rate of neuron-like PC12
cells were observed among the groups at 2 and 24 h, and no
apoptosis peak was observed in any of the groups. Under the
experimental conditions, PTZ had no influence on the survival rate
of neuron-like PC12 cells and apoptosis was not observed. At 24 h,
p38 expression in the PTZ group was significantly higher than that
in the control group, indicating that PTZ induces p38 expression.
Chronic stimulation with sub-doses of PTZ may influence neuronal
plasticity, potentially by modulating p38 protein expression.
NF-κB is a dimer protein composed of two Rel family
proteins and its activation is a key step in epileptic
pathogenesis. Animal studies indicate that the epileptic
seizure-induced intracerebral inflammatory response is one of the
main reasons for the pathological changes observed in the brain
tissue of patients following epileptic seizures, particularly
hippocampal structural damage (11,12).
It has been suggested that the NF-κB signalling pathway has an
important role in the expression and regulation of genes encoding
cytokines and inflammatory mediators, and that overexpression of
NF-κB may cause severe inflammation and tissue injury (13). A specific antagonist of NF-κB,
pyrrolidine dithiocarbamate, inhibits epileptic seizures and
intracerebral NF-κB expression in rats (14). Under the experimental conditions of
the present study, the effect of PTZ on p38 expression and neuronal
plasticity was examined, and NF-κB activity was determined using
CLSM. PTZ was used to directly intervene in the functioning of
neuron-like PC12 cells in vitro.
Wang et al (9,15)
demonstrated that CLSM shows the location of NF-κB as well as its
activation level on the basis of fluorescence intensities. An
additional advantage of CLSM is that it shows cell morphology. The
results of the present study showed that at 2 h, NF-κB activity in
the PTZ group was significantly higher than that in the control
group, indicating that PTZ was able to activate NF-κB. Therefore,
it may be inferred that chronic stimulation with a sub-dose of PTZ
affects neuronal plasticity, possibly by influencing NF-κB
activity. Lubin (16) performed
immunohistochemical analyses on brain sections obtained
post-operatively from patients with temporal lobe epilepsy
accompanied by hippocampal sclerosis. Overexpression of NF-κB was
observed in gliacytes and pyramidal cells, indicating that epilepsy
was induced by an NF-κB-mediated inflammatory reaction. This study
also revealed that the inflammatory reaction was chronically active
or transiently reinduced by repeated epileptiform seizures.
Hippocampal neuron activation, in particular nuclear translocation
of the p65 subunit of NF-κB, is an important mechanism of
PTZ-kindled epilepsy formation in rats. Epileptic seizures are
capable of inducing nuclear translocation of NF-κB in hippocampal
tissue as well as interleukin-1β and cyclooxygenase-2 expression
(17). PTZ increases protein
expression of the p65 subunit of NF-κB in the brain tissue of rats
with epilepsy (18,19). Another study indicated that
epileptic seizures cause autophagic death of astrocytes via a
pathway involving tumour necrosis factor-α and phosphorylated
p65/RelA-Ser529 (20).
As a transcription factor, NF-κB contributes to
variations in epileptic plasticity by regulating the expression of
multiple genes. Therefore, studies on target genes being regulated
by this transcription factor are important. One study indicated
that insular epilepsy is closely associated with the hippocampus
(18,19). This study also showed that
growth-associated protein 43 and p38 are the pathological bases for
this condition and the key molecular mechanisms in synaptic
plasticity. Previous studies have shown that epilepsy-induced
neuronal death is associated with various activations or
deactivations of p38 and certain extracellular signal-regulated
kinases (12,22). In particular, sesamin protects
against kainic acid-induced brain damage in status epilepticus and
inhibits the mitogen-activated protein kinase pathway through
anti-inflammatory and partially anti-inflammatory mechanisms.
NF-κB and p38 participate in epileptic plasticity
variation. As a presynaptic terminal-specific protein, p38 is a
marker of synaptic plasticity; however, prior to this study the
role of NF-κB in p38 regulation was yet to be elucidated. In the
present study, an immunoblot assay was used to simultaneously
determine p38 expression in neuron-like PC12 cells prior to and
following exposure to PTZ, a missense NF-κB decoy ODN and an NF-κB
decoy ODN. CLSM was used to assess variations in NF-κB activity.
NF-κB decoy ODNs were artificially synthesised and competitively
bound to the specific consensus sequence of NF-κB, reducing the
binding of NF-κB to a target gene promoter and downregulating the
double-stranded ODN expressed by the target gene. The decoy
strategy has become a powerful tool for in vitro and in
vivo studies of gene regulation due to the fact that it is
associated with greater efficiency and selectivity than antisense
ODNs (23). The missense NF-κB
decoy ODN is a double-stranded ODN with a similar structure to that
of the NF-κB decoy ODN. It differs in terms of key bases; thus, it
is not able to competitively interact with the specific consensus
sequences of NF-κB and has no regulatory effects on target genes.
The results showed that following PTZ exposure, p38 expression in
neuron-like PC12 cells was significantly increased. NF-κB activity
in neuron-like PC12 cells decreased following NF-κB decoy ODN
transfection; however, p38 expression levels remained constant. The
missense NF-κB decoy ODN had no influence on p38 expression or
NF-κB activity. These results suggested that NF-κB does not
regulate p38 expression. Epileptic plasticity variation is a
complex pathological process and further studies are necessary to
determine the specific mechanisms involved.
