Introduction
Bone metastasis is one of the most common
complications in late stage malignancies, including in lung,
breast, prostate and renal cancer. Approximately 20~70% patients
with malignancy have bone metastases in the later stages and bone
metastatic pain is a highly discomforting condition for patients
(1). Effectively relieving the
pain of bone metastasis improves the life quality of patients and
should be considered an important part of integrative therapy for
malignancy (2–4). Patients with bone metastasis may have
the possibility for complete remission (no clinical or radiography
evidence of disease) if they accept the most suitable localized
therapy (5–7). Bone lesions due to metastatic disease
destroy the structural integrity of the bone and increase the
morbidity of adverse bone-related events (8). These adverse bone-related events
severely impact on the quality of patients’ lives (9). At present, there are a number of
treatment strategies for the therapeutic management of bone
metastasis, including surgery, percutaneous thermal ablation,
radiation, chemotherapy and medicines promoting the reconstruction
of bone lesions (10,11).
Radiotherapy and surgery had been used for the
relief of bone metastatic pain. However, there are limitations to
these approaches, particularly the injury of normal tissue
surrounding the diseased lesions. Percutaneous ablation offers an
effective minimally invasive alternative therapy to treat patients
with limited bone metastases. Ablation may also be considered as an
alternative to, or used in conjunction with, systemic therapies.
Cryoablation with accurate ablation extent monitoring is an
excellent form of ablation for eliminating the lesions of bone
metastases (12,13).
Bisphosphonates are analogs of pyrophosphates that
are able to improve bone metabolism and inhibit several components
of the bone resorptive process. Bisphosphonates currently have an
important role in the treatment of skeletal complications
associated with metastatic bone disease. Zoledronic acid is a
later-generation bisphosphonate that has been identified as having
the most potent inhibitory activity as an antiresorptive drug. To
the best of our knowledge, there are no other studies concerning
the use of cryoablation in combination with zoledronic acid
treatment in bone metastatic pain (14–16).
The purpose of this prospective case-controlled
study was to determine the safety and efficacy of percutaneous
cryoablation combined with zoledronic acid for the reduction of
bone metastatic pain, with the aim of improving the quality of life
for patients with painful metastatic tumors involving bone.
A total of 84 cases of malignant tumor bone
metastases with pain between June 2008 and October 2012 were
recruited into the study. Patients were randomly divided into three
groups. Group A patients were subject to targeted argon-helium
cryoablation once and were monthly administered an injection of
zoledronic acid (4 mg) dissolved in 0.9% sodium chloride injection
(100 ml), by intravenous drip for >15 min, for a total of >6
times. Group B patients were subject to targeted argon-helium
cryoablation of metastatic lesions once. Group C patients were
monthly administered an injection of zoledronic acid (4 mg)
dissolved in 0.9% sodium chloride injection (100 ml), by
intravenous drip for >5 min, for a total of >6 times.
Materials and methods
Patient inclusion criteria
The inclusion criteria of this prospective study
were: i) a metastatic bone tumor confirmed by histological or
cytological examination and/or imaging, including systemic computed
tomography (CT) and magnetic resonance imaging (MRI), and bone
emission computed tomography, with moderate to severe pain; ii) a
life expectancy of >6 months; iii) blood routine examination was
normal and serum Ca2+ levels were >2.00 mmol/l; iv)
the functions of heart, liver, kidney and other vital organs were
mostly normal; v) physical Karnofsky performance status (KPS) was
>60. 0%; vi) patients enrolled signed an informed consent form;
and vii) subjects were able to tolerate preoperative and
postoperative plain and enhanced CT scanning.
Exclusion criteria
The exclusion criteria of this prospective study
were: i) patients diagnosed with primary bone cancer by pathology;
ii) patients with impending fractures; iii) unwilling to accept
cryoablation and/or zoledronic acid therapy; iv) intolerant of
targeted argon-helium cryoablation due to severe dysfunction of
vital organs, including heart, liver and kidney; v) blood
coagulation disorders; and vi) serious hypocalcemia.
Demographic data of subjects
A total of 84 cases of malignant tumor bone
metastatic pain in patients aged between 37 and 72 years were
enrolled. Among them, there were 44 male cases and 40 female cases.
The patients suffered from lung cancer in 30 cases, breast cancer
in 23 cases, digestive system cancer in 7 cases, kidney cancer in 9
cases, nasopharyngeal carcinoma (NPC) in 4 cases and other tumor
types in 11 cases. Patients were randomly divided into three
groups: group A (28 cases) argon-helium cryoablation combined with
zoledronic acid), group B (28 cases, argon-helium cryoablation) and
group C (28 cases, zoledronic acid). There were no statistically
differences in gender, age, pain intensity and activity ability
among the three groups, as determined by a Student’s t-test and
χ2 test. The present study was conducted in accordance
with the Declaration of Helsinki, and with approval from the Ethics
Committee of the First Hospital of Lanzhou University (Lanzhou,
China). Written informed consent was obtained from all
participants. The detailed demographic data are summarized in
Table I.
 | Table IDemographic characteristics and
baseline clinical features in the three groups. |
Table I
Demographic characteristics and
baseline clinical features in the three groups.
| Group | n | Age (years) | Male, n (%) | Pain score | KPS score | Pain medication
(n) |
|---|
| Group A | 28 | 56.6±11.33 | 14 (50.0) | 8±1.2 | 70±0.9 | 15 |
| Group B | 28 | 54.8±10.52 | 15 (53.6) | 8±1.1 | 70±1.3 | 14 |
| Group C | 28 | 51.8±9.31 | 15 (53.6) | 9±0.7 | 70±1.1 | 15 |
| χ2 | - | 0.699 | 0.095 | 0.000 | 0.087 | 0.095 |
| P-value | - | 0.514 | 0.757 | 1.000 | 0.900 | 0.766 |
Equipment and therapeutic regimens
A minimally invasive, targeted argon-helium
cryoablation operating system was used, which comprised an
argon-helium cryoablation system, and cryoprobes with diameters
1.7, 2.4 and 3.8 mm (Endocare Cryocare System; HealthTronics, Inc.,
Austin, TX, USA) and a 16- or 64-slice CT instrument (Siemens,
München, Germany).
All patients were informed of the relevant
precautions and operational risk and provided informed consent.
Preoperative plain CT scanning was obtained to confirm tumor range
and select the freezing levels, and to identify the feeding angle
and direction. Metal markers were used as guides to determine the
puncture point. The group A patients were provided targeted
argon-helium cryoablation to metastatic lesions once and were
monthly administered an injection of zoledronic acid (4 mg)
dissolved in 0.9% sodium chloride injection (100 ml) by intravenous
drip for >15 min, for a total of >6 times. Group B patients
were subject to targeted argon-helium cryoablation to metastatic
lesions once. Group C patients were monthly administered an
injection of zoledronic acid (4 mg), as described for group A.
Pretreatment patient assessment
Prior to therapy with cryoablation, the effect of
focal painful bone metastasis was assessed by use of the verbal
rating scale (VRS), and the KPS was used for assessment of the
patient’s quality of life. Analgesic medicine use was also
recorded. Each patient was instructed to specifically respond to
the VRS questions with respect to the focal painful metastasis that
was to be treated. Patients were physically examined by an
interventionalist prior to treatment to determine whether the site
or sites of focal pain correlated with the available imaging,
including CT, MRI and ultrasound imaging, which was obtained
immediately following entrance into the study. A complete blood
count and prothrombin time were obtained within one week of the
ablation procedure. Each patient’s history of previous chemotherapy
and radiation therapy was recorded. Complications were recorded
throughout the follow-up period and classified via Common
Terminology Criteria for Adverse Events (CTCAE, version 4.03)
(17).