References
|
1
|
Eastwood SL, Burnet PW, McDonald B,
Clinton J and Harrison PJ: Synaptophysin gene expression in human
brain: a quantitative in situ hybridization and immunocytochemical
study. Neuroscience. 59:881–892. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Proper EA, Oestreicher AB, Jansen GH, et
al: Immunohistochemical characterization of mossy fibre sprouting
in the hippocampus of patients with pharmaco-resistant temporal
lobe epilepsy. Brain. 123:19–30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies KG, Schweitzer JB, Looney MR, Bush
AJ, Dohan FC Jr and Hermann BP: Synaptophysin immunohistohemistry
densitometry measurement in resected human hippocampus: implication
for the etiology of hippocampal sclerosis. Epilepsy Res.
32:335–344. 1998. View Article : Google Scholar
|
|
4
|
Rauch BH, Weber AA, Braun M, Zimmermann N
and Schrör K: PDGF-induced Akt phosphorylation does not activate
NF-kappa B in human vascular smooth muscle cells and fibroblasts.
FEBS Lett. 481:3–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitz ML, Bacher S and Kracht M: I kappa
B-independent control of NF-kappa B activity by modulatory
phosphorylations. Trends Biochem Sci. 26:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blondeau N, Widmann C, Lazdunski M and
Heurteaux C: Activation of the nuclear factor-kappaB is a key event
in brain tolerance. J Neurosci. 21:4668–4677. 2001.PubMed/NCBI
|
|
7
|
Savolainen KM, Loikkanen J, Eerikäinen S
and Naarala J: Interactions of excitatory neurotransmitters and
xenobiotics in excitotoxicity and oxidative stress: glutamate and
lead. Toxicol Lett. 28:363–367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lerner-Natoli M, Montpied P, Rousset MC,
Bockaert J and Rondouin G: Sequential expression of surface
antigens and transcription factor NFkappa B by hippocampal cells in
excitotoxicity and experimental epilepsy. Epilepsy Res. 41:141–154.
2000. View Article : Google Scholar
|
|
9
|
Wang KY, Ruan XZ, Zhang ZH and Zeng SQ:
Activation of nuclear factor κB assayed by laser scanning confocal
microscope. Chinese Journal of Physical Medicine and
Retabulitation. 23:141–143. 2001.(In Chinese).
|
|
10
|
Qu H, Eloqayli H and Sonnewald U:
Pentylenetetrazole affects metabolism of astrocytes in culture. J
Neurosci Res. 79:48–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samland H, Huitron-Resendiz S, Masliah E,
Criado J, Henriksen SJ and Campbell IL: Profound increase in
sensitivity to glutamatergic- but not cholinergic agonist-induced
seizures in transgenic mice with astrocyte production of IL-6. J
Neurosci Res. 73:176–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voutsinos-Porche B, Koning E, Kaplan H, et
al: Temporal patterns of the cerebral inflammatory response in the
rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol
Dis. 17:385–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O‘Neill LA and Kaltschmidt C: NF-kappaB: a
crucial transcription factor for glial and neuronal cell function.
Trends Neursci. 20:252–258. 1997.PubMed/NCBI
|
|
14
|
Yu N, Di Q, Liu H, Hu Y, Jiang Y, Yan YK,
Zhang YF and Zhang YD: Nuclear factor-kappa B activity regulates
brain expression of P-glycoprotein in the kainic acid-induced
seizure rats. Mediators Inflamm. 2011:6706132011.PubMed/NCBI
|
|
15
|
Wang KY, Ruan XZ, Zhu SQ and Wang W: The
role of the neuronal activation of hippocampus in epileptogenesis
of pentylenetetrazol kindling rat. Journal of Apoplexy and Nervous
Diseases. 18:347–349. 2001.(In Chinese).
|
|
16
|
Lubin FD, Ren Y, Xu X and Anderson AE:
Nuclear factor-kappa B regulates seizure threshold and gene
transcription following convulsant stimulation. J Neurochem.
103:1381–1395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SJ, Chi ZF, Wang SH, Chi LZ and Zhao
XH: Poly adenosine diphosphate-ribose polymerase regulates the
expression of nuclear factor-κB and related inflammatory factors in
rat hippocampus after epilepsy. Chin J Pathophysiol. 26:86–90.
2010.(In Chinese).
|
|
18
|
Liu QZ, Wang F, Mu QC, Zhang PS and Sun T:
Expressions of GAP43, P38 mRNA and protein in insular electrical
kindled rats and its significance. Zhonghua Yi Xue Za Zhi.
90:1348–1352. 2010.(In Chinese).
|
|
19
|
Liu XW, Cai AM, Tian BX, Han K and Zhang
XJ: Study on activation of NF-κB p65 in rat hippocampal formation
after experimental seizure and neuroprotective effects of W-7. Acta
Universitatis Medicinalis Nanjing (Natural Science). 12:1691–1695.
2010.(In Chinese).
|
|
20
|
Ryu HJ, Kim JE, Yeo SI and Kang TC:
p65/RelA-Ser529 NF-κB subunit phosphorylation induces autophagic
astroglial death (Clasmatodendrosis) following status epilepticus.
Cell Mol Neurobiol. 31:1071–1078. 2011.PubMed/NCBI
|
|
21
|
de Lemos L, Junyent F, Verdaguer E, et al:
Differences in activation of ERK1/2 and p38 kinase in Jnk3 null
mice following KA treatment. J Neurochem. 114:1315–1322.
2010.PubMed/NCBI
|
|
22
|
Hsieh PF, Hou CW, Yao PW, et al: Sesamin
ameliorates oxidative stress and mortality in kainic acid-induced
status epilepticus by inhibition of MAPK and COX-2 activation. J
Neuroinflammation. 8:572011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crinelli R, Bianchi M, Gentilini L and
Magnani M: Design and characterization of decoy oligonucleotides
containing locked nucleic acids. Nucleic Acids Res. 30:2435–2443.
2002. View Article : Google Scholar : PubMed/NCBI
|