Cryoablation procedure
Following routine sterile preparation, 0.2%
chloroprocaine was used to anesthetize the puncture point. The 1.7,
2.4 or 3.8 mm cryoprobes were placed into a 6, 9 or 11F sheath tube
and inserted into the metastatic lesions; the feeding direction and
depth were under the guidance of plain CT scanning. A single
cryoprobe was placed for lesions ≤3 cm in diameter. For larger
lesions, two to five additional cryoprobes were systematically
placed with CT guidance. Cryoablation treatments were focused on
the margin of the lesion involving bone to treat the
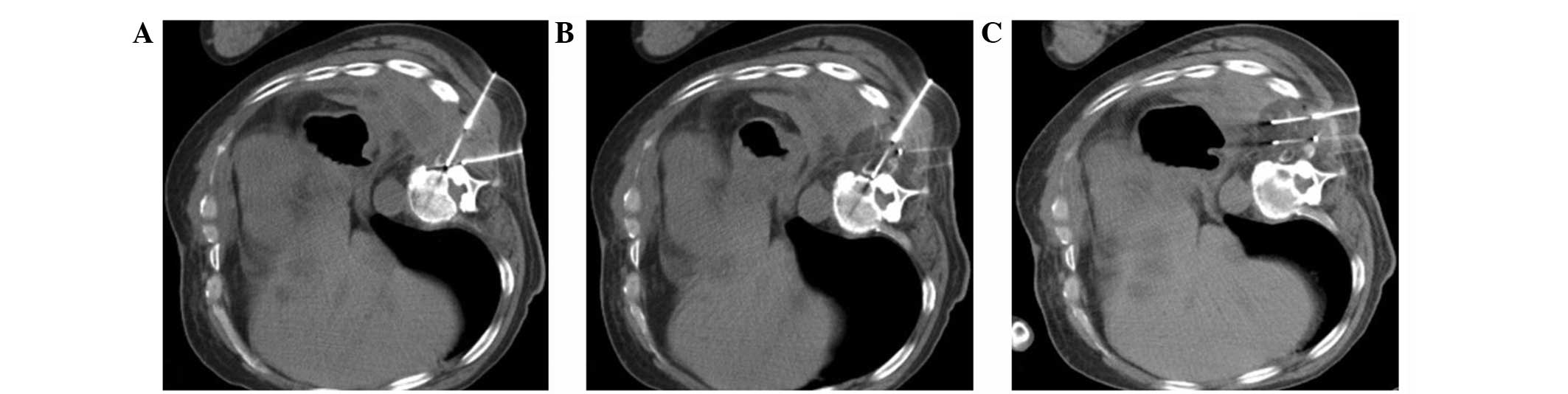
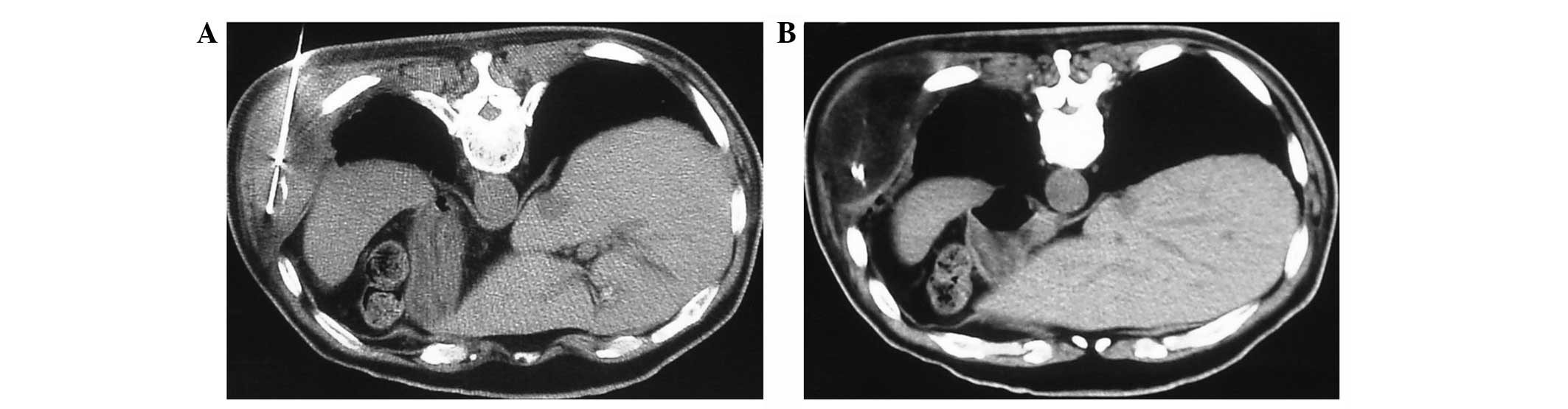
soft-tissue-bone interface (Fig.
1). Plain CT scanning was performed approximately every 2 min
throughout the freezing portions of the cycle to monitor the growth
of the ice ball (Fig. 2). Each
lesion was subject to three freeze-thaw-freeze cycles, 20 min per
cycle. Following each freezing cycle, the cryoprobes were warmed
with active heating using helium gas until the temperature reached
>20°C. The cryoprobes were then withdrawn (Fig. 3).
Test items
The pain improvement was continuously observed for
180 days following the treatments. One day prior to treatment and
7, 14 and 21 days following treatment, the general condition, blood
calcium, blood routine, liver function, renal function, blood
biochemistry, urine routine and electrocardiogram of patients were
measured. The normal range of blood Ca2+ is 2.0–2.6
mmol/l.
Efficacy assessment criteria
The VRS was presented to the patient as a series of
descriptions, ranked and numbered as follows: no pain, 0; mild
pain, 1; moderate pain, 2; intense pain, 3; extremely intense pain,
4. The primary endpoints were complete response (CR) defined as the
absence of pain without the need for increasing analgesic relief,
and partial response (PR) defined as an improvement ≥2 on the
ordinal scale with no requirement for increasing analgesic relief.
The patients with the same or worse pain level at three weeks were
considered to have no response (NR). The responses were assessed by
follow-up or with telephone interviews. The responses were examined
at 3 and 24 weeks. The response durations were calculated from the
first date evaluated at 3 weeks to the date of relapse, or in
absence of relapse to the date of last assessment or mortality
(18,19).
Adverse reactions
Potential adverse reactions of the therapies include
active bleeding, frostbite, fever, muscle pain, nausea and
vomiting, skin rash, hypocalcemia and dysfunction of the kidneys
and liver.
Statistical analysis
Student’s t-test was used to assess the differences
in age, KPS score and VRS score of each group. χ2 test
was used to assess the differences in gender, malignant
hypercalcemia, pain medication and primary tumor location and type.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cryoablation combined with zoledronic
acid exerted evident analgesic effects
Following 180 days of treatment, according to the
efficacy assessment criteria, the CR, PR and OR were counted in
each group. In group A, the OR was 85.7% (24/28), the CR was 35.7%
(10/28) and the PR was 50.0% (14/28). In group B, the OR was 50.0%
(14/28), the CR was 14.3% (4/28) and the PR was 35.7% (10/28). In
group C, the OR was 67.9% (19/28), the CR was 21.4% (6/28) and the
PR was 46.4% (13/28). Next, the therapeutic effects were compared
between each of the groups. The statistical results demonstrated
that the analgesic effect in group A was the highest, compared with
that in groups B and C (P<0.05). No distinct difference in
analgesic effect was observed between groups B and C (Table II).
 | Table IIAnalgesic evaluation of the three
groups after 180 days. |
Table II
Analgesic evaluation of the three
groups after 180 days.
| Group | n | CR, n (%) | PR, n (%) | NR, n (%) | CR+PR, n (%) | Z | P-value |
|---|
| Group A | 28 | 10 (35.7) | 14 (50.0) | 4 (14.3) | 24 (85.7) | 4.729 | 0.000 |
| Group B | 28 | 4 (14.3) | 10 (35.7) | 14 (50.0) | 14 (50.0) | 3.116 | 0.032 |
| Group C | 28 | 6 (21.4) | 13 (46.4) | 9 (32.1) | 19 (67.9) | 3.887 | 0.002 |
| χ2 | | | 22.699 | | | | |
| P-value | | | 0.000 | | | | |
Onset time and response duration of the
three groups
The results revealed that in group A the onset time
of pain relief was 1–4 days, averaging at 1.96±2.26 days, with the
fastest onset time in a patient noted as 1 day. In group B, the
onset time was 1–3 days, averaging at 1.43±1.79 days. In group C,
the onset time was 6–14 days, with an average of 11.67±3.14 days.
The onset time was significantly different among the three groups
(P<0.05). The fastest onset times in group A and B were markedly
shorter than that in group C (Table
III). The response duration was 146.68±1.89 days in group A,
71.60±2.94 days in group B and 112.99±1.37 days in group C. There
were significant differences among the three groups (P<0.05).
The response durations of treatment for groups A and C were longer
compared with that in group B (Table
III).
 | Table IIIOnset time and duration time of pain
relief following treatment. |
Table III
Onset time and duration time of pain
relief following treatment.
| Group | ST (days) | OT (days) | DT (days) |
|---|
| Group A | 1 | 1.96±2.26 | 146.68±1.89 |
| Group B | 1 | 1.43±1.79 | 71.60±2.94 |
| Group C | 6 | 11.67±3.14 | 112.99±1.37 |
| χ2 | 3.495 | 8.289 | 1.536 |
| P-value | 0.001 | 0.000 | 0.016 |
Adverse effects and complications
The incidence of adverse effects and complications
was 85.7% in group A, 82.1% in group B and 14.3% in group C. The
adverse effects and complications were considered to arise mainly
due to the argon-helium cryoablation; therefore, they were
significantly higher in groups A and B compared with those in group
C (all P<0.05). The majority of the adverse effects and
complications were relatively mild and the majority were alleviated
following short-term treatment (Table
IV).
 | Table IVAdverse reactions. |
Table IV
Adverse reactions.
| Group | Fever, n (%) | Fatigue, n (%) | Muscle pain, n
(%) | GT, n (%) | Rash, n (%) | Frostbite, n
(%) | Total, n (%) |
|---|
| Group A | 16 (57.1) | 3 (10.7) | 2 (7.1) | 1 (3.57) | 1 (3.57) | 2 (7.1) | 24 (85.7) |
| Group B | 15 (53.57) | 2 (7.1) | 3 (10.7) | 0 | 0 | 3 (10.7) | 23 (82.1) |
| Group C | 2 (7.1) | 0 | 2 (7.1) | 0 | 0 | 0 | 4 (14.3) |
Discussion
Bone metastasis is one of the common complications
in late malignant tumors. Approximately 50% of patients who develop
bone metastases will develop poorly controlled pain during the
course of their disease (20–22).
The present study reported significant evaluation of
analgesia and improvement in quality of life for patients with
focal painful bone metastases following percutaneous cryoablation
combined with zoledronic acid treatment. Profound analgesic relief
was reported in the three groups of patients, with rates of 85.7%
in group A (24/28), 50.0% in group B (14/28) and 67.9% in group
C(19/28). All of these strategies relieved the pain associated with
bone metastases, but cryoablation combined with zoledronic acid
appeared to have more efficacy than that observed for either
treatment alone. The response duration for the patients was
146.68±1.89 days in group A, 71.60±2.94 days in group B and
112.99±1.37 days in group C. The analgesic relief provided by
percutaneous cryoablation combined with zoledronic acid lasted
longer than that in the other two groups.
Bone metastasis itself is not fatal in the short
term. However, it may develop into pathological fracture and spinal
cord compression resulting in severe complications, including
paraplegia, if it is not effectively treated and well
controlled.
Zoledronic acid has been reported to be the most
effective of all bisphosphonate drugs. The mechanisms of zoledronic
acid in the treatment of malignant tumor bone metastases include:
i) inhibiting the maturation of osteoclasts; ii) restraining the
gathering and functioning of osteoclasts; iii) reducing the
production of cytokines (such as IL-6); iv) direct antitumor
activity (restraining cell proliferation and increasing cell lysis;
v) inhibiting tumor cell adhesion and infiltration in the bone
matrix; and vi) antiangiogenic effects (23–25).
Previous studies have reported that zoledronic acid
has a strong effect on bone metastatic pain, prolonged analgesic
activity and mild adverse reactions; therefore, it has become one
of the main analgesics used to relieve the pain of bone metastases.
Zoledronic acid is the first bisphosphonate that has demonstrated
effectiveness in all types of malignant tumor bone metastases. In
the present study, groups A and C were administered zoledronic acid
to treat metastatic bone pain, and the duration of the effect was
longer than that observed in group B (cryoablation alone) without
zoledronic acid. By contrast, the onset time of zoledronic acid
alone was slower than that of cryoablation, and its effect was
poorer than that for its combination with cryoablation.
Argon-helium cryoablation has a number of unique advantages in
treating cancer-associated pain, particularly bone metastatic pain
(26,27).
There are numerous causes of pain in cancer
patients; the primary causes are invasion and oppression of the
neighboring bone, nerves, skin, viscera and pleura by tumors, which
often cause continuous and or severely irritant pain. As
argon-helium cryoablation has been confirmed to be effective in
destroying tumor lesions locally by freezing, it may relieve or
reduce the invasion and oppression of neighboring tissues and
organs by the tumor. Therefore, cryoablation possesses potential
analgesic and pain-relieving properties. Cancer pain due to tumor
development and invasion is the main diagnostic indicator for the
initiation of cryoablation therapy. The effective treatment of
cancer-associated pain by argon-helium cryoablation is based on its
ability to directly destroy tumors. Compared with other therapies,
cryoablation may not only relieve pain but also control and
regulate the pathological effects of the tumor. Furthermore, it has
a confirmed effect, causes only mild injury, has fewer
complications and has no toxic adverse effects, amongst other
advantages (28,29). In the present study, groups A and
B, (a total of 56 cases) underwent percutaneous argon-helium
cryoablation. The results demonstrated that the pain of 38 cases
was significantly relieved, while 18 cases exhibited a poor
response to the therapy. No severe complications occurred in any of
the patients, which demonstrated that cryoablation has an improved
clinical effect and fast onset time, and when combined with
zoledronic acid, the response duration was markedly prolonged.
Multislice CT-guided percutaneous cryoablation has the advantage of
precise positioning and exactly monitoring of the ablation extent
during the treatment of malignant bone tumors; therefore, it may
clinically minimize complications and improve the success rate.
This, this technique is worth extending clinically for its safety
and accuracy.
In the present study, argon-helium cryoablation was
applied to treat bone metastatic pain. A CR was achieved in 85.7,
50.0 and 67.9% of patients in the groups treated with cryoablation
combined with zoledronic acid, cryoablation alone and zoledronic
acid alone, respectively. There were statistically significant
differences among the three groups (P<0.05). The results
demonstrated that cryoablation combined with zoledronic acid
exerted significantly fast responses and durable effects on bone
metastatic pain, which was superior to that of cryoablation or
zoledronic acid alone as this combination remedies the demerits of
both therapies. Additionally, no severe adverse effects and
complications were observed for this combination, suggesting that
this combined treatment is an acceptable therapeutic option for
patients with bone metastatic pain. However, further large-scale
studies are required to confirm these results and determine their
clinical utility in the treatment of bone metastatic pain.
References
|
1
|
Boyle P and Levin B: World cancer report
2008. International Agency for Research on Cancer; Lyon: World
Health Organization; Geneva: 2008
|
|
2
|
Berruti A, Dogliotti L, Bitossi R, et al:
Incidence of skeletal complications in patients with bone
metastatic prostate cancer and hormone refractory disease:
predictive role of bone resorption and formation markers evaluated
at baseline. J Urol. 64:1248–1253. 2000. View Article : Google Scholar
|
|
3
|
Meuser T, Pietruck C, Radbruch L, Stute P,
Lehmann KA and Grond S: Symptoms during cancer pain treatment
following WHO-guidelines: a longitudinal follow-up study of symptom
prevalence, severity and etiology. Pain. 93:247–257. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van den Beuken-van Everdingen MH, de Rijke
JM, Kessels AG, et al: Prevalence of pain in patients with cancer:
a systematic review of the past 40 years. Ann Oncol. 18:1437–1449.
2007.PubMed/NCBI
|
|
5
|
Weichselbaum RR and Hellman S:
Oligometastases revisited. Nat Rev Clin Oncol. 8:378–382. 2011.
|
|
6
|
Ollila DW, Gleisner AL and Hsueh EC:
Rationale for complete metastasectomy in patients with stage IV
metastatic melanoma. J Surg Oncol. 104:420–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh D, Yi WS, Brasacchio RA, et al: Is
there a favorable subset of patients with prostate cancer who
develop oligometastases? Int J Radiat Oncol Biol Phys. 58:3–10.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coleman R: Management of bone metastases.
Cancer Treat Rev. 23(Suppl 1): S69–S75. 1997. View Article : Google Scholar
|
|
9
|
Lipton A: Implications of bone metastases
and the benefits of bone-targeted therapy. Semin Oncol. 37(Suppl
2): S15–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rose AA and Siegel PM: Emerging
therapeutic targets in breast cancer bone metastasis. Future Oncol.
6:55–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McMenomy BP, Kurup AN, Johnson GB, et al:
Percutaneous cryoablation of musculoskeletal oligometastatic
disease for complete remission. J Vasc Interv Radiol. 24:207–213.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Callstrom MR and Kurup AN: Percutaneous
ablation for bone and soft tissue metastases - why cryoablation?
Skeletal Radiol. 38:835–839. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brace CL: Radiofrequency and microwave
ablation of the liver, lung, kidney, and bone: what are the
differences? Curr Probl Diagn Radiol. 38:135–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ford J, Cummins E, Sharma P, et al:
Systematic review of the clinical effectiveness and cost
effectiveness and economic evaluation of denosumab for the
treatment of bone metastases from solid tumors. Health Technol
Assess. 17:1–386. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80(8 Suppl): 1588–1594. 1997. View Article : Google Scholar
|
|
16
|
Delea T, Langer C, McKiernan J, et al: The
cost of treatment of skeletal-related events in patients with bone
metastases from lung cancer. Oncology. 67:390–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Cancer Institute and National
Institutes of Health. Common Terminology Criteria for Adverse
Events (CTCAE), Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdfuri.
Accessed February 10, 2009
|
|
18
|
Ripamonti CI and Brunelli C: Comparison
between numerical rating scale and six-level verbal rating scale in
cancer patients with pain: a preliminary report. Support Care
Cancer. 17:1433–1434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian J, Chen ZC and Hang LF: Effects of
nutritional and psychological status of the patients with advanced
stomach cancer on physical performance status. Support Care Cancer.
17:1263–1268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marks RM and Sachar EJ: Undertreatment of
medical inpatients with narcotic analgesics. Ann Intern Med.
78:173–181. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peteet J, Tay V, Cohen G and MacIntyre J:
Pain characteristics and treatment in an outpatient cancer
population. Cancer. 57:1259–1265. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Portenoy RK, Miransky J, Thaler HT, et al:
Pain in ambulatory patients with lung or colon cancer. Prevalence,
characteristics, and effect. Cancer. 70:1616–1624. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amadori D, Aglietta M, Alessi B, et al:
Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid
for prolonged treatment of patients with bone metastases from
breast cancer (ZOOM): a phase 3, open-label, randomised,
non-inferiority trial. Lancet Oncol. 14:663–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosen LS, Gordon D, Tchekmedyian S, et al:
Zoledronic acid versus placebo in the treatment of skeletal
metastases in patients with lung cancer and other solid tumors: a
phase III, double-blind, randomized trial - The Zoledronic Acid
Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol.
21:3150–3157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saad F: Clinical benefit of zoledronic
acid for the prevention of skeletal complications in advanced
prostate cancer. Clin Prostate Cancer. 4:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi J and Raghavan M: Diagnostic imaging
and image-guided therapy of skeletal metastases. Cancer Control.
19:102–112. 2012.
|
|
27
|
Hatoum HT, Lin SJ, Smith MR, Barghout V
and Lipton A: Zoledronic acid and skeletal complications in
patients with solid tumors and bone metastases: analysis of a
national medical claims database. Cancer. 113:1438–1445. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beland MD, Dupuy DE and Mayo-Smith WW:
Percutaneous cryoablation of symptomatic extraabdominal metastatic
disease: preliminary results. AJR Am J Roentgenol. 184:926–930.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Callstrom MR, Atwell TD, Charboneau JW, et
al: Painful metastases involving bone: percutaneous image-guided
cryoablation - prospective trial interim analysis. Radiology.
241:572–580. 2006. View Article : Google Scholar : PubMed/NCBI
